Effectiveness of conjunctival bleb scarring by knockdown of heat shock protein 47 in rat model
Wei-Wei Wang, Hai-Yan Li, Huan-Huan Yan
Shaanxi Eye Hospital, Xi’an People’s Hospital (Xi’an Fourth
Hospital), Affiliated Guangren Hospital, School of Medicine,
Xi’an Jiaotong University, Xi’an 710004, Shaanxi Province,China
Abstract
● KEYWORDS: heat shock protein 47; filtration surgery;conjunctival bleb; scar; transforming growth factor-β1
INTRODUCTION
Glaucoma is the leading cause of irreversible blindness globally.It is believed that increasing intraocular pressure (IOP) is the primary risk that drives the pathological progression of this disease[1].Currently the only effective therapeutic option to treat glaucoma is to reduce IOP[2].It is well known that glaucoma filtration surgery (GFS) can successfully decrease IOP[2-3].However, conjunctival bleb scarring remains the leading cause of GFS failure[4-5].
The process of fibroproliferation eventually causes physiological challenges within the subconjunctival filtration region[4],leading to conjunctival bleb scarification and uncontrolled IOP.Mitomycin C (MMC) and 5-fluorouracil are clinically used to prevent scar tissue formation and increase surgery success[6-7].However, these therapies can cause complications, including endophthalmitis, corneal toxicity as well as bleb leakage[6-7].Therefore, it is of significant clinical importance to target a novel molecule that could play a vital role during postoperative wound healing in order to minimize GFS failure.
Heat shock protein (HSP47), a collagen-specific molecular chaperon, is closely related to fibrotic and ocular fibrosis diseases[8-14].Recent studies have shown that excessive collagen accumulation is a key pathological process of bleb scarring[15-16].Furthermore, we have shown that increased levels of HSP47 correlates with the growing deposition of collagen I and III in a rat conjunctival bleb model, suggesting that HSP47 may increase conjunctival bleb scarification[17].Recent evidence demonstrated that HSP47 may be a candidate as a therapeutic target against fibrotic diseases and breast cancer metastasis[9-10,18-19].However, mechanism of HSP47 in reducing conjunctival bleb scarring postoperatively remains unknown.To solve this issue, we have designed this study to evaluate the effect of HSP47 knock-down against conjunctival bleb scarring.Moreover, we have detected the expression levels of specific growth factors in order to further elucidate the scarification signaling pathways of conjunctival bleb scarring.
MATERIALS AND METHODS
Ethical ApprovalThis research was approved by the Ethics Committee of Xi’an Jiaotong University (No.2015-107).Male Sprague-Dawley rats (250-300 g weight) from Beijing Vital River Laboratory Animal Technology Co., Ltd.were used for this study.The rats were treated in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals.
Filtration Surgery in RatsFollowing acclimatization for seven days, the rats were randomly divided into five groups determined using a software-based random quantity generator prior to the experiments.They consisted of: normal control,phosphate buffered solution (PBS), shControl, sh-HSP47, and MMC-treatment groups.The PBS and MMC-treatment groups were used as the negative and positive control groups.
The techniques for developing the filtration surgical procedure model can be found in our previous research[17].Erythromycin was administered into the conjunctival sac at the end of the surgery to prevent infection.The rats were euthanizedviacervical dislocation at 2, 5, 8, and 11d after GFS to extract protein and mRNA.The method of collecting blebs and normal conjunctivas followed our previous study’s protocol[17].
Knock-down by AdenovirusWe cloned the annealed set of oligonucleotides that encoded the short hairpin transcripts for HSP47 in order to create the HSP47 knock-down vector.Bacterial colonies were collected for plasmid preparation.Prior to plasmid preparation, we measured the efficacy of the constructs ability to decrease the HSP47 mRNA transcripts in a cell cultured system.Next, we constructed an adenovirussiRNA vector using an AdEasyTMKit (MP Biomedicals).Briefly, to create a polymerase chain reaction (PCR) fragment,the H1-RNA promoter with 21nt siRNA templates were cloned at the Xho I and Not I restriction enzyme sites using pAdTrack.The adenoviral pAdEasy-1 and the resultant DNA were linearized with PacI, followed by cellular transfection utilizing a calcium phosphate method for adenovirus packaging.The shControl was constructed in the same way and the performance of the adenoviral infection used was the same method developed by Heet al[20].Cesium chloride ultracentrifugation was used to purify the adenovirus-siRNA vector.After dialyzing with PBS and 10% glycerol, the viruses were stored at -70℃ and used for all downstream experiments.
A microsyringe was inserted into the bleb of 0.1 mL liquid containing either PBS, shControl, sh-HSP47, or 0.04%MMC immediately after GFS followed by administration of erythromycin into the conjunctival sac.We then explored the silencing effect of HSP47 and the potential signaling pathways of HSP47 against scarification 11d after GFS.
Filtering Bleb MorphologyWe observed the size, shape,height, and surface blood vessel of the filtering blebs.According to the Kronfeld bleb classification[5]: Type I: the filtering vesicles are microcystic with thin walls; Type II: the blebs are flat, diffuse with thicker walls; Type III: filtering blebs disappear or bulge conjunctiva with hyperemia and multi-vascular appearance; Type IV: the filtering vesicles are limited dome-shaped with polycystic hyperplasia.As follows,type I and type II are defined as functional filtering blebs, and type III and type IV are classified as nonfunctional filtering blebs.Western Blot AnalysisUsing a lysis buffer we extracted total protein from the conjunctival blebs or control conjunctivas and used a bicinchoninic acid protein assay to quantify total protein concentration.Next, the samples were transferred onto a nitrocellulose membrane, and blocked using 10% dry milk and 0.1% bovine serum albumin (Fraction V) in PBS for 1h at room temperature.The membrane was then incubated at 4℃overnight with the primary antibodies.β-actin was used as the internal control and the protein bands were visualized using chemiluminescence.The relative intensities were calculated as the densitometric proportion between the protein band and β-actin using Image J.Each test was performed in triplicate.
Reverse Transcription Quantitative Polymerase Chain ReactionAn RNA-isolation kit (Takara, Japan) was used to extract RNA from the conjunctival blebs.A Prime Script RT Reagent Kit (Takara) was utilized for reversetranscription, followed by amplification with an ExTaq Kit (Takara).The PCR protocol is described as follows:denaturation at 95℃ for 30s twice, annealing and elongation at 60℃ for 10s followed by 75℃ for 15s.The entire process was repeated 40 times.Primers sequences used:HSP47: 5’-TGCTAGTCAACGCCATGTTC-3’ (forward),5’-ATCATGACACCCACGGTATAGG-3’ (reverse);collagen I: 5’-GCAATGCTGAATCGTCCCAC-3’ (forward),5’-CAGCACAGGCCCTCAAAAAC-3’ (reverse); and collagen III: 5’-AGGGCAGGGAACAACTGATG-3’(forward), 5’-GTCGCCATTTCTCCCAGGAA-3’ (reverse).β-actin was used to normalize the expression levels of HSP47 and collagen I and III.
Statistical AnalysisAll data in the current study were performed in triplicate and are presented as the mean±standard deviation.The normally distributed statistics were analyzed using a one-way ANOVA, and aPvalue <0.05 was considered statistically significant.
RESULTS
Blebs and Anterior Segment ObservationBleb formation was examined and functional filtration blebs were visible in all eyes immediately after GFS (Figure 1).Hyphema was observed in 6 eyes and was completely absorbed after 1-3d.No case of bleb leak, corneal oedema or endophthalmitis was detected.
At 2d after GFS, all groups of filtering blebs were swollen and diffuse.In the PBS and shControl groups, scarring of the blebs was prominent over time, showing conjunctival hyperemia and vascularization on the surface of the blebs.While sh-HSP47 treatment and MMC-treatment significantly prolonged the functional filtering bleb retention than the PBS and shControl treatment.At 5, 8, 11d after GFS, the filtering blebs remained bulged and diffuse in both sh-HSP47 and MMC-treatment groups, with mild conjunctival hyperemia and low-grade vascularization.
Highly Expressed and Silenced of HSP47 in Conjunctival Blebs after GFSUsing reverse transcription quantitative polymerase chain reaction (RT-qPCR), we analyzed the alterations of HSP47 gene expression within the conjunctival blebs.Figure 2A shows that the expression levels of HSP47 were increased for an extended time period after surgery.Compared to the normal control group, the changes varied significantly in a time-dependent manner within the surgical group (Figure 2B).The expression levels of HSP47 protein at day 11 postoperatively were significantly down-regulated after HSP47 silencing using adenovirus transfection with sh-HSP47,as shown in Figure 2C.
Silencing of HSP47 Inhibiting the Expression of Collagen I and IIICollagen induces scar formationviaabnormal expression and deposition onto the conjunctival bleb.To determine HSP47’s potential effect on scar formation, we explored the alteration of collagen I and III expression levels within the conjunctival blebs that were harvested 11d after administration of the different treatments.The RT-qPCR data showed that sh-HSP47 and MMC significantly down-regulates the expression of collagen I and III, revealing significant differences compared to the control groups (Figure 3A, 3B).The results of the Western blot analysis are shown in Figure 3C and 3D and confirm the results of the RT-qPCR analysis.
Effects of HSP47 on Related Signaling PathwaysTo further understand the mechanisms of HSP47 effects on scar formation at a molecular level, we found that transforming growth factor (TGF)-β1 is essential for the transdifferentiation of myofibroblasts.Figure 4A revealed that the knock-down of HSP47 diminishes the expression of TGF-β1 with MMC treatment following a similar trend.Moreover, p-Smad2,p-Smad3, as well as p-p38 expression levels in the absence of HSP47 were inhibited compared to the control groups,as shown in Figure 4B-4D.We determined that HSP47 may inhibit scar formation after GFSviaTGF-β1, Smad2/3, and p38 molecular signaling pathways.
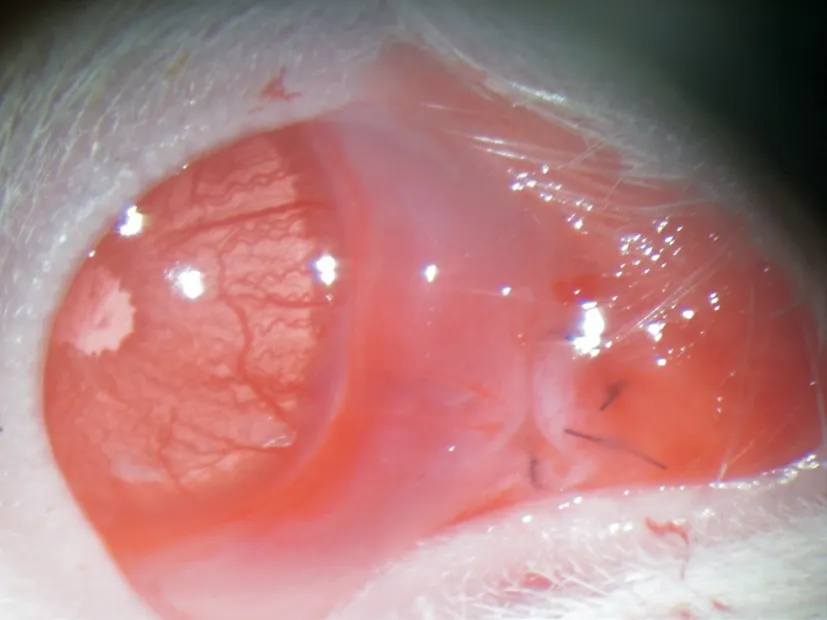
Figure 1 The filtering bleb after trabeculectomy A swollen and diffuse vesicle was visible with the black sutures on the fornix conjunctiva.
DISCUSSION
The principal pathological progression of fibrotic illnesses is due to the disorganized deposition of collagen[15-16].Approaches that slow or stop the synthesis and deposition of collagen may hold promise as an anti-fibrotic therapy.Recent data suggests that increased HSP47 is closely related to the immoderate deposition of collagen in several fibrotic diseases[8-14].Our research revealed that up-regulated HSP47 correlates with the elevated expression of collagen I and III within the conjunctival blebs after trabeculectomy[17].Therefore, we assume that HSP47 may play a role in conjunctival bleb scar formation.However, it is still unclear whether HSP47 is a therapeutic target that mediates conjunctival bleb scarring postoperatively.In this study, we demonstrated that silencing of the HSP47 gene reduces the deposition of collagen I and III.It has been shown that elevated levels of HSP47 in fibrotic illnesses aid in the excessive assembly and intracellular processing of procollagen molecules, resulting in the formation of fibrotic injuries.Conjunctival bleb scarification after GFS is a wound-healing process, with collagen I and III being valuable contributors during this process.In a functioning bleb,broadly spaced collagen is loosely organized.However, in non-functioning blebs, compact collagenous connective tissue was found[21].Furthermore, collagen I and III found in blebs progressively increase postoperatively[22].Our data suggest that silencing the HSP47 gene could lower the expression of collagen I and III, and alleviate conjunctival scarring after GFS.Postoperatively day 11 revealed that the expression of HSP47 had reached a maximum.Therefore, we picked this time to investigate our adenovirus construct’s silencing effect.Guoet al[12]revealed that decreased expression of HSP 47 gave rise to down-regulated expression levels of collagen in scleral fibroblasts.Wanget al[23]found that antisense treatment against the HSP47 gene suppressed the accumulation of collagen I during wound healing of neonatal rat skin, and assumed that silencing of the HSP47 gene had a therapeutic potential to inhibit skin scarification.Bianchiet al[24]demonstrated that lowering the expression of HSP47 down-regulated the levels of secreted collagen peptides, suggesting that HSP47 is a novel target in preventing the formation and growth of amyloid plaques.Nishinoet al[25]revealed that HSP47 antisense therapy reduced the expression levels of collagen I and III, indicating that inhibition of HSP47 expression may be a beneficial therapy for peritoneal fibrosis in continuous ambulatory peritoneal dialysis patients.These studies and our findings indicate that HSP47 is likely to be a novel therapeutic target against fibrosis by regulating the assembly and synthesis of collagen.

Figure 2 Heat shock protein 47 (HSP47) is high expression in conjunctiva tissue after glaucoma filtration surgery (GFS) A: The elevated levels of HSP47 genes expression are detected by real-time quantitative PCR in conjunctiva tissue at days 2, 5, 8, 11 after GFS; B: The elevated levels of HSP47 protein are detected using Western blot in conjunctiva tissue at days 2, 5, 8, 11 after GFS; C: Western blot analysis of HSP47 protein level in conjunctiva tissue after GFS transfection with shHSP47 or shControl.aP<0.05, bP<0.01, cP<0.001.PCR: Polymerase chain reaction.

Figure 3 Knocked down of heat shock protein 47 (HSP47) inhibit the expression of type I and type III collagen Real-time quantitative PCR analysis of type I collagen (A) and type III collagen (B).Western blot analysis of type I collagen (C) and type III collagen (D) at day 11 in conjunctiva tissue after glaucoma filtration surgery.bP<0.01.PCR: Polymerase chain reaction; MMC: Mitomycin C; PBS: Phosphate buffered solution.
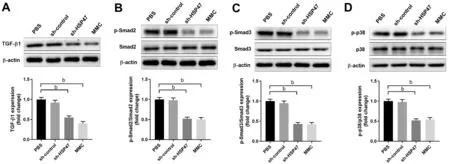
Figure 4 Knocked down of heat shock protein 47 (HSP47) inhibit the expression of transforming growth factor-β1 (TGF-β1)/phospho-Smad2,3 (p-Smad2, 3)/phospho-p38 (p-p38) signaling pathway Western blot analysis of TGF-β1(A), p-Smad2 (B), p-Smad3 (C), and p-p38 (D)at day 11 in conjunctiva tissue after glaucoma filtration surgery.bP<0.01.PCR: Polymerase chain reaction; MMC: Mitomycin C; PBS: Phosphate buffered solution.
Moreover, collagen has been recognized as a prognostic marker and is associated with cancer recurrence[26], with the HSP47/collagen axis playing a significant role in breast cancer metastasis[18].HSP47 silencing reprogrammed human breast cancer cells to a structured growth-arrested and/or a non-invasive construct, leading to the restriction of tumor growth by decreasing collagen deposition[26].This suggests that the research regarding the HSP47/collagen axis is of great significance and further studies are needed to solve bleb scarring after filtration surgery.
Recent studies have shown that the TGF-β1/Smad pathway and the MAPK signaling pathway play important roles in tissue fibrosis[27-28].Smad2 and 3 are two principal downstream regulators that promote TGF-β1 mediated tissue fibrosis, and p38 is a key MAPK signaling protein.Therefore, we detected the levels of Smad2, Smad3, p38, and the phosphorylation levels of the three proteinsviaWestern blotting.We found that knock-down of HSP47 significantly decreases TGF-β1,p-Smad2, p-Smad-3 as well as p-p38 expression levels,indicating that the TGF-β1/Smad pathway and the MAPK signaling pathway are involved in HSP47-induced scar formation.
The limitations of our study should be mentioned.Postoperatively day 11 time point was the only time point analyzed; therefore, and further studies are required to investigate the effect of HSP47 antisense therapy in a time dependent manner postoperatively.
To the best of our knowledge, this is the first research to explore the potential effect of HSP47 knock-down as a potential mechanism for scar formation after filtration surgery.Although the specific mechanisms of inhibition were not thoroughly studied, the research offers an alternative strategy to improve the surgical success of filtration surgery.
ACKNOWLEDGEMENTS
The authors thank AiMi Academic Services (www.aimieditor.com) for English language editing and review services.
Foundations:Supported by the National Natural Science Foundation of China (No.81500719); Shaanxi Science and Technology Project (No.2022SF-434); Xi’an Science and Technology Project (No.21YXYJ0044).
Conflicts of Interest: Wang WW,None;Li HY,None;Yan HH,None.
 International Journal of Ophthalmology2023年10期
International Journal of Ophthalmology2023年10期
- International Journal of Ophthalmology的其它文章
- A novel pathogenic splicing mutation of RPGR in a Chinese family with X-linked retinitis pigmentosa verified by minigene splicing assay
- Vault predicting after implantable collamer lens implantation using random forest network based on different features in ultrasound biomicroscopy images
- Multiple evanescent white dot syndrome relapse following BNT162b2 mRNA COVID-19 vaccination
- Acute micro-macular hole associating with extensive intraoperative rotation of implantable collamer lens without ophthalmic viscosurgical device assistance: a case report
- Effect of miR-27b-3p and Nrf2 in human retinal pigment epithelial cell induced by high-glucose
- lnfluence of hypoxia on retinal progenitor and ganglion cells in human induced pluripotent stem cell-derived retinal organoids
