Method for Vitamin D Deficiency Screening
1 Scope
This standard specifies the indicators,reference values,and measurement methods for vitamin D deficiency and insufficiency screening in the people.
This standard is applicable to the determination of vitamin D nutritional status in the people.
2 Normative references
The following documents are essential for the application of this document.For the references dated,only their dated versions are applies to this document;for the references undated,their latest versions,including all amendments,are applicable to this document.
GB/T 603 Chemical reagent Preparation of formulations and products used in test methods
GB/T 6682 Analysis of laboratory water specifications and test methods
WS/T 225 Collection and processing of blood samples for clinical chemical testing
3 Terms and definitions
The following terms and definitions apply to this document.
3.1 25-hydroxyvitamin D;25(OH)D
The main circulating form of vitamin D in the blood,with good stability,is recognized as a reliable indicator for evaluating the nutritional status of vitamin D in the human body.It mainly includes two forms: 25 (OH) D2and 25 (OH) D3,of which 25 (OH) D3is the main form of vitamin D present in the blood.
3.2 Vitamin D deficiency
Vitamin D deficiency can be determined when the content of 25-hydroxyvitamin D in human serum (or plasma) is lower than the reference values for deficiency.
3.2 Vitamin D insufficiency
Vitamin D insufficiency can be determined when the content of 25-hydroxyvitamin D in human serum (or plasma) is lower than the reference values for the normal people,but higher than that for deficiency.
4 Determination indicators and reference values for vitamin D nutritional status in the people
Determination indicators and reference values for vitamin D nutritional status in the people are shown in Table 1.
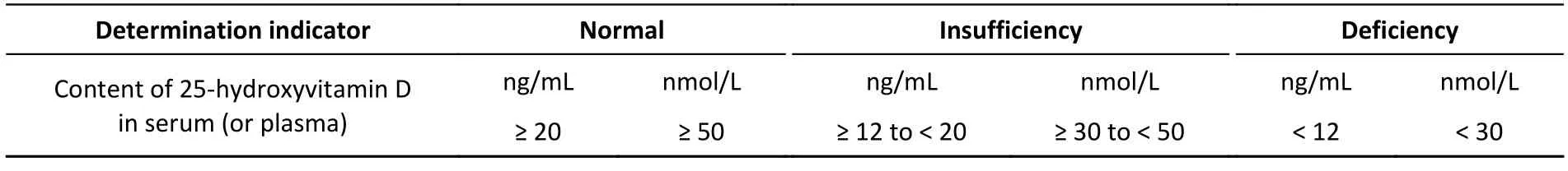
Table 1. Determination indicators and reference values for vitamin D nutritional status in the people
5 Measurement methods
5.1 Collection and preservation of blood specimens
Follow the methods specified in Appendix A.
5.2 First: Liquid chromatography tandem mass spectrometry
Follow the methods specified in Appendix B.
5.3 Second: Chemiluminescence immunoassay
Follow the methods specified in Appendix C.
5.4 Third: Enzyme linked immunosorbent assays
Follow the methods specified in Appendix D.
 Biomedical and Environmental Sciences2023年8期
Biomedical and Environmental Sciences2023年8期
- Biomedical and Environmental Sciences的其它文章
- The China Cardiovascular Health Index 2023 was Grandly Released
- Method for Folate Deficiency Screening
- A Campylobacteriosis Outbreak Caused by One Asymptomatic Food Handler Carrier*
- Exploring Gender Differences in Adolescent Dissociative Symptoms via A Structural Equation Model*
- A Risk Assessment Model for Pancreatic Cancer Based on Cuproptosis-related Genes and Clinical Characteristics
- Risk of Developing Non-Alcoholic Fatty Liver Disease Over Time in a Cohort of the Elderly in Qingdao,China*
