Methanolic pomegranate dried peel extract improves cryopreserved semen quality and antioxidant capacity of rams
Amal M.Aboelmaaty ,Mohamed S.Kotp ,Aya M.Fadl ,Elshymaa A.Abdelnaby ,Islam E.El-Seadawy ,Hossam R.El-Sherbiny
1Animal Reproduction and Artificial Insemination Department,Veterinary Research Institute,National Research Centre,Cairo,Egypt
2Theriogenology Department,Faculty of Veterinary Medicine,Cairo University,Cairo,Egypt
ABSTRACT Objective:To select the appropriate concentrations of methanolic pomegranate extract supplemented in rams’ semen extender for obtaining the best-cryopreserved semen quality.Methods:Tris-based semen extender was supplemented with 0.0,0.40,0.48,and 0.56 mg/mL pomegranate peel methanolic extract to extend semen collected from five native rams twice weekly for two months (n=80).Pooled (n=16) post-thaw semen characteristics were determined.Thawed seminal plasma of all supplemented and control groups were used to measure malondialdehyde (MDA),nitric oxide (NO),glutathione peroxidase (GPx),superoxide dismutase(SOD),ascorbic acid,zinc,copper,total cholesterol,low-density lipoproteins (LDL),lactate dehydrogenase (LDH),and alkaline phosphatase (ALP).Results:The supplementation of Tris-based semen extender with 0.48 mg/mL semen extender resulted in the highest post-thaw sperm total motility (P<0.001),sperm progressive motility (P<0.001),live sperm (P<0.001),sperm plasma membrane integrity (P<0.001),acrosome integrity (P<0.001),SOD (P<0.05),zinc (P<0.001),total cholesterol (P<0.001),and LDL (P<0.001) with the lowest percentage of abnormal sperm morphology (P<0.001),the lowest lipid peroxidation (MDA,P<0.01),ascorbic acid (P>0.05),and LDH (P>0.05).Conclusions:Pomegranate peel methanolic extract 0.48 mg/mL supplemented to Tris-based semen extender of rams is the best enrichment in preserving the sperm post-thaw characteristics via improving biochemical profiles and antioxidant capacity.
KEYWORDS: Oxidants-antioxidants;Pomegranate peel ethanolic extract;Post-thaw semen characteristics;Tris-based semen diluents;Rams
1.Introduction
The semen freezing process decreases post-thaw sperm motility,viability,and fertilizing capacity compared to non-frozen one.Several decades ago,semen extenders were invented for the semen cryopreservation of many domesticated mammals aimed to preserve high post-thaw semen characteristics,viability,and fertilizing capacity[1].Sheep sperms are incredibly vulnerable to temperature changes during freeze-thawing processes that require supplementing freezing semen extenders with suitable additives to improve postthaw motility,viability and fertility potentials[1].The main goal of selecting ingredients in any cryopreservative semen extender is getting the best semen quality by keeping intact sperm plasma membrane and DNA integrities and decreasing the lipid peroxidation of the lipoproteins in the sperm plasma membrane[1].The increased polyunsaturated fatty acids in the sperm plasma membrane associated insufficient cytoplasmic antioxidants rendered mammalian sperms unable to survive the lipid peroxidation caused by increasing the production of reactive oxygen species resulted from the increased metabolic process and energy consumption started after semen collection[1,2].The over production of free radicals impaired sperm motility,viability,and fertilizing capacity[3].Semen cryopreservation increased the activities of total lactate dehydrogenase (LDH),superoxide dismutase (SOD),catalase,and glutathione peroxidase(GPx) enzymes in seminal plasma and decreased their activities in spermatozoa[4].Addition of plant extracts rich in antioxidants[5] or micro-minerals[6] to semen extenders before sperm cryopreservation were indispensable to compensate the over production of oxidants and lipid peroxidation induced during semen processing and the decreased temperature.
Plant and fruit extracts are rich in antioxidants and have been used for improving cryopreserved sperm characteristics[7].Pomegranate fruit is rich in several natural antioxidant compounds in its juice[8] and peels[9].Pomegranate peel is a rich source of water-soluble sugars,polyphenols,phenolic acids,flavonoids,and proanthocyanins[8] that could not only scavenge free radicals but also possess antimicrobial,antioxidative capacities,anti-atherosclerotic,anti-inflammatory,anti-cancer,immunomodulatory,and health-promoting properties[8].Pomegranate peels demonstrated better methane activity in ruminant stomachs,and improved feed intake and weight gain in bull calves[9].Pomegranate seeds contain conjugated fatty acids,punicic acid,phytoestrogens,sterols,γ-tocopherol,and hydroxyl benzoic acids[10].Conjugated fatty acids in pomegranate seeds possess antioxidant and anti-inflammatory activity in vitro and in lactating dairy cows[10].Pomegranate juice byproducts,including pomegranate peel,contain high amounts of non-fibrous carbohydrates and had indicated immunomodulatory,antibacterial,and antioxidant activities[10].Pomegranate byproducts were incorporated into the diet of goats[11]and dairy cows[12].The water-soluble polyphenols,including hydrolysable tannins (punicalagin and penicillin’s),phenolic acids,flavonoids,and proanthocyanins in pomegranate showed antioxidant and antibacterial effects in diseased pets[13].Pomegranate(Punica granatum) possesses antioxidant properties as it contains polyphenols,including flavonoids (anthocyanins),and tannins,such as punicalagin and ellagic acid[14].Tannins,such as punicalagin,exhibited antimicrobial effects[14,15].Pomegranate extracts showed beneficial effects on oral bacterial strains in humans[14,16].
Feeding pomegranate seeds improved the goat’s post-thaw semen characteristics[17].Pomegranate peels fed to rabbits for 90 days increased total sperm motility[18],and those constituted 3% and 5% in their diet for 60 days increased the percentage of progressive motile sperms,sperm concentration,and testicular enzymes[19].Feeding pomegranate juice decreased oxidative stress,improved the antioxidant scavenging power in supplemented rats[20],and improved their semen parameters[21].To our knowledge,few studies have included pomegranate juice in the semen extenders of rams[22],cattle[23],and buffalo bulls[24],but no studies have used the dried pomegranate peel methanolic extract as a supplement to the semen extenders.This study investigated the effect of supplementing Trisbased extender with three concentrations of pomegranate peels methanolic extract on the cryopreserved semen quality,oxidants,antioxidants,trace minerals,and enzymes.
2.Materials and methods
2.1.Animals
Fertile and clinically healthy native rams (Ovis aries;n=5) weighing 50-65 kg and of 3-5 years old came from the research farm of Theriogenology Department (Faculty of Veterinary Medicine,Cairo University).Rams were kept in semi opened yards under the natural environmental temperature and daylight and were supplied with their maintenance ration following National Research Council,(NRC,2007) recommendations (each ram fed daily 1.25 kg concentrated ration composed of 850 g wheat straw,green forage and 400 g pelleted concentrated ration with clean fresh water ad libidum.The study was conducted during March 2019 to April 2019.
2.2.Pomegranate peels collection
For preparing the pomegranate (Punica granatum L.) peels,fruits were collected during October 2015 from El-Behira governorate,Egypt.The pomegranate peels were removed,washed with distilled water,and dried in hot-air oven for 16 h at 50 ℃.The dried peels were grinded,passed through a mesh sieve,packed in polyethene bags and stored at -20 ℃.
2.3.Preparation,phenolic and antioxidants characterization of pomegranate peel methanolic extracts
After mixing 30 g of the dried and grinded pomegranate peels with 300 mL methanol,an Ultra-Turrax homogenizer was performed.The homogenate was kept at 4 ℃ for 24 h and then filtered using filter paper Whatman No.1.The homogenate was dried using a rotary evaporator under reduced pressure at 40 ℃[5].Each dried extract was reconstituted in 10 mL dimethyl sulfoxide (DMSO) and stored at-80 ℃.
2.4.Solvents and chemicals
Acetonitrile (HPLC grade) was purchased from Aldrich Chemical(GmbH &Co KG,Steinheim,Germany).Petroleum ether,diethyl ether,ethyl acetate,tetrahydrofuran,and methanol (analytical grades) were purchased from Tedia Company,Inc.,Fairfield,OH 45014,USA.Sodium hydroxide,potassium peer sulphate,dinitro-salicylic acid,aluminum chloride,sodium nitrite,sodium carbonate,hydrochloric acid,sulphuric acid,and acetic acid were analyzed.For the characterization of the phenolic composition in dried pomegranate peel methanolic extracts,gallic,protocatechuic,gentisic,chlorogenic,vanillic,caffeic,syringic,p-coumaric,ferulic,sinapic,rosmarinic and cinnamic acid,catechins,scopolamine,rutin,naringenin,hesperidin,myricetin,quercetin,apigenin and kaempferol standards were purchased from Sigma-Aldrich,Inc.(Louis,USA).For assaying radical precursor in the extracted total phenolics,2,2-diphenyl-1-picryl-hydrazyl (DPPH),2,2-azino-bis/3-ethil-benothiazoline-6-sulfonic acid (ABTS),2,4,6-tripyridyl-striazine (TPTZ),and Folin-Ciocalteu were purchased from Sigma-Aldrich,Inc.(Louis,USA).
For determining the total phenolic content,Folin-Ciocalteu procedure[5] was used by using a calibration curve prepared with gallic acid equivalent and expressed as mg GAE/g pomegranate peel methanolic extracts.Aluminum chloride (AlCl3) colorimetric assay[5] was used to assay the total flavonoid content using a calibration curve prepared with catechins equivalent and expressed as mg CE/g pomegranate peel methanolic extracts.Stable DPPH,ABTS,and ferric reducing activity power (FRAP)[5]were assayed to measure the free radical scavenging capacity of pomegranate peel methanolic extracts.The standard curve using Trolox equivalent mg TE/g pomegranate peel methanolic extract was performed.The phenolic acids were identified by high performance liquid chromatography (HPLC) by injecting pomegranate peel methanolic extracts automatically into an HP 1100 series HPLC system (Hewlett-Packard,GmbH,Germany) equipped with a diode array detector (DAD) and an Xterra RP18 reverse phase column (4.6 mm×250 mm) with a spherical particle size of 5 µm,and were kept at 25 ℃.The mobile phase was composed of 1% formic acid (A) and acetonitrile (B) with keeping the elution gradient from 2% to 100% (B) in 40 min at a flow rate of 0.5 mL/min and 25 ℃.The injection volume was 20 µL[5].All absorption spectra of the prominent peaks were recorded at 280 nm.
2.5.Semen collection and processing
Semen was collected from all rams (n=5) twice weekly for two successive months using a lubricated and pre-warmed [(40-42) ℃]short artificial vagina (total 80 semen collections).After collecting fresh semen ejaculates,semen was pooled to obtain 5.0 mL (n=16)for evaluating the fresh semen motility,live spermatozoa percentage and sperm cell concentration.Samples showing ≥75% motility,≥75% live sperm,and 300×106sperm cells/mL were extended and frozen.Pooled semen samples were divided into 12 equal aliquots and diluted 1:5 (V/V) with Tris-citrate-glucose extender[5,6].Dried pomegranate peel methanolic extracts in concentrations of 0,0.40,0.48,and 0.56 mg/mL were added to the semen extenders[6].Chilled diluted semen samples were kept in the refrigerator at 5 ℃ for 90 min.Cryopreserved diluted pooled semen was equilibrated at 5 ℃ for 15 min,loaded in 0.25 mL straws,and sealed with polyvinyl powder.After sealing,the straws were placed horizontally at 6.5 cm over liquid nitrogen for 10 min and then plunged into liquid nitrogen tank for storage.Frozen ram semen straws were thawed by taking two straws from the liquid nitrogen container and dropping them into water bath at 40 ℃ for 30 s[6].
2.6.Post-thaw semen evaluation
Post-thaw motility was assessed immediately after thawing and expressed in percentages by using a heated stage at 37 ℃ using optical microscope (Olympus BH-2,Olympos Optical Co.Ltd.,Japan) and 400× magnification[1-3].
Sperm viability and sperm abnormalities were evaluated by mixing one drop of frozen-thawed semen with two drops of eosin-nigrosine on a warm glass slide[3] to count the percentage of live sperm (not stained) and the percentage of abnormal spermatozoa in the head,tail,or neck-mid piece in two hundred sperm cells using a bright field microscope (1000×).
For determining the functional plasma membrane integrity (FPMI)of the spermatozoa by the hypo-osmotic swelling test[3,5,6],thawed semen (30 µL) was mixed with 100 mOsm/kg hypo-osmotic solution(300 µL) composed of 0.9% fructose and 0.49% sodium citrate/100 mL distilled water (W/V),then incubated at 37℃for 1 h and 0.2 mL of the incubated mixture was evaluated under the bright field microscope (400×) to count the percentage of sperm cells with normal membranes showing a swelling around the tail and those sperms with a swollen ‘bubble’ around the curled flagellum in 200 spermatozoa in at least five different microscopic fields.
After fixing the dried sperm smear in 10% formalin for 10 min,acrosome integrity was evaluated by immersing the slides in three successive jars containing Spermac stain for 1 min at room temperature followed by air drying and counting the percentage of spermatozoa with intact acrosome from two hundred sperm cells under oil immersion (×1000) bright microscope[5,6].
2.7.Biochemical evaluation
For determination of malondialdehyde (MDA,nmol/mL),nitric oxide (NO,umol/L),ascorbic acid (mg/L),GPx (mU/mL),SOD(U/mL),zinc,copper,total cholesterol (mg/dL),low-density lipoproteins (LDL,mg/dL;Biodiagnostics,Egypt),LDH,and alkaline phosphatase (ALP;MG,Science and Technology Center”STC”) commercial kits were purchased.The intra-and inter-test precisions were <5%.
2.8.Statistical analysis
IBM-SPSS version 20.0 (2016) was used to analyze the collected data.After testing the normal distribution of the parameters,the effect of supplementing different concentrations of the pomegranate peel methanolic extract (0.0,0.40,0.48,0.56 mg/mL) to Trisbased semen diluents on the semen parameters and seminal plasma biochemicals,oxidants,antioxidants,trace minerals,and enzymes was tested using simple one-way analysis of variance (ANOVA)and Duncan’s multiple range test was used to differentiate between significant means at P<0.05.Results of statistical analysis are presented in tables as mean±standard deviation (mean±SD).The partial Pearson correlation coefficient was also processed.
2.9.Ethics statement
The study was obtained from the Ethics Committee of the Faculty of Veterinary Medicine,Cairo University (Vet CU 8/3/2022/399).
3.Results
3.1.Composition and antioxidant capacity of pomegranate peel methanolic extracts
The results of pomegranate peel methanolic extracts interpreted using HPLC analysis against 24 standard metabolites revealed the presence of 68.29 mg GAE/g total phenolic compounds and 72.53 mg CE/g total flavonoids.The common compounds in 1 g pomegranate peel methanolic extracts from the highest to the lowest concentrations were pyrogallol (2 667.31 µg/g),protocatechuic acid (1 634.61 µg/g),rutin (530.45 µg/g),catechin (523.47 µg/g),gallic acid (307.90 µg/g),P-coumaric acid (285.2 µg/g).Traces of naringeen (80.01 µg/g),scoplatine (78.97 µg/g),myrcetin(67.03 µg/g),hisperdin (56.40 µg/g),and rosmarinic acid(31.71 µg/g) (Figure 1).The antioxidant activity of pomegranate peel methanolic extracts indicated 324.03 mM TE for ABTS,273.17 mM TE for DPPH and 215.75 mM TE/g for FRAP.
3.2.Effect of pomegranate peel methanolic extracts on postthaw semen characteristics
Sperm post-thaw total motility for the Tris-based extenders supplemented with 0.48 mg/mL and 0.56 mg/mL were significantly higher compared to either non-supplemented one or that supplemented by 0.40 mg/mL pomegranate peel methanolic extracts (P<0.001).The post-thaw sperm progressive motility was optimum for the Tris-based extender supplemented with 0.48 mg/mL pomegranate peel methanolic extracts compared to those other concentrations (P<0.001) including the control.The live sperm percent achieved the highest value for the Tris-based extender supplemented with 0.48 mg/mL pomegranate peel methanolic extracts compared to the two other supplemented concentrations (P<0.001) and the control.The lowest abnormal sperm percent was recorded when the Trisbased extender was supplemented with 0.48 mg/mL compared to either control or semen extender supplemented with 0.40 mg/mL pomegranate peel methanolic extracts.The Tris-based extender supplemented with 0.56 mg/mL had significantly higher abnormalsperm % than 0.48 mg/mL (P<0.001).The FPMI kept the highest percentage for the Tris-based extender supplemented with 0.48 mg/mL pomegranate peel methanolic extracts compared to the other supplemented concentrations (P<0.001) and control.The sperm acrosome integrity reached the highest percentage for the Trisbased extender supplemented with 0.48 mg/mL pomegranate peel methanolic extracts compared to the control and other supplemented concentrations (P<0.001) (Table 1).
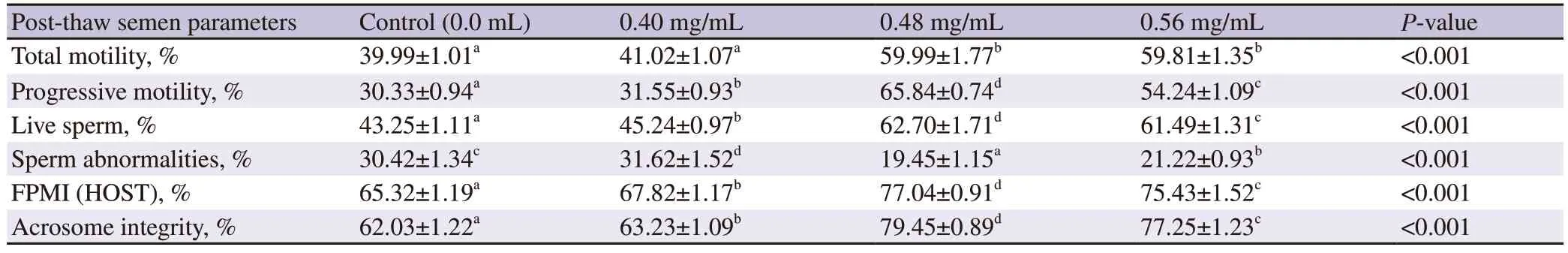
Table 1.Sperm parameters of frozen-thawed ram semen supplemented with pomegranate peel methanolic extract.

Table 2.Seminal plasma lipid peroxide products of post-thaw semen supplemented with pomegranate peel methanolic extract.
3.3.Effect of pomegranate peel methanolic extracts on seminal plasma biochemicals,micro-minerals,enzymes,oxidants,and antioxidants
The concentrations of cholesterol in the non-supplemented control extender and that supplemented with 0.48 mg/mL pomegranate peel methanolic extracts were significantly higher (P<0.001) than those supplemented with 0.40 mg/mL,and 0.56 mg/mL.The LDL concentration in the post-thaw semen extender supplemented with 0.48 mg/mL pomegranate peel methanolic extracts was the highest one (P<0.001) compared to either extenders supplemented with either 0.40 mg/mL or 0.56 mg/mL pomegranate peel methanolic extracts and is slightly higher than the control.The highest value of zinc in the post-thaw semen extender increased (P<0.001) linearly with increasing the concentration of pomegranate peel methanolic extracts from 0.40 mg/mL,0.48 mg/mL to 0.56 mg/mL in the semen extender compared to the control.The level of copper reached the highest value (P=0.008) in the post-thaw semen extender supplemented with 0.40 mg/mL compared to the control and those extenders supplemented with either 0.48 mg/mL or 0.56 mg/mL pomegranate peel methanolic extracts.Tris-based extender supplemented with 0.56 mg/mL pomegranate peel methanolic extracts had the highest(P<0.05) MDA compared to 0.48 mg/mL,0.40 mg/mL,and control.Tris-based extender supplemented with 0.40 mg/mL had the highest NO concentration compared to the control,0.48 mg/mL and 0.56 mg/mL pomegranate peel methanolic extracts (P<0.01).The LDH activity tended to be low (P>0.05) in the semen extender supplemented with 0.48 mg/mL compared to the nonsupplemented control and that supplemented with 0.40 mg/mL pomegranate peel methanolic extracts.The ALP significantly(P<0.001) increased when semen extender was supplemented with 0.56 mg/mL compared to the control and other two treatments (Table 2).Ascorbic acid levels tended to decrease(P>0.05) in Tris-based extender supplemented with 0.48 mg/mL of pomegranate peel methanolic extracts compared to the control.The highest GPx activity can be noticed (P<0.05) when the semen extender was supplemented with 0.48 and 0.0 mg/mL pomegranate peel methanolic extracts and declined in the other two supplemented extenders.All treated semen extenders showed increased SOD activity (P<0.05) with increasing pomegranate peel methanolic extracts concentration compared to the control (Table 2).
3.4.Correlations between seminal plasma biochemicals,micro-minerals,enzymes,oxidants,and antioxidants and semen characteristics
MDA tended to correlate negatively with the sperm progressivemotility (r=-0.24;P>0.05) but has a negative fair and significant correlation with the plasma membrane integrity (r=-0.29;P<0.05).NO had negative correlations with sperm total motility (r=-0.49;P<0.0001),sperm progressive motility (r=-0.30;P<0.05),live sperm% (r=-0.46;P<0.001),FPMI (r=-0.45;P<0.001),and acrosome integrity (r=-0.45;P<0.001).Zinc has negative correlations with sperm total motility (r=-0.38;P<0.01) and live sperm % (r=-0.26;P>0.05) and a positive one with abnormal sperm % (r=0.28;P<0.05).Copper has a negative correlation with sperm total motility (r=-0.32;P<0.05),live sperm % (r=-0.27;P>0.05),and acrosome integrity(r=-0.27;P>0.05).Copper correlated positively with abnormal sperm% (r=0.31;P<0.05).Cholesterol and LDL have positive correlations with sperm total motility,progressive motility,live sperm %,plasma membrane integrity,and acrosome integrity and have a negative correlation with abnormal sperm %.The increase in ALP activity correlated with a decrease in the sperm plasma membrane integrity(r=-0.35;P<0.05).The increase in LDH activity strongly associated the decreased total sperm motility,progressive motility,live sperm %,FPMI,and acrosome integrity.The increased GPx activity associated increased sperm total motility,progressive motility,live sperm %,FPMI,acrosome integrity and decreased abnormal sperm %.The supplementation of the Tris-based extender with three concentrations of pomegranate peel methanolic extracts correlated positively with sperm total motility (r=0.90;P<0.001),progressive motility(r=0.78;P<0.001),live sperm % (r=0.89;P<0.001),FMPI (r=0.87;P<0.001),acrosome integrity (r=0.87;P<0.001),zinc (r=0.79;P<0.001),ALP (r=0.41;P<0.01),SOD (r=0.42;P<0.01),and MDA(r=0.27;P<0.05) but inversely related to abnormal sperm % (r=-0.81;P<0.001) (Table 3).
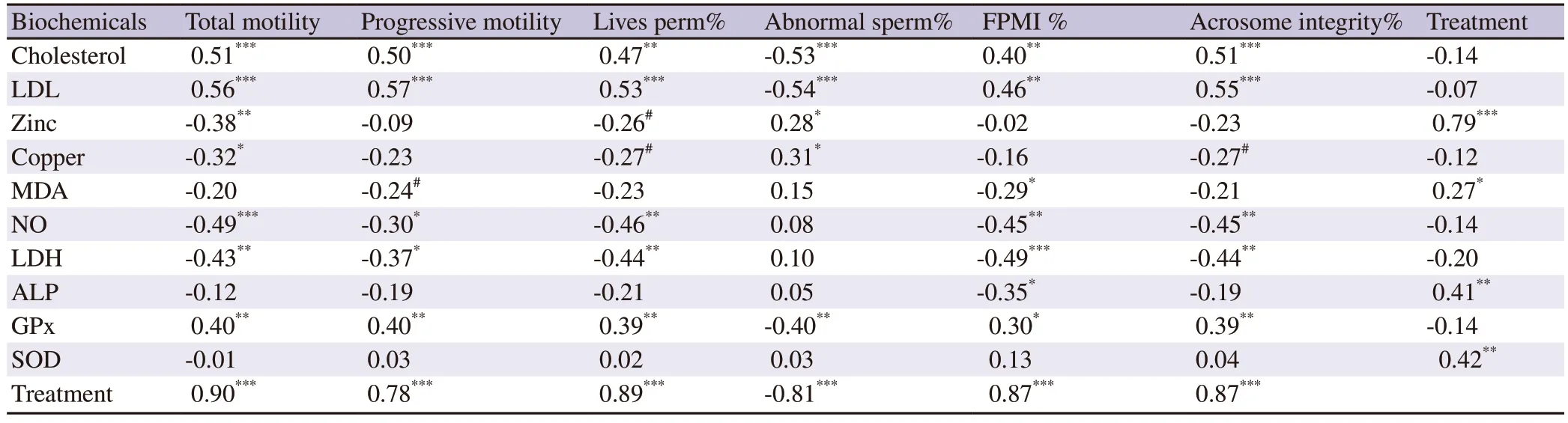
Table 3.Two-tailed partial correlation coefficients (r) between post-thaw semen characteristics with seminal plasma lipid peroxide products and treatment.
4.Discussion
Compared to another dried pomegranate peels extract using several solvents,the total phenols recorded in the current study are nearly four times greater than the methanolic extract of another pomegranate peel,seven times higher than its ethanolic extract,and more than eight times greater than the aqueous extract[25].The total flavonoid content in the pomegranate peel methanolic extracts of our study is five times higher than the methanolic extract of another pomegranate peels which refer to the different origin of the plant cultivar area[25,26],different varieties,and seasons[27].The higher total phenolic and total flavonoid content of the methanolic pomegranate peel extract compared to ethanolic and water extracts of the pomegranate peels indicate that methanol was the most suitable solvent[25].
In addition to pyrogallol,gallic acid,protocatechuic acid,catechin,scoplatine,rutin,P-coumaric acid,naringeen,hisperidine,myrcetin,and rosmarinic acid compounds detected in the current study,the methanolic pomegranate peels extract contained several sugars such as Apig6-rhamnose8-galactose,Apig6-arabinose8-galactose,Luteov7-glucose,Quercetrin-3-O-glucose,and Kaemp.3-(2-p-coumaroyl) glucose[25].The presence of glucose,galactose,and arabinose in pomegranate peel methanolic extracts play synergistic roles with the glucose in the Tris-citrate semen extender for preserving sperm viability.During the cryopreservation process,sugars in pomegranate peel methanolic extracts act as energy substrates,oxidative stress scavengers,non-penetrating cryoprotectants,and minimize excessive dehydration of spermatozoa during the freezing and thawing process.Sugars decrease sperm injury by linking with hydrogen ponds in the macromolecules,interacting with the phospholipids in the plasma membrane,and replacing water[28].
Similar to the optimized post-thaw sperm total motility,progressive motility,viability,acrosome integrity,and plasma membrane integrity with the lowest abnormal sperm morphology observed in the current study after supplementing Tris-based semen extender of rams with 0.48 mg/mL pomegranate peel methanolic extracts,the supplementation of the Tris-based extender with 0.0%,2.5%,5.0%,7.5%,and 10.0% pomegranate juice for the cryopreservation of buffalo sperms showed the optimum total sperm motility,progressive motility,plasma membrane integrity,acrosome integrity,DNA integrity,and pregnancy rate with the lowest abnormal sperm morphology[24].In cattle bulls,the chilled semen kept optimum motility for seven days when the Tris-based extender was supplemented with 10% pomegranate juice while increasing the concentrations to 50% declined it compared to the control semen extender[23].In contrast to the chilled semen,the cryopreserved semen of cattle,including more than 30% pomegranate juice in a Tris-based extender deteriorated the percentage of post-thaw sperm motility,plasma membrane integrity and live sperm but did not influence the abnormal sperm morphology[23].Similar to our results,where 0.48 mg/mL Tris-based semen extender achieved the post-thaw semen quality,supplementing Tris-based extender of cattle[23] and buffalo bulls[24] with 10% pomegranate juice reached the optimum post-thaw semen quality.In rams,supplementing Tris-based semen extender with 5% pomegranate juice achieved the highest sperm motility,viability,plasma membrane integrity and mitochondrial activity[22].Similar to our results,increasing the concentrations of pomegranate juice in the semen extender of cattle[23] and rams[22],in concentrations higher than the optimum one,resulted in deteriorated post-thaw semen quality.
Glycerol in freezing semen extenders acts as a membranepermeable cryoprotectant for freezing spermatozoa[29].LDL alone as a component in egg yolk protected spermatozoa from cold shock during processing freezing by various mechanisms to maintain the integrity of sperm membrane[29].The increased LDL and total cholesterol in supplemented semen extenders with pomegranate peel methanolic extracts indicated better post-thaw sperm motility,viability,hypo-osmotic swelling test,and acrosome integrity parameters.The addition of 20% egg yolk regardless of the presence/absence of permeable cryoprotectant or addition of cholesterol or phosphatidylcholine indicated offered no additional benefits of LDL in post-thaw sperm motility[30],the increase of cholesterol and LDL levels in supplemented extender with 0.48 mg/mL pomegranate peel methanolic extracts associated better motility.The declined cholesterol and LDL in the seminal plasma of preserved semen supplemented with 0.40 and 0.56 mg/mL pomegranate peel methanolic extracts than the control one and that supplemented with 0.48 mg/mL pomegranate peel methanolic extracts may refer to their compensation in repairing plasma membrane injuries in response to increased oxidative stress production and lipid peroxidation.The positive correlations of both cholesterol and LDL concentrations in the seminal plasma of post-thaw semen with sperm total motility,sperm progressive motility,live perm and both plasma membrane and acrosome integrities and the negative one with the abnormal sperm morphology confirm their role in protecting spermatozoa during cryopreservation.Similar to the improved post-thaw sperm motility,viability,plasma membrane and acrosome integrities,and sperm morphology in the semen supplemented with 0.48 mg/mL pomegranate peel methanolic extracts,this improvement could be referred to increased cholesterol and LDL in the seminal plasma than the control non-supplemented semen extender.As well as,the increase of LDL from 2% to 8% in modified Tris-glucose based extender improved all post-thaw semen parameters in rams[30].This slight increase in LDL preserved polyunsaturated fatty acids in the sperm membranes from oxidation by free radicals and peroxides and minimized their adverse effects in reducing the sperm motility or the activation of the apoptotic process culminated in DNA fragmentation[31].
In agreement with supplementing the Tris-based semen extender of rams with 5% pomegranate juice,our study recorded a decline in the lipid peroxidation marker (MDA) to the minimum value after supplementing 0.48 mg/mL pomegranate peel methanolic extracts[22].This decrease in lipid peroxidation could be referred to the protective effects of phytoestrogens,sterols,and γ-tocopherol present in the pomegranate extract[11].Similarly,0.48 mg/mL methanolic extract of Moringa leaves reduced the MDA[5].Oral supplementation of 0.5 and 1.0 mL pomegranate juice for seven weeks reduced lipid peroxidation in the semen of rats that was associated with posted levels of vitamin A,E,and C in the blood plasma of supplemented rats[20].The negative correlation between NO and post-thaw semen quality conforms to the role of NO in physiological levels regulating spermatogenesis,sperm motility,and maturation[5,6] that have been noticed in rams after supplementing their semen extender with NO precursors[32].In agreement with the negative correlations between NO and semen parameters in our study,increasing NO than the physiological limit induced reactive nitrogen species and resulted in reduced spermatozoa motility,viability and the rate of abnormal spermatozoa in the treated rams with NO precursors[32].In contrast to the negative correlations among abnormal sperm morphology,viability,plasma membrane and acrosome integrities and NO observed in rams supplemented with pomegranate peel methanolic extracts,NO levels were positively correlated to progressive motility and viability in buffaloes[33].In our results,the concentration of NO in the seminal plasma of cryopreserved semen supplemented with 0.0,0.48,and 0.56 mg/mL pomegranate peel methanolic extracts is lower than that supplemented with 0.40 mg/mL,and these average physiological concentrations protected sperm DNA and decreased their apoptosis through free radical scavenging,deactivation,and inhibition the production of superoxide anions[34].
Supplementing rams’ semen extender with pomegranate peel methanolic extracts correlated positively with increased SOD activity compared to the non-supplemented control.SOD-increased activity was observed when the ram semen extender was supplemented with 2.5% and 5.0% pomegranate juice[22].In our study,the absence of correlation between SOD and post-thaw sperm parameters was also recorded in rams[35].Though the three concentrations of pomegranate peel methanolic extracts improved the SOD activity in the cryopreserved semen in our rams,only 2.5% and 5% pomegranate juice insignificantly enhanced it[22].Parallel to the insignificant decrease in GPx activity after supplementing pomegranate juice in concentrations of 2.5% and 5% and its increased activity after adding 7.5%,0.48% pomegranate peel methanolic extracts significantly optimized GPx activity.At the same time,0.40 and 0.56 mg/mL pomegranate peel methanolic extracts reduced it[22].The increased GPx activity in post-thaw semen of rams supplemented with 0.48 mg/mL pomegranate peel methanolic extracts in the Tris-based semen extender is similar to its increase after supplementing 7.5% pomegranate juice to ram semen extender[22].Similar to buffaloes,positive correlations were found between GPx activity in seminal plasma and all post-thaw semen parameters except the abnormal sperm morphology[36].
The significant decrease of ascorbic acid in the semen extender supplemented with 0.48 mg/mL pomegranate peel methanolic extracts than the control and other two supplemented extenders indicates its consumption in scavenging oxidants as a water-soluble non-enzymatic antioxidant[37].In agreement with our results,ascorbic acid supplemented with bovine semen diluents reduced sperm damage during cryopreservation due to its reactive oxygen species scavenging property[37].Lowered ascorbic acid in the current study associated with the best post-thaw semen quality agrees with the addition of 4.5 mg/mL ascorbic acid to bovine semen extender improved the motility and viability and achieved the highest membrane and acrosome integrities[38].
The significant effect of pomegranate peel methanolic extracts on zinc levels in semen extenders is indicated by the increased zinc concentrations in the seminal plasma of the thawed semen of the supplemented extenders and agreed with the involvement of zinc in sperm maturation[39].In contrast to the negative correlation between zinc and total motility,the increased release of zinc from pomegranate peel methanolic extracts correlated with motility[39].Many antioxidant enzymes contain copper like copper-and zinc-SOD to protect sperm cells against reactive oxygen species.Cytochrome-c oxidase enzyme responsible for cellular energy supply contains copper[37-39].Elevated copper concentrations in semen extender supplemented with 0.40 mg/mL pomegranate peel methanolic extracts reduced oxidative processes,glycolysis,mobility,and viability.The negative non-significant low correlation of copper with all ram semen characteristics of this study indicated that higher copper concentrations to the toxic levels disrupted sperm motility and viability[5,6].
The antioxidant power of pomegranate peel methanolic extracts in semen supplemented extender reduced oxidative stress and the levels of ALP and LDH strongly associated with reduced sperm viability,sperm membrane integrity and morphology[40].The negative correlation between ALP and plasma membrane integrity agreed with the significant positive effect of the treatment on the increased ALP activity in the frozen-thawed seminal plasma that correlated to poor sperm characteristics[41].The leakage of ALP in the seminal plasma of frozen-thawed semen in the current study after supplementing semen extender with 0.56 mg/mL pomegranate peel methanolic extracts associated poor semen quality due to the acrosomal damage[5,6,41].Similar to rams of this study,increasing seminal plasma ALP activity in cryopreserved semen was considered a bio-marker for sperm viability due to its negative correlation with poor sperm quality parameters[42].In agreement with our results,the activities of total LDH activity increased in seminal plasma and decreased in spermatozoa after cryopreservation[4].The substantial increase in LDH activity in the control non-supplemented semen extender and those supplemented with 0.40 and 0.56 mg/mL pomegranate peel methanolic extracts and the significant negative correlations with all post-thaw semen parameters in the current study conformed with the increased release of LDH enzyme from dead and abnormal sperm[5,6,43].
There are some limitations in the study.The type,the season,the locality of pomegranate collected for the study differ in composition than recorded for other studies in different localities.Dark red pomegranate peels were selected for conducting this study.The season of semen collection and preservation was selected to have the maximum semen quality.The selection of antioxidants,traceminerals,and enzymes for this study depended on their relation to the composition of the extract and their role in protecting spermatozoa.
In conclusion,pomegranate by-products can be used for dietary and semen extender supplementation.The methanolic extract of pomegranate peels has the highest antioxidant,minerals,and vitamins suitable for protecting spermatozoa during cryopreservation.The supplementation of semen extender with 0.48 mg/mL pomegranate peel methanolic extracts resulted in the best semen quality,antioxidant and enzyme activities.
Conflict of interest statement
The authors declare that they do not have any conflict of interest.
Funding
This study received no extramural funding.
Authors’ contributions
Amal M.Aboelmaaty performed the clinical analysis,made the statistical analysis,helped in writing the manuscript and submission for publication.Mohamed S.Kotp helped in clinical and statistical analysis.Islam E.El-Seadawy collected the fruits,prepared the extract,and characterized the phenolic composition and the scavenging properties and helped in writing the manuscript.Aya M.Fadl,Elshymaa A.Abdelnaby,and Hossam R.El-Sherbiny collected semen and evaluated it,prepared the semen extenders and defined the concentrations,cryopreserved semen,and analyzed the post-thaw semen characteristics.The manuscript has been read and approved by all the authors.
 Asian Pacific Journal of Reproduction2023年5期
Asian Pacific Journal of Reproduction2023年5期
- Asian Pacific Journal of Reproduction的其它文章
- The role of small non-coding RNAs (sncRNAs) in male infertility: A scoping review
- Reproductive outcomes of water pipe smoking: A scoping review
- Predictors of antenatal health service utilization among left-behind wives of male outmigrants: Evidence from Patna District,India
- Effect of taxifolin on cold-shock damages in spermatozoa in rabbits
