Effectiveness and safety of COVID-19 vaccines in patients with oncological diseases: State-of-the-art
Nedelcho Ivanov,Boris Krastev,Dimitrina Georgieva Miteva,Hristiana Batselova,Radostina Alexandrova,Tsvetelina Velikova
Abstract Although the coronavirus disease 2019 (COVID-19) pandemic was declared to be no longer “a public health emergency of international concern” with its wide range of clinical manifestations and late complications,severe acute respiratory syndrome coronavirus 2 infection proved to be a serious threat,especially to the elderly and patients with comorbidities.Patients with oncologic diseases are vulnerable to severe infection and death.Indeed,patients with oncohematological diseases have a higher risk of severe COVID-19 and impaired post-vaccination immunity.Unfortunately,cancer patients are usually excluded from vaccine trials and investigations of post-vaccinal immune responses and the effectiveness of the vaccines.We aimed to elucidate to what extent patients with cancer are at increased risk of developing severe COVID-19 and what is their overall case fatality rate.We also present the current concept and evidence on the effectiveness and safety of COVID-19 vaccines,including boosters,in oncology patients.In conclusion,despite the considerably higher mortality in the cancer patient group than the general population,countries with high vaccination rates have demonstrated trends toward improved survival of cancer patients early and late in the pandemic.
Key Words: COVID-19;COVID-19 vaccines;RNA vaccines;Cancer;Oncological;Safety;Efficacy;Immunogenicity
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic has considerably impacted the lives of cancer patients.Their medical care has been challenging because of the competing risks of death from cancer or serious complications from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the likely higher lethality in immunocompromised hosts[1,2].Furthermore,patients diagnosed with malignancies are at higher risk of developing severe COVID-19[3] and fatal outcomes due to the disease.Studies have demonstrated variable mortality rates among subjects with hematological cancers and solid tumors,with some reporting fatality cases of as much as 40% of the infected subjects[4].Despite this considerably higher mortality than the one observed in the general population,trends towards improved survival during the evolution of the pandemic have already been demonstrated in Europe,and much of this could be a direct result of the rigorous COVID-19 vaccination in this region[5].
Since the beginning of the pandemic,hundreds of different therapeutic options have been studied,including those well-known in the treatment of other diseases,such as reoriented drugs.Amongst them are remdesivir (initially developed for hepatitis C treatment,tocilizumab-rheumatoid arthritis,hydroxychloroquine-malaria,lupus,etc),corticosteroids,plasma from donors who have recovered from COVID-19,monoclonal antibodies (casirivimab+imdevimab,bamlanivimab,sotrovimab,cilgavimab+tixagevimab,etc),Janus kinase inhibitors (baricitinib),and even mesenchymal stem cells[6,7].Targeting both the virus itself and the host’s immune response with variable effectiveness during the different stages of the disease.However,prevention in the form of COVID vaccines remains the most desirable option for the general population both in long-term health-related and financial terms.Cancer patients are no exception in this regard.But exactly how effective are vaccines in cancer patients compared to the general population? This is the question we will try to answer.
In this review,we elucidated to what extent patients with cancer are at increased risk of developing severe COVID-19 and what is their overall case fatality rate.We also present the current concept and evidence on the effectiveness and safety of COVID-19 vaccines,including boosters,in oncology patients.
SEARCH STRATEGY
We performed a modified form of a biomedical narrative review according to recent recommendations for writing[8].First,we thoroughly searched the scientific bibliographic databases Medline (PubMed) and Scopus.We used relevant free-text and Medical Subject Headings terms,as follows: (“COVID-19” OR “SARS-CoV-2”) AND (“cancer patients” OR“oncological patients”) AND (“COVID-19 vaccine” OR “mRNA vaccine”).We confined the search from January 1,2020 to June 20,2023.Then we identified additional papers using the search engine Google Scholar.Information from advisory committee meetings was also added.
COVID-19 AND PATIENTS WITH ONCOLOGICAL DISEASES
Patients with oncologic diseases are affected by SARS-CoV-2 in many different ways.Similar to many other infections,COVID-19 poses an additional risk of a fatal outcome for cancer patients.However,it is challenging to say to what extent patients with malignancies are threatened by complications of severe infections.As oncological diseases and treatment protocols are extremely diverse,it can be expected that the course of SARS-CoV2 infection would also be quite different[9-11].
The stage of disease,type of malignancy,and the sort and phase of the applied treatment modalities (surgery,chemotherapy,radiation therapy,and immunotherapy) introduce even more variables and more superimposing confounding factors,making this group of patients even more heterogeneous and difficult for overall risk assessment.Cancer patients who have recently undergone surgery or chemotherapy (especially during the induction phase with high-dose intensive regimens) are at a dramatically increased risk of death from COVID[12,13].Side effects of chemotherapy,such as secondary immunodeficiency due to severe leukopenia and specific tissue toxicity due to some chemo-and immunotherapeutics,can significantly alter the course of COVID-19 infection,from worsening the patient’s overall condition and increasing the risk of complications and death to masking or mimicking the radiological pulmonary signs (e.g.,immune checkpoint related pneumonitis)[13].Finally,another confounding factor is the various therapeutic regimens used to treat infection in hospitals and intensive care units worldwide.Cancer patients are treated as high risk by default,which carries a risk (polypharmacy,drug interactions,adverse drug effects,acute kidney or liver failure,etc)[14].
Below,we present data from several studies that attempt to measure and objectify this risk.The first large-scale metaanalysis by 2020 done by Zhangetal[11] of 15 studies involving a total of 3019 patients from Europe,the United Kingdom,the United States,Canada,and Asia detected 22.4% circulating free RNAs (CFRs) in cancer patients with COVID-19,compared to 5.9% in noncancer patients.As in other patients,risk factors influencing the course and mortality are: Being over 65 years old,male sex,and having comorbidities (especially hypertension and diabetes).No significant difference in mortality was found between different continents.The study found that mortality in patients with lung cancer and hematological malignancies was highest,although the incidence of complications did not differ[11].
A study by Yangetal[15] involving 1575 patients,of whom 52 with various cancers (lung,colorectal,breast,cervical,thyroid,etc) showed that oncologic patients are at higher risk of presenting as severe/critical cases and are more likely to develop acute respiratory distress syndrome.Also,other life-threatening complications such as myocardial infarction and shock are significantly increased in frequency.Lower lymphocyte count,as well as higher concentrations of C-reactive protein,D-dimer,procalcitonin,interleukin 6 (IL-6),and lactate dehydrogenase,were reported to reachP< 0.05.Cancer patients are also more likely to have comorbidities,which,as it becomes clear in this study,contributes seriously to the overall higher CFR[15].
A meta-analysis of 122 papers and 9 studies,including a total of 805 patients by Afsharetal[16],demonstrated how heterogeneous the data on mortality in cancer patients are.They showed that cancer patients are more likely to be admitted to intensive care units,need invasive ventilation,and are more likely to die.The published CFR in the analyzed studies ranges from 5.5% to 60.0%,with a pooled CFR of 21%.However,the authors warn that these data should be interpreted cautiously due to the high heterogeneity and the small number of patients in most studies[16].
Large-scale survival analysis by Lietal[9] based on data from United Kingdom Biobank followed 4606 cancer patients(288 positives) and 4606 noncancer patients (275 positives) for 21 mo after the SARS-CoV2 test.The cumulative CFR of the positive cancer patients was six times higher than the negative ones.The hazard ratio was assessed for each specific malignancy in the study,and the results showed that hematological malignancies,melanoma,kidney,and uterine cancer had particularly high CFRs (up to 10 times higher than the noncancer controls).The authors emphasize the importance of timely vaccination in these groups of patients[9].
In contrast to the data above,a study by Braretal[17] included 585 patients,117 with active malignancies.It showed no statistically significant difference in morbidity or mortality in cancer patientsvsthe general population.Furthermore,the authors argued that the studies claiming the opposite did not consider confounding factors such as age,sex,and comorbidities.According to this study,cytotoxic treatment within 90 d of admission is not associated with worse outcomes[17].
A team from London published a study in onco-hematology patients,where 40% (14 of 35) of patients hospitalized with COVID-19 had succumbed to the infection[18].In general,COVID-19 appears to have an increased risk of complications and mortality in a large proportion of cancer patients.In addition,besides the virus itself,the pandemic and the restrictive measures were associated with disrupted access to medical care,hindered timely diagnosis and treatment,the lack of follow-up of many patients and lower quality of life[19].Studies have shown that since the beginning of the pandemic,the total number of newly diagnosed cancers has dropped substantially[20].As many authors warned,this inevitably led to an increased frequency of advanced cancers at diagnosis.Delaying diagnosis and treatment resulted in lower chances of survival[21].Yongetal[22] conducted a study in Canada using microsimulation models,which estimated that for colorectal cancers,suspending primary screening for only 6 mo will increase cancer incidence by about 2200 cases,of which about 960 will be lethal over time.Consequences that otherwise would be prevented by the screening program and early detection.
Furthermore,there are many other indirect ways the COVID-19 pandemic affects cancer patients’ quality of life and mortality[13].At the same time,the standard of living,the structure and stability of the health care system,and even political factors in connection with dealing with the pandemic play roles that should not be underestimated[23].Knowing risk factors for the severity and mortality of COVID-19,cancer patients have their unique risk factors.They may include active and progressing cancer,type of cancer,administration of cytotoxic chemotherapy,radiation therapy,impaired immune system due to leukocytopenia,low immunoglobulin levels,long-lasting immunosuppression,comorbidities,and others.
Malignancies reported as comorbidities in patients hospitalized with confirmed COVID-19 in different countries are:(1) Malignancies in 7.2% in a cohort study with 138 adults with confirmed COVID-19 pneumonia in Wuhan,China,in January 2020[24];(2) malignancies in 8% at admission in a cohort study with 1591 patients with laboratory-confirmed COVID-19 in Lombardy,Italy between February 20 and March 18,2020[25];and (3) Malignancies reported in 5.6% at admission in a cohort study with 5700 patients with confirmed COVID-19 infection hospitalized in 12 New York City hospitals between March 1 and April 4,2020[26].
In a cohort study of 928 adults with COVID-19 and current or past cancer diagnosis,solid tumors were found in 82%,including breast (21%),hematologic (22%),prostate (16%),gastrointestinal (12%),thoracic (10%),gynecologic (5%),and renal cell carcinoma (5%)[27,28].The estimated overall mortality in the research was 13%: 20% for patients with multiple cancers,18% for patients with hematological malignancies,and 12% for patients with solid tumors[27].
Zhangetal[11] showed the COVID-19 fatality rates in subgroup analysis: (1) By cancer type: 32.9% in patients with lung cancer;34.2% in patients with hematologic cancer;17.2% in patients with solid cancer;and (2) By cancer treatment:25.6% in patients with chemotherapy,27.6% in patients with surgery,24.3% in patients with immunotherapy,21.3% in patients with targeted therapy,and 20.5% in patients with radiation therapy[11].
Children with cancer and positive for COVID-19 are at higher risk of severe illness than children without cancer.The cohort study found that about 20% of pediatric cancer patients with COVID-19 experienced a severe infection,compared to 1%-6% of children in the general population[29].Among patients with hematologic malignancy and laboratoryconfirmed COVID-19,mortality was reported in 34% of adults and 4% of children[4].
We can summarize that the main challenges in cancer patients regarding COVID-19 are the often immunocompromised state (e.g.,due to leukocytopenia,low immunoglobulin levels,long-lasting immunosuppression),the treatment(e.g.,severe chemotherapy,radiation therapy),progression of cancer,comorbidities,and others.
IMMUNE RESPONSE IN CANCER PATIENTS
Cancer cells induce an immune suppressive microenvironment and use various mechanisms to “escape” the body’s immune response.As a systemic disease,cancer causes a wide range of functional and compositional changes in the immune system and can affect the body’s defenses against various pathogens[30,31].
Dendritic cells (DCs) are antigen-presenting cells with an essential role in originating and directing cellular and humoral immune responses,converging innate and adaptive immunity.DCs have been recognized as the most potent professional antigen-presenting cells[32].
Tumors use different strategies to alter DC maturation and function,such as: (1) The ability to influence the capacity of hematopoietic progenitor cells to differentiate into functional DCs[33,34];(2) production of various immunosuppressive factors that block the maturation of CD34+stem cells into DCs[35];and (3) spontaneous apoptosis of DCs in peripheral blood of patients with breast cancer[36].Quantitative and functional DC deficiencies have been widely observed in patients with several types of cancer including breast cancer[37,38],prostate cancer[38],non-small cell lung cancer[39,40],colon cancer[41],and melanoma[42].
Data have revealed that tumors disrupt normal hematopoiesis,leading to extramedullary hematopoiesis and myeloid skewing.The three branches of terminally differentiated myeloid cells (macrophages,DCs,and granulocytes) are essential for normal innate and adaptive immune response functioning.The tumor microenvironment alters myeloid cells and can convert them into potent immunosuppressive cells[43,44].Lymphopenia caused by disease or treatment is frequent in oncology patients and affects their prognosis[45,46].
T cells,one of the primary arms of the adaptive immune response,are also affected in oncology patients.Cancer cells express various membrane and soluble T-cell inhibitory signals.For example,programmed cell death protein-ligand 1 linking to programmed cell death protein 1 on T cells results in decreased activation,proliferation,survival,and cytotoxicity[47].The last discovery led to the development of checkpoint inhibitors,a breakthrough in immuno-oncology,which led to the 2018 Nobel Prize for Physiology or Medicine.Indoleamine 2,3-dioxygenase,a soluble enzyme physiologically expressed in many tissues,is overproduced in some cancers leading to tryptophan depletion in the tumor microenvironment.T cells,being highly sensitive to tryptophan deprivation,suffer significant functional impairment,promoting tumor growth[48].An increased rate of CD4+CD25+regulatory T cells with potent immunosuppressive properties in the peripheral blood of individuals with cancer diseases has been reported[49,50].
Additionally,regulatory B cells (Bregs) are a newly designated subset of B cells that play a central role in regulating immune responses associated with inflammation,autoimmunity,and cancer.Increased Bregs express immunosuppressive properties in gastric cancer through the secretion of anti-inflammatory molecules,such as IL-10,and facilitating the conversion of T cells to regulatory T cells[44,51,52].Additionally,tumor progression is associated with the dysfunction of natural killer cells due to the combined action of tissue-specific and systemic factors[53].All of these immune alterations in cancer patients contribute to the differences in immune response after vaccination,including after COVID-19 vaccine administration.Before the COVID-19 pandemic,we had an experience with influenza vaccine administration in patients with oncological diseases.Infectious complications resulting from bacterial,fungal,and viral (often due to reactivation of latent disease,primarily in patients with hematological malignancies) diseases are a severe cause of morbidity and mortality in cancer patients[54].Oncology patients receiving chemotherapy are at increased risk of influenza virus infection and serious post-influenza complications.Cancer patients are eligible for influenza vaccination,although their response may be suboptimal due to immunosuppression associated with cancer itself and/or its treatment[55,56].Data have shown that cancer patients receiving chemotherapy can respond to influenza vaccination[57].
Breast cancer patients receiving influenza vaccination during FEC (5-fluorouracil,epirubicin,and cyclophosphamide)-containing treatment regimens have exhibited significantly lower responses to influenza virus vaccination than healthy controls.Vaccination early during the chemotherapy cycle (day 4) induces better responses than vaccination on day 16[58].The summary of the available evidence reveals that immunization of individuals with malignancies is critical to their care and may protect them from significant morbidity and mortality associated with vaccine-preventable diseases[59].
COVID-19 VACCINES FOR PATIENTS WITH ONCOLOGICAL DISEASES-DATA ON OUTCOMES AND EFFECTIVENESS
Several available COVID-19 vaccines are now in use all over the world.Moderate or severely immunocompromised people should receive a vaccination to protect them from severe COVID-19 disease[60,61].
The efficacy of COVID-19 vaccines in cancer patients is a question of continuous research,with most studies using immunological parameters as surrogate endpoints for clinical outcomes.Clinical trials investigating immune response after COVID-19 vaccination often use seroconversion to SARS-CoV-2 spike (S) protein as an endpoint for vaccine efficacy.Other parameters such as anti-spike antibody titers,detection of neutralizing antibodies,and cellular immune response are usually explored as secondary endpoints[62].Some authors,however,underscore the role of neutralizing antibodies as the immunological parameter,which probably best correlates with the level of protection after COVID-19 vaccination[63-65].
Both humoral and cellular immune responses to COVID-19 vaccines differ in patients with malignancies compared to noncancer patients;this is not only attributed to the immunosuppressive nature of the oncologic disease but also to the antitumor therapy itself and its direct impact on immune cells.While patients with solid tumors have seroconversion rates similar to the general population,the most significant concern regarding post-vaccination and post-infectious COVID-19 immunity lies with hematological malignancies,especially those where lymphocyte-depletion therapy is used.In support of this is the research of Moninetal[66],who presented interim results of a prospective observational study that explores the immunogenicity of one compared to receiving two doses of the COVID-19 vaccine in patients with cancer by assessing the humoral immune response between 151 patients (95 with solid tumors and 56 with hematological malignancies) and 54 healthy controls.Authors reported efficacy after the first dose in 94%,38%,and 18% of control subjects,patients with solid tumors and hematological cancer,respectively.After the second dose,the response increased to 100% in controls,95% in patients with solid cancers,and only 60% in the group with hematological malignancies[66].
When considering post-vaccination immunity in patients with cancer,we should consider that those with hematological malignancies are expected to show different levels of antibody response to COVID-19 vaccines compared to patients with solid tumors.One of the most substantial pieces of evidence in corroboration came from the CAPTURE trial[67].This prospective clinical study assessed the humoral response after COVID-19 vaccination in more than 700 subjects with solid tumors or hematologic neoplasms,585 of whom did not have previous SARS-CoV-2 infection.The trial demonstrated 85% and 54% seroconversion rates for anti-spike antibodies after the second dose in patients with solid tumors and hematological malignancies,respectively.However,the response observed among participants was not the same for all SARS-CoV-2 variants[68].
The authors announced substantial differences in neutralizing antibodies concerning viral genotypes from the CAPTURE trial: 83% of patients developed detectable levels of the original SARS-CoV-2 and only 54% of the delta variant.And while nearly two-thirds (62%) of patients with solid tumors elicit humoral response against delta variant,only 31% of those with hematologic malignancies did so[67].The prospective cohort study of immune response to COVID-19 vaccination in cancer patients CAPTURE (NCT03226886) also showed that among 585 patients,the antibody rates after two doses of BNT162b2 or AZD1222 vaccines given over 12 wk were assessed.The results showed that seroconversion was 85% and 59% after two doses in patients with solid and hematological malignancies,respectively.Neutralizing antibodies against SARS-CoV-2 VOCs were detected in a small proportion of patients,mainly with solid cancers.Vaccine-induced T-cell responses were found in 80% of patients regardless of the vaccine or type of cancer[67].
In an attempt to overcome this relatively low rate of seroconversion in patients with blood cancers,Greenbergeretal[69] conducted a large prospective cohort trial on nearly 700 patients vaccinated with three doses of the COVID-19 vaccine.It was estimated that antibody response indeed increased with the 3rd(booster) dose,so 43% of those without detectable antibodies after the 2nddose demonstrated humoral response after the booster.However,about 20% of all hematological patients still failed to achieve a response even with 3 doses of vaccine[69].In contrast to the plethora of research on humoral immunity after COVID-19 vaccination in cancer patients,the cellular immune response in this setting is considerably less studied.In a review article by Rüthrichetal[70],the authors tried to summarize what is currently known about the issue in patients with solid tumors and hematological malignancies,comparing data from COVID-19 vaccines and other ” classical” vaccines.Although the assessment of T-cell immune response in the reviewed studies varied,most research used methods based on quantifying and characterizing pathogen-specific T cells and/or estimating T-cell function by cytokine measurement[70].
Observations on immune responses in patients with hematological malignancies revealed that although this population may lack adequate levels of neutralizing viral antibodies,especially after treatment with B cell-depleting agents such as anti-cluster of differentiation 20 monoclonal antibodies,COVID-19 vaccines are still able to produce protective cellular immunity.Solid evidence for the sufficient efficacy of T-cell response comes from a trial in patients with agammaglobulinemia who demonstrated improved COVID-19 infection outcomes after vaccination.However,cellular immunity could also be impaired in this specific patient population,and some of the significant factors for this are age,disease activity,immunosuppressive treatment,and low lymphocyte counts in circulation[70].
This discordance between humoral and cellular immune response could also be seen In patients with solid tumors.In this population,T-cell responses vary among different cancer subtypes and are determined mainly by the type of systemic antitumor treatment.Various studies have demonstrated wide ranges in terms of cellular immunity achieved after COVID-19 vaccination ranging from about 50% to nearly 90% of the vaccinated cases[71,72].
However,despite being generally higher than those observed in blood cancer patients,T-cell response in those with solid tumors remains significantly lower than in healthy controls.One of the most extensive trials reporting data on immune response in patients with solid tumors receiving systemic anticancer treatment is the VOICE study[73].After recruiting nearly 800 subjects (240 without cancer),the authors assessed cellular immunity by measuring the SARS-CoV-2 spike-specific interferon gamma T-cell response after two vaccine doses.They reported cellular responses in 67%,66%,and 53% of patients treated with chemotherapy,immunotherapy,or chemoimmunotherapy,respectively.Another interesting trial finding was that more than 40% of patients who did not elicit a humoral immune response could develop a T-cell response,highlighting the vaccine’s ‘double-edge sword’ efficacy in this specific population.Similar to the model observed with the humoral response,whether the cellular response is affected by a booster dose is still an open question since there are conflicting data.Some studies have reported significant enhancement of the T-cell response after the 3rddose,whereas others refute such assertions[73].
To date,most trials reporting COVID-19 vaccine efficacy in cancer rely on immunological endpoints and not so much on clinical outcomes.However,a recent study on infection rate and outcomes in vaccinated patients with solid tumors and hematologic malignancies has raised concern that despite vaccination,these patients remain at risk of worse outcomes compared to the general population[74].Among fully vaccinated cancer patients,who experienced breakthrough SARS-CoV-2 infection,the hospitalization rate,intensive care unit admission (or required mechanical vaccination),and death rate are 65%,19%,and 13%,respectively.This is mainly attributed to patients’ comorbidities and the much worse COVID-19 prognosis in those with hematological malignancies.
In a prospective study conducted by Goshen-Lagoetal[75],it was shown that patients with solid tumors demonstrated short-term efficacy and safety of the BNT162b2 vaccine.A follow-up study evaluated these outcomes at 6 mo after vaccination[76].Participants were 154 patients with solid tumors and 135 controls (health workers).At 6 mo after vaccination,122 patients were seropositive compared with 114 controls,and the serologic titers dramatically decreased almost equally in both cohorts.Efficacy and safety evidence of BNT162b2 vaccines shows that the serological profile in cancer patients after 6 mo resembles that of the general population[76].
A similar study was conducted by Barrièreetal[77],who evaluated the immunogenicity of the BNT162b2 vaccine in patients with solid tumors.Serological analyses were performed during the first vaccination,during the booster dose(w3-w4),and 3-4 wk after the booster dose (w6-w8).The study reported the results for 122 of 194 evaluable patients with solid tumors who had at least two doses from January 2021 to March 2021.In the first analysis (w3-w4),58 patients had neutralizing antibodies,although the median levels were significantly lower than in the control group.In the following analysis (w6-w8),the data showed the same anti-S seroconversion rate,demonstrating impaired immunogenicity of the BNT162b2 vaccine in cancer patients[77].
Shroffetal[78] also compared anti-S seroconversion to the BNT162b2 mRNA vaccine in patients with solid tumors on active cytotoxic anticancer therapy with healthy control participants.Neutralizing antibodies were found in 67% of cancer patients after the first immunization,and a follow-up analysis found a threefold increase in titers after the second or third doses.European Union Drug Regulating Authorities Clinical Trials (EudraCT) Number 2021-000291-11 was conducted in patients with solid cancers,multiple myeloma,and inflammatory bowel disease[79].The study is a prospective,openlabel,phase four trial to monitor vaccine-specific antibody and cellular responses after booster vaccination with mRNA-1273 or BNT162b2.The data show that booster vaccination against SARS-CoV-2 reverses the lack of response and early antibody weakening in immunocompromised patients.
Another study on the efficacy and safety of heterologous booster vaccination with Ad26.COV2.S after BNT162b2 mRNA vaccine in cancer patients without antibody response was conducted in 2022[80].The assessment was done directly before vaccination and 4 wk after.Ad26.COV2.S booster vaccination resulted in a serological response in 31% of nonresponders after a double dose of BNT162b2.Clinical trials with the number NCT04368728 reported results from individuals with a history of past or active neoplasms and up to 6 mo of follow-up after dose 2 of a placebo-controlled,observer-blinded trial of the BNT162b2 vaccine[81].In participants with past or active neoplasms,two doses of the BNT162b2 vaccine improved efficacy and safety profile as in the overall trial population.No vaccine-related deaths were reported.
One of the first evaluations of the effectiveness of vaccination against breakthrough SARS-CoV-2 infections in cancer patients at a population level was done by Leeetal[82].Analysis was performed in the cancer cohort by vaccine type(BNT162b2,ChAdOx1 nCov-19,or mixed,and other),cancer type and subtype,stage,date of cancer diagnosis,and anticancer treatment or radiotherapy.Data show that vaccination with different COVID-19 vaccines is effective in people with cancer,providing varying levels of protection against SARS-CoV-2 infection.However,it is lower in cancer patients than in the general population[82].
A single-arm prospective clinical trial was conducted with 106 cancer patients by Thakkaretal[83].They received two doses of mRNA followed by one dose of AD26.CoV2.S vaccine or a third dose of mRNA vaccine.The results showed that a third dose induced immunity in cancer patients.Seroconversion was also assessed in 57% of patients who did not respond to primary vaccination.A fourth dose boosted the immune response by two-thirds.Some patients have neutralizing activity against the omicron variant[83].
In conclusion,all of these studies confirm that people with cancer are at increased risk of severe COVID-19 disease,hospitalization,and death after SARS-CoV-2 infection compared to the general population.The above data show that cancer patients have impaired overall vaccine effectiveness to the approved COVID-19 vaccines.Seroconversion in them decreases faster than in the control population.Although vaccination provides different levels of protection,there should be a global prioritization of the programs to boost vaccination for cancer people,considering the impact of other treatments.
There are still a lack of data on vaccine efficacy in cancer patients concerning novel virus variants like omicron[68].Table 1 presents the studies on the effectiveness and safety of COVID-19 vaccination with different approved COVID-19 vaccines in oncological patients with solid tumors[5,63,66,67,69-71,73,75-84,84].
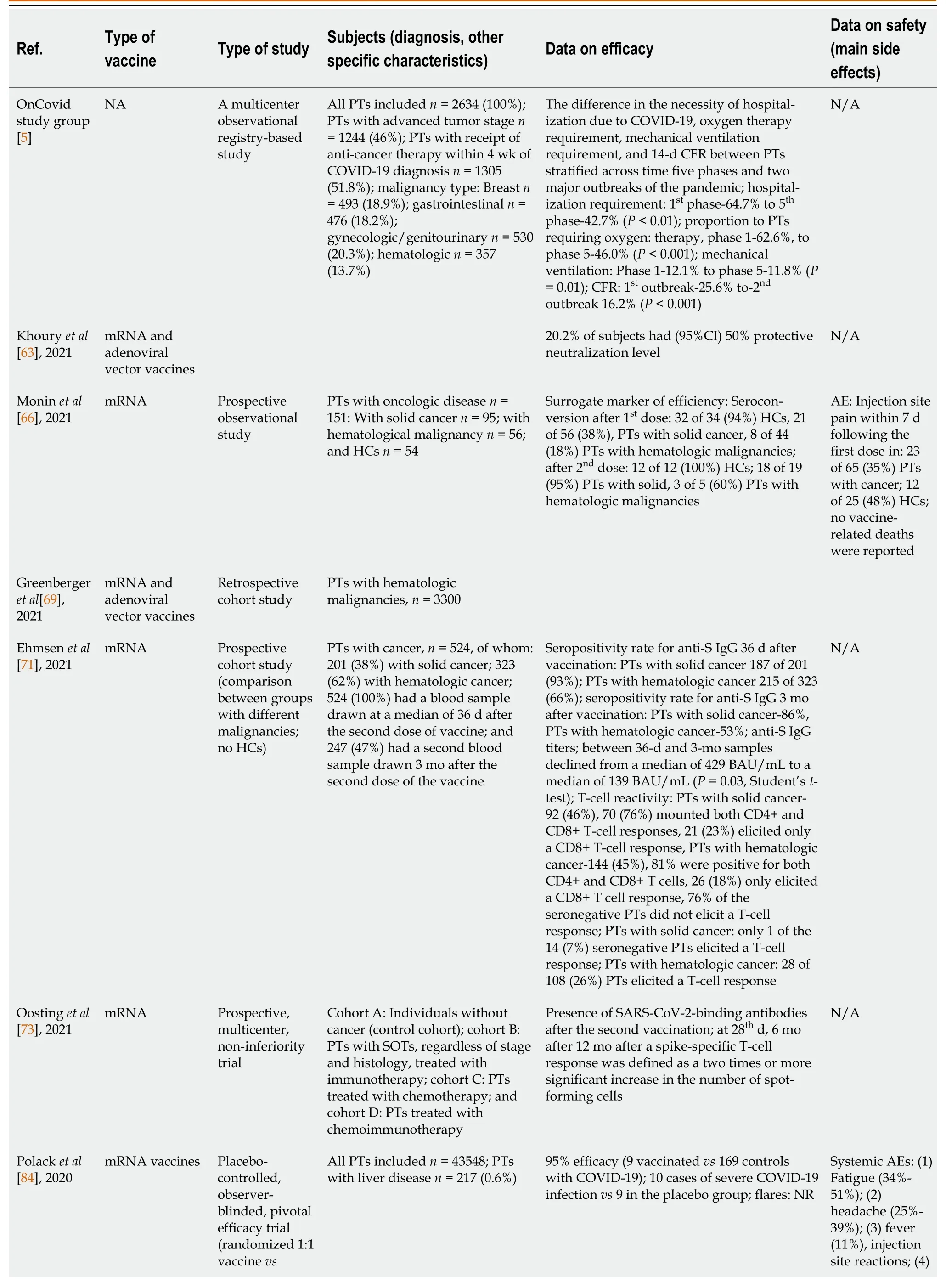
Table 1 Some of the more significant studies conducted on the efficacy and safety of COVID-19 vaccination with different approved COVID-19 vaccines in oncological patients with solid tumors
COVID-19 VACCINES AND CHEMOTHERAPY INTERACTIONS
People with cancer often have an increased susceptibility to infections due to various factors,including cancer itself and/or,in some cases,the applied therapy,poor nutrition,and damaged physiological barriers.In addition,the incidence of neoplasia is highest in individuals aged 65 and over.When the immune system’s effectiveness is weakened,the elderly often have concomitant diseases for which they can also take medications[54,85].
Regarding cancer chemotherapy,conventional antitumor chemotherapeutic agents kill actively proliferating cells,including bone marrow cells,and myelosuppression is one of clinical oncology’s most common side effects[86].Chemotherapy-induced neutropenia is a significant cause of hematological and dose-limiting toxicities of chemotherapy[87].Some currently available anticancer drugs,such as methotrexate and cyclophosphamide,express immunosuppressive effects and impair peripheral T cells’ proliferative and/or effector functions.Methotrexate is an antimetabolite of the antifolate type developed in 1947 and is included in the World Health Organization’s List of Essential Medicines.Currently,it is widely used not only in clinical oncology (in the treatment of acute lymphoblastic leukemia,acute myeloid leukemia,meningeal leukemia and lymphoma,osteosarcomas,non-Hodgkin’s lymphoma,breast and bladder cancers,etc.) but also as a first-line treatment in autoimmune,inflammatory diseases such as rheumatoid arthritis,psoriasis and Crone’s disease[88-90].Methotrexate has been found to disturb antibody response after pneumococcal vaccination[91,92];the drug reduces circulating T helper 17 (Th17) cells and impairs plasmablast and memory B-cell expansion following pneumococcal conjugate immunization in patients with rheumatoid arthritis[93].
Cyclophosphamide is an alkylating agent synthesized in 1958 and used for decades in clinical practice in the therapy regimens of neoplasms (malignant lymphomas,multiple myeloma,sarcoma,breast cancer,disseminated neuroblastomas,retinoblastoma,ovarian adenocarcinoma,etc) and as an immunosuppressive agent for the treatment of autoimmune and immune-mediated diseases such as multiple sclerosis.Cyclophosphamide shows selectivity for T cells and is an immunosuppressant to prevent transplant rejection and graft-vs-host complications[94].Cyclophosphamide has been associated with suppressing helper Th1 activity and enhancing Th2 responses[95].This drug inhibits Th1/Th17 responses and increases the cells secreting anti-inflammatory cytokines such as IL-4,IL-10,and transforming growth factor beta[96].A single administration of low-dose cyclophosphamide selectively suppresses regulatory T cells.The low-dose cyclophosphamide promotes antitumor immunity by selectively depleting regulatory T cells and enhancing effector T cell function.However,cyclophosphamide can also increase the number of myeloid-derived suppressor cells[97,98].
Treatment with tyrosine kinase inhibitors imatinib,dasatinib,and nilotinib applied in the treatment of chronic myeloid leukemia is associated with loss of memory B-cell subsets and impaired humoral immune responses to 23-valent polysaccharide pneumococcal vaccine,likely due to the off-target kinase inhibitory activity of these drugs[99].
CONCLUSION
Data so far show that patients with cancer are at increased risk of severe COVID-19 and developing various complications mainly due to their immunocompromised state,type of treatment and comorbidities.Although cancer patients were excluded from vaccine trials,the investigations of post-vaccinal immune responses and the effectiveness of the vaccines showed that both humoral and cellular immune responses to COVID-19 vaccines differ in patients with malignancies compared to noncancer patients,and this is being attributed not only to the immunosuppressive nature of the oncologic disease but to the antitumor therapy itself and its direct impact on immune cells.
The evidence indicates that the efficacy of vaccinations could be impaired in cancer patients in line with a reduced rate of seroconversion and shorter duration compared to healthy controls.Despite these data,when focusing on the clinical outcomes instead of immunological endpoints regarding vaccine efficacy,COVID-19 vaccines demonstrated high effectiveness in preventing severe COVID-19 and infection-related death,and safety profile with comparable to healthy controls adverse effects in patients with solid tumors and hematological malignancies.
Despite the considerably higher mortality in the cancer patients group from COVID-19 than the general population,countries with high vaccination rates have demonstrated trends toward improved survival of cancer patients early and late in the pandemic.Nevertheless,vaccination of these patients and overall vaccination of the population has proven to significantly reduce the risk of complications and mortality of COVID-19 and should be promoted worldwide.
FOOTNOTES
Author contributions:Ivanov N and Velikova T contributed to conceptualization;Krastev B contributed to methodology;Miteva DG contributed to software;Ivanov N,Alexandrova R,and Velikova T contributed to validation;Ivanov N contributed to formal analysis;Krastev B contributed to investigation;Batselova H contributed to resources;Miteva DG contributed to data curation;Ivanov N,Krastev B,and Miteva DG contributed to writing-original draft preparation;Batselova H contributed to writing-review &editing;Miteva DG contributed to visualization;Velikova T contributed to supervision,project administration,and funding acquisition;and all authors revised and approved the final version of the manuscript.
Supported bythe European Union-Next Generation EU,through the National Recovery and Resilience Plan of the Republic of Bulgaria,No.BG-RRP-2.004-0008.
Conflict-of-interest statement:The authors have no conflicts of interest to declare.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Bulgaria
ORCID number:Nedelcho Ivanov 0000-0002-7471-7368;Boris Krastev 0000-0003-4196-0828;Dimitrina Georgieva Miteva 0000-0002-5931-2426;Hristiana Batselova 0000-0002-6201-848X;Radostina Alexandrova 0000-0002-7699-6479;Tsvetelina Velikova 0000-0002-0593-1272.
S-Editor:Chen YL
L-Editor:Filipodia
P-Editor:Chen YL

