Diabetes mellitus and atrial fibrillation-from pathophysiology to treatment
Marianna Leopoulou, Panagiotis Theofilis, Athanasios Kordalis, Nikolaos Papageorgiou, Marios Sagris,Evangelos Oikonomou, Dimitris Tousoulis
Marianna Leopoulou, Panagiotis Theofilis, Athanasios Kordalis, Nikolaos Papageorgiou, Marios Sagris, Dimitris Tousoulis, 1st Cardiology Clinic, ‘Hippokration’ General Hospital, National and Kapodistrian University of Athens, School of Medicine, Athens 11527, Greece
Evangelos Oikonomou, 3rd Cardiology Clinic, ‘Sotiria’ Chest Diseases Hospital, National and Kapodistrian University of Athens, School of Medicine, Athens 11527, Greece
Abstract Type 2 diabetes mellitus (T2DM) is a leading risk factor for cardiovascular complications around the globe and one of the most common medical conditions. Atrial fibrillation (AF) is the most common supraventricular arrhythmia, with a rapidly increasing prevalence. T2DM has been closely associated with the risk of AF development, identified as an independent risk factor. Regarding cardiovascular complications, both AF and T2DM have been linked with high mortality. The underlying pathophysiology has not been fully determined yet; however, it is multifactorial, including structural, electrical, and autonomic pathways. Novel therapies include pharmaceutical agents in sodium-glucose cotransporter-2 inhibitors, as well as antiarrhythmic strategies, such as cardioversion and ablation. Of interest, glucose-lowering therapies may affect the prevalence of AF. This review presents the current evidence regarding the connection between the two entities, the pathophysiological pathways that link them, and the therapeutic options that exist.
Key Words: Atrial fibrillation; Diabetes mellitus; Pathophysiology; Treatment
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a leading risk factor for cardiovascular complications around the globe and one of the most common medical conditions[1]. Atrial fibrillation (AF) is the most common supraventricular arrhythmia, with a rapidly increasing prevalence[2]. T2DM has been closely associated with the risk of AF development, being identified as an independent risk factor for AF. Furthermore, T2DM has been linked with an increased symptom burden for patients that suffer from AF, leading to impaired life quality and increased hospitalization[3].
The risk of AF development in patients with T2DM has been established by large studies and metaanalyses showing a clear link between AF and T2DM. Based on the association between the two medical conditions and the high risk of cardiovascular morbidity and mortality that their combination presents, literature has concluded that underlying pathophysiology is related to structural, electrical-electromechanical, and autonomic remodeling as well as metabolic parameters[4,5]. Furthermore, their association has highlighted the need for surfacing therapeutic models that can alter the risk of the AF and T2DM combination or lower the risk of AF development in the diabetic population.
In this review, we present the pathophysiologic mechanisms that may combine the two entities, and the therapeutic options that are available for diabetic patients with AF.
AN ASSOCIATION BETWEEN AF AND T2DM
The Women's Health Study established T2DM as a significant predictor of risk for AF[6]. Similarly, a 2010 study suggested a 40% higher risk of developing AF, for diabetic patients, with the overall risk increasing by 3% for every year of T2DM[7]. In 2011, the risk of developing AF in patients with T2DM was identified at 34% over the non-diabetic population[8], while in a 2017 meta-analysis, higher serum glycated hemoglobin levels (HbA1c) were associated with incident AF in prospective cohort studies[9]. In a prospective study, T1DM was associated with a modest increase in the risk of AF in men and a 50% increased risk of AF in women; the risk was proportional to worse glycemic control and renal complications[10]. Similarly, in the prospective cross-sectional observational NOMED-AF study, researchers concluded that AF affects one in four patients with T2DM, highlighting the excessive need for AF screening amongst the diabetic population[11]. Interestingly, a recent Swedish cohort revealed an overall 35% higher risk of AF compared to age- and sex-matched controls from the general population for patients with T2DM; renal complications or poor glycemic control increased the risk of AF[12]. In a Danish study, T2DM was associated with a relative 19% increased risk of incident AF, especially in the 18-39-year-old group[13], while a case-control study concluded that T1DM modestly increases the risk of AF in men but elevates the risk for women by 50%, especially in the cases of poor glycemic control and renal complications[10]. Interestingly, prediabetes, a condition that is also associated with heart failure[14], cardiovascular and all-cause mortality[15], may drive the development of AF[16]. While there is significant evidence pointing concerning the high rates of AF among individuals with T2DM, there is no data on the prevalence of T2DM among AF populations. Thus, the bidirectional relationship between those two entities could only be speculated at present.
The presence of both T2DM and AF can present more complications than each individual entity. In 2022, a meta-analysis of 21 studies concluded that AF patients with T2DM run a higher cardiovascular and all-cause mortality risk[17]. Similarly, in the much earlier ADVANCE study, T2DM patients with AF had an increased risk of major cardiovascular and cerebrovascular events, as well as of cardiovascular and all-cause mortality death, when compared to diabetic patients without AF[18]. Similar results were presented by the ORBIT-AF study, as high symptom burden, low life quality, cardiovascular and overall mortality were higher for AF patients with T2DM compared to AF patients without T2DM[3]. The 2021 Swiss-AF study also claimed that AF patients with T2DM are less self-aware of AF symptoms and maybe should be systematically screened for silent AF[19]. Moreover, although individuals with T2DM may exhibit a higher thrombotic risk, the rates of electrical cardioversion and catheter ablation use are significantly lower compared to non-T2DM individuals, as shown in the EORPAF general pilot registry report[1].
PATHOPHYSIOLOGY
Structural remodeling
All pathophysiologic mechanisms are depicted in Table 1 and Figure 1. The most prominent structural modification that AF causes is atrial dilatation and fibrosis. Interestingly, atrial dilatation and fibrosis can result in AF development. In this context, as myocardial fibrosis is independently associated with T2DM, diabetic patients have a prominent substrate for developing AF[4,20]. More specifically, the cellular and molecular underlying mechanisms linking T2DM to myocardial fibrosis include inflammation and oxidative stress deriving from prolonged hyperglycemia[20]. Both increased production of reactive oxygen species (ROS) and decreased expression of enzymes that downregulate ROS have been revealed in diabetic patients, suggesting a high oxidative stress burden[21,22]. A high oxidative stress burden can both result in and aggravate pre-existing inflammation and inflammatory markers such as C-reactive protein and tumor necrosis factor-α, associated with left atrial dilatation and increased AF incidence[23-25]. Furthermore, high levels of ROS result in the activation of fibrotic pathways (i.e., nuclear factor-kappaB pathway) that can result in atrial fibrosis[21].

Figure 1 Pathophysiologic mechanisms of diabetes mellitus-induced atrial fibrillation.

Table 1 Pathophysiologic mechanisms connecting type 2 diabetes mellitus and Atrial Fibrillation
Furthermore, T2DM upregulates the expression of profibrotic growth factors, such as transforming growth factor (TGF)-B, which activates profibrotic pathways[20,26]. In addition, the increased production of advanced glycation end-products (AGE)s and AGE receptors that derive from T2DM also contributes to atrial fibrosis by upregulating connective tissue growth factors[27]. Fibrosis can slow down atrial conduction and create the substrate for re-entry[28]. Notably, diabetic hearts exhibit enhanced levels of collagen synthesis and high fibroblast activity[29]. We should also mention that the levels of myocardial fibrosis biomarkers, including ST2 and galectin-3, could indicate structural remodeling[25].
In addition, the renin-angiotensin-aldosterone system has also been implicated in promoting fibrosis through the TGF-B signaling pathway[4,20]. Angiotensin II is known to induce cardiac fibrosis[30]. Besides the atria, myocardial fibrosis can also occur in the ventricular myocardium of diabetic patients, resulting in stiffening and diastolic dysfunction of the left ventricle, which is associated with left atrium enlargement[31].
Adiposity may also contribute to atrial interstitial fibrosis and concomitant conduction abnormalities[30]. Obesity is associated with T2DM and lipomatous metaplasia of the heart[31]. In an animal model of a high-caloric diet, authors reported left atrial enlargement, bi-atrial conduction abnormalities, and an increased propensity for inducible and spontaneous AF among the findings[32,33].
Electrical remodeling
Another pathway that may lead to the development of AF in diabetic patients is electrical and electromechanical remodeling. Patients with abnormal glucose metabolism may present conduction abnormalities, such as longer activation times[34]. Experimental data from animal studies suggest that T2DM is linked to abnormal electrical current densities, atrial conduction, and refractory periods, all increasing susceptibility to AF[26,35]. In addition to the electrical and conduction remodeling, T2DM can affect the atrial excitation-contraction coupling, resulting in electromechanical delay (EMD) and arrhythmogenesis, as EMD is an independent predictor of both new and recurrent AF[36,37]. Interestingly, diabetic patients tend to have a higher recurrence of AF after ablation, possibly due to a proarrhythmic substrate caused by electrical remodeling[34]. Furthermore, prolonged conduction times were found in patients with abnormal fasting glucose[38], while EMDs in the atrium are higher in patients with T2DM[37].
Atrial action potential morphology altercations due to ionic currents can alter conduction velocity or susceptibility to triggered activity. In addition, gap junction function may also be affected in the atria of diabetic patients, possibly due to changes in the expression or localization of connexins[30].
Autonomic remodeling
Autonomic dysfunction can also contribute to the development of AF in diabetic patients. Cardiac autonomic neuropathy caused by T2DM contributes to the downsizing of parasympathetic and upregulation of the sympathetic stimuli, resulting in an autonomic imbalance that can excite an arrhythmia, such as AF[39]. A cross-sectional controlled study of 1992 T2DM patients suggested a strong relationship between autonomic dysfunction and silent AF in T2DM originating from autonomic dysfunction[40].
Glycemic parameters
Patients with T2DM may suffer from hypoglycemia, which can propagate sympathetic activation and overdrive, resulting in an increased risk of AF[41]. The fact that intensive glycemic control does not lower the risk of AF may be attributed to the sympathetic overdrive caused by severe hypoglycemia[42]. On the other hand, chronic hyperglycemia also creates a substrate for atrial remodeling and initiation of AF[4,26]. Hyperglycemia is also associated with enhanced angiotensin II signaling ROS production[43]. Furthermore, high glucose levels can enhance fibrosis through the production of AGEs, which can regulate cardiac fibroblasts by activating their surface receptors[27]. Studies have found, though, that it is actually glycemic fluctuations, rather than chronic hyperglycemia, that may increase the risk of AF, as they can cause oxidative stress and atrial fibrosis[42,44]. Moreover, a 2017 study revealed that long-term glycemic variability is associated with new-onset AF[45]. It has been suggested that AF and T2DM may share thrombotic pathways. Patients with T2DM suffer from insulin resistance as part of their metabolic profile. In itself, insulin resistance is associated with hypercoagulability, platelet hypersensitivity, endothelial dysfunction, and impaired fibrinolysis, all of which result in high thromboembolic risk[46]. Last, adipokines, signaling modules produced in the epicardial fat layer, have been implicated in the pathophysiology of AF in diabetic patients[30]. Leptin has been found to be associated with atrial fibrosis and AF susceptibility[47]. Other adipokines, such as secreted frizzledrelated protein 5, may represent important biomarkers in the risk prediction and management of diabetic complications such as heart failure[48], since they are implicated in mitochondrial energetics, oxidative stress, and apoptosis pathways[49]. However, their role in AF has not been thoroughly assessed. Insulin resistance and adiposity are also considered the main contributors to nonalcoholic fatty liver disease development, a condition that is linked to AF development[50].
TREATMENT
Antidiabetic drugs
Regarding the treatment of diabetic patients, medication should aim to lower blood glucose levels and prevent glycemic fluctuations. Various oral medications are currently being used to treat T2DM, several of which have been associated with a lower risk of AF, as shown in Table 2[4]. Metformin is the most commonly prescribed oral medication. By inhibiting hepatic gluconeogenesis, opposing the action of glucagon, and increasing insulin sensitivity, it exerts its glucose-lowering action. Moreover, its use has been associated with a lower risk for new-onset AF[51]. Several mechanisms have been implicated, including the prevention of the structural and electrical remodeling of left atriumviaattenuating intracellular ROS, activation of 5′ adenosine monophosphate-activated protein kinase, improvement of calcium homeostasis, attenuation of inflammation, increase in connexin-43 gap junction expression, and restoration of small conductance calcium-activated potassium channels current[52]. Thiazolidinediones (TZD) increase insulin sensitivity by acting on adipose, muscle, and, to a lesser extent, liver to increase glucose utilization and decrease glucose production. Antioxidant effects may be additionally evident, through proliferator-activated receptor-γ agonism and stimulation of catalase[53]. They are also associated with a lower risk of new-onset AF, possibly due to their anti-fibrotic effect[54]; a metaanalysis identified that the risk was reduced by 27% for patients treated with TZDs compared to the control group, especially pioglitazone[55]. On the other hand, sulfonylureas, a widely prescribed second-line hypoglycemic drug category that directly stimulates insulin release from pancreatic beta cells, is not associated with a lower risk for AF[56]. Of interest, sulfonylureas are associated with severe hypoglycemic effects, a substrate for AF development[57]. Insulin therapy has been associated with an increased risk for AF occurrence, possibly due to its hypoglycemic effect[58]. A large study, however, reported no increase in AF incidence with the use of insulin glarginevsstandard care[59].
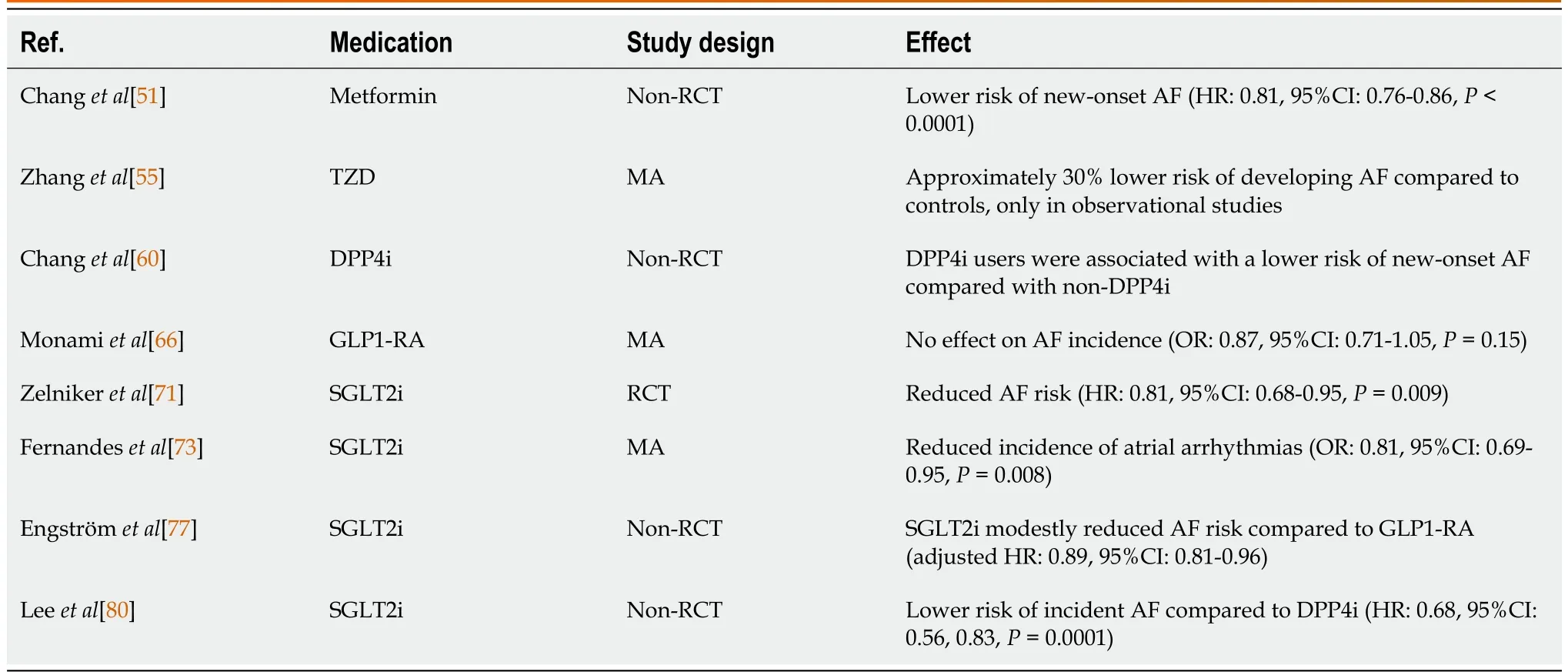
Table 2 The effect of antidiabetic medication in atrial fibrillation
Moving to novel antidiabetic agents, dipeptidyl peptidase-4 (DPP-4) inhibitors are glucose-lowering agents that inhibit DPP-4 activity in peripheral plasma, which prevents the inactivation of the incretin hormone glucagon-like peptide-1 (GLP1) in the peripheral circulation. Those agents were found to produce a lower risk of AF when compared to other antidiabetic medications, as shown in a previous study[60]. However, large trials have not revealed a correlation between DPP-4 inhibitors and the incidence of AF[61,62]. Another new class of antidiabetic drugs, GLP1 receptor agonists, are a potent glucose-lowering option by stimulating glucose-dependent insulin release from the pancreatic islets. They exhibit many cardioprotective effects, including antioxidant responses through the upregulation of antioxidant substances (catalase, glutathione peroxidase)[63]. However, they have not been associated with the incidence of AF in large trials; thus, no association between them and AF has been established[64-66].
Sodium-glucose cotransporter-2 (SGLT2) inhibitors lower plasma glucose levels by blocking the reabsorption of filtered glucose at the level of the kidneys. These agents have established cardioprotective effects[67,68], which are dependent on numerous molecular mechanisms, including restoration of beneficial autophagy, antioxidant[63], anti-inflammatory[69,70], and anti-fibrotic responses. SGLT2 inhibitors appear to affect the AF burden. A post-hoc analysis of the DECLARE-TIMI 58 trial reported decreased AF and atrial flutter episodes in individuals with T2DM on dapagliflozin regardless of AF history[71]. Even though the findings from the canagliflozin trial program were neutral[72], recent meta-analyses of randomized controlled trials point to a significant reduction of atrial arrhythmias compared to placebo[73-75]. It also has to be noted that treatment with an SGLT2 inhibitor that was accompanied by a greater than 30% initial decline in the estimated glomerular filtration rate led to a higher risk of AF incidence[76]. In a recently reported Scandinavian cohort study of 79343 new users of SGLT2 inhibitors and 57613 new users of GLP1 receptor agonists, the former was associated with a modestly reduced risk of new-onset AF[77]. Similar findings have also been reported in large registry data analyses[78-80]. Moreover, in elderly individuals with T2DM, the initiation of an SGLT2 inhibitor was accompanied by a lower incidence of AF across the follow-up[81].
Experimental studies have been conducted to assess the antiarrhythmic mechanisms of SGLT2 inhibitors. Shaoet al[82] initially demonstrated the reversal of atrial structural and electrical remodeling induced by T2DM in rats following treatment with empagliflozin. This effect was possibly mediated by the peroxisome proliferator-activated receptor-c coactivator 1α/nuclear respiratory factor-1/mitochondrial transcription factor A signaling pathway[82]. Moreover, the administration of canagliflozin in an experimental model of rapid atrial pacing resulted in a diminished atrial refractory period reduction, suppressed AF inducibility, attenuated atrial interstitial fibrosis, and oxidative stress[83]. A decreased inducibility and duration of pacing-induced AF were also reported in a rat model of mitral regurgitation following treatment with dapagliflozin[84]. Overall, the published preclinical and clinical data regarding the effect of SGLT2 inhibitors on AF appears promising, while appropriately designed randomized controlled trials are warranted to provide further insight into their antiarrhythmogenic potential.
Stroke prevention
Anticoagulants:While AF is independently associated with a high risk of stroke, it seems that DM has an additive effect on the established risk. More specifically, T2DM is associated with a 70% relative increase in the risk of stroke for patients with AF[85]. Of importance, T2DM, as a comorbidity, is included in CHAD2DS2-VASc risk score, which is the pillar of risk assessment and anticoagulation management[86]. A cohort of 37358 diabetic patients with AF demonstrated that elevated HbA1c levels were associated with an increased risk of stroke[87]. A nationwide cohort study concluded that while in AF patients with T2DM, long-lasting T2DM was associated with a higher risk of thromboembolism, it was not associated with a higher risk of anticoagulant-related bleeding[88]. In addition, the duration of T2DM for over three years was independently associated with a high risk of ischemic stroke for AF patients in the ATRIA study[89]. Insulin-dependent patients exhibit a worse prognosis regarding the incidence of stroke or systemic embolism when compared to diabetic patients who do not require insulin therapy[90]. In an observational cohort, prediabetes was also associated with increased risk for stroke for patients with incident non-valvular AF, even after accounting for other CHA2DS2-VASc risk factors[91]. It was also shown that T2DM in AF patients seems to increase the risk of both all-cause and cardiovascular mortality, as well as stroke. Furthermore, HbA1c values of < 6.2% for patients with both conditions predict significantly decreased all-cause and cardiovascular mortality[92].
Based on the CHAD2DS2-VASc risk score, anticoagulant treatment should be considered in every diabetic patient by default. When contemplating the anticoagulant of choice in this patient population, it has been shown that T2DM affects the time of therapeutic range for AF patients that receive warfarin, a fact that raises safety issues[93]. On the other hand, direct oral anticoagulants (DOACs) use resulted in a 20% reduction in stroke incidents and a 43% reduction in intracranial bleeding compared to warfarin[85]. Furthermore, a study showed that DOACs are as safe and efficient for people with T2DM as for non-diabetic people[94]. A study proposed that dabigatran had the lowest risk for T2DM among AF patients compared to warfarin[95]. For patients with T2DM and CHA2DS2-VASc scores ≥ 2, DOACs may be recommended over warfarin[4]. For a CHA2DS2-VASc score of 1 in AF patients with T2DM, the optimal type of coagulation has not yet been determined[4]. A 2021 systematic review examining the safety (hypoglycemia or bleeding) and efficacy (stroke or systemic embolism) of OACs in diabetic patients concluded that DOACs have a better clinical profile than warfarin[96].
Atrial appendage closure:Because of their improved safety and effectiveness profile, DOACs (apixaban, rivaroxaban, dabigatran, edoxaban) have replaced warfarin as the cornerstone of stroke prevention in AF patients. However, alternative treatments must be considered for the subset of individuals at extremely high risk of bleeding. It has long been demonstrated that the great majority (> 90%) of thrombi in nonvalvular AF originate in the left atrial appendage (LAA)[97]. This is a structure of variable form and size with neurohormonal and reservoir functions. Left atrial remodeling with changes in shape, blood flow (stasis), and the presence of trabeculations is thought to be involved in LAA thrombogenesis in AF[98]. T2DM has been associated with adverse LAA remodeling, with important prognostic implications regarding embolic events. Such alterations include the enlargement of the LAA orifice and the reduction of orifice flow velocity, as shown by Yosefyet al[99] in a retrospective study of 242 individuals with AF[99]. Interestingly, this appears to be unrelated to the coexistence of AF, as indicated by the experimental study of the same research group[100]. The reduced LAA flow velocity is proportional to the degree of T2DM control, measured by HbA1c[101].
LAA closure (LAAC) is a therapeutic option that is gaining ground in the field of stroke prophylaxis for AF[102]. Surgical LAAC is a technique with confirmed effectiveness, as demonstrated in the recently completed LAAOS-III randomized trial and a recent meta-analysis, for patients with AF who are having cardiac surgery for another cause[103,104]. However, no subgroup analysis according to T2DM status was made, and no safe conclusions can be drawn based on those studies. Percutaneous LAAC has also gained attention recently due to the safety and efficacy of the Watchman and Amplatzer devices, with noninferior outcomes compared to direct OACs in a randomized trial[105]. When examining the devices separately, the landmark trial comparing the Watchman device to warfarin in nonvalvular AF with CHADS2score ≥ 1 revealed a decreased rate of the primary endpoint (stroke, systemic embolism, and cardiovascular/unexplained mortality) after a 3.8-year follow-up with the device implantation[106]. However, no subgroup analysis based on the presence of T2DM was performed. An upgraded version, the Watchman FLX, is also available and is associated with superior sealing, together with similar safety[107-109], but limited data on the impact of T2DM. Concerning the Amplatzer devices (Cardiac Plug and Amulet), no dedicated large randomized trials are currently available.
The outcomes of LAAC in patients with T2DM have been inconsistent across the reported cohort studies. Litwinowiczet al[110] demonstrated similar rates of thromboembolism, mortality, and bleeding events after LAAC between T2DM and non-T2DM individuals[110]. However, in a study of 807 patients undergoing LAAC, T2DM emerged as an independent predictor of the incident early mortality[111]. T2DM was also an independent determinant of hospital readmission 30 and 90 d after LAAC[112]. These T2DM-related readmissions could be more likely associated with gastrointestinal bleeding[113]. Additionally, according to a recent report from the National Cardiovascular Data Registry of 36681 patients receiving the Watchman device, T2DM was an independent variable associated with incident ischemic stroke[114]. To our knowledge, no studies with the Amplatzer devices have assessed the role of T2DM in its safety and efficacy.
Antiarrhythmic strategies
Electrical and pharmacologic cardioversion:T2DM is associated, as comorbidity, with less efficacy of cardioversion. So far, various studies have shown that T2DM results in a lower cardioversion immediate success rate and lower success of sinus rhythm maintenance at 74.5 d follow-up, while it has also been identified as an independent risk factor for cardioversion failure within 30 d[115-117]. Interestingly, T2DM, higher HbA1c, digoxin treatment, and structural and functional cardiac abnormalities were identified as independent risk factors for cardioversion failure and AF recurrence in a 2018 retrospective outcome analysis[117]. In another study, however, this finding was not confirmed[118]. It should also be noted that although spontaneous cardioversion may be seen in a significant proportion of patients with AF, the rates are significantly lower in individuals with coexisting T2DM[119].
Similarly, antiarrhythmic drugs seem less effective for T2DM patients in experimental studies[120], although the evidence is scarce in the clinical setting. Krizet al[121] did not detect a significant association between T2DM and the failure of pharmacologic cardioversion in a single-center study of 236 patients with recent-onset AF[121]. Moving to specific drug classes, in a study of 50 consecutive patients with recent-onset AF, the presence of T2DM did not affect the efficacy of cardioversion with propafenone[122]. Regarding dronedarone use in T2DM, it has been favorably associated with a lower rate of cardiovascular hospitalizations and mortality, as well as greater freedom from AF, compared to placebo[123]. At the same time, no data are available for the specific subgroup of AF patients with T2DM who receive amiodarone. However, a previous study has suggested a delayed antiarrhythmic effect of amiodarone in individuals with T2DM, partly attributed to diabetic autonomic neuropathy[124]. Often, due to concomitant QTc prolongation, silent coronary artery disease, or renal failure, patients with T2DM may be at higher risk of developing adverse effects from antiarrhythmic drug therapy[62,125]. Despite that, a study by D’Angeloet al[126] observed that patients with T2DM were less likely to discontinue the prescribed antiarrhythmic regimen[126].
Ablation:Regardless of symptoms, early rhythm management is critical in lowering the burden of AF consequences[127,128]. Percutaneous catheter AF ablation is an appealing technique for rhythm regulation. The most often used ablation treatment in electrophysiology is radiofrequency catheter ablation. It mainly consists of pulmonary vein isolation, which is thought to be a key trigger of paroxysmal AF[129]. Catheter ablation is a well-established treatment for drug-refractory, symptomatic AF with a variety of clinical benefits and better AF control for diabetic patients when compared to antiarrhythmic drugs[130]. Despite that fact, individuals with T2DM may be less likely to receive catheter ablation, as pointed out by the recent study of Quirozet al[131]. However, the rate of T2DM patients receiving this treatment has increased over the years[132].
There have been reports of a lower efficacy of catheter ablation in individuals with T2DM than in those without T2DM. This could be due to the fact that the induced scar may impair atrial relaxation, promoting a stiff left atrial phenotype in individuals with T2DM[133]. Wanget al[134] highlighted that T2DM was associated with lower arrhythmia-free intervals in patients with T2DM after a median 29.5-mo follow-up[134]. A recent study of 369 patients with AF reported that T2DM was a predictor of AF recurrence in patients with paroxysmal AF[135]. This has not been the case in persistent AF, where the already established fibrotic changes may account for the increased risk of recurrence[136]. The performance of a second-generation, cryoballoon-based procedure may be accompanied by similar success rates in T2DM and non-T2DM patients, as pointed out by the study of Amret al[137]. Moreover, T2DM is among the variables of the DR-FLASH score that has been utilized to identify individuals with a greater burden of arrhythmogenic substrates that may benefit from extensive ablation beyond the pulmonary veins[138,139]. T2DM is also an independent predictor of pulmonary vein stenosis after catheter ablation, as shown by the ADVICE trial[140]. It should also be mentioned that individuals with T2DM may be less likely to receive catheter ablation, as pointed out by the recent study of Quirozet al[131]. However, the rate of T2DM patients receiving this treatment has increased over the years[132].
Other studies have concluded that there is no difference in post-ablation recurrence between diabetic and non-diabetic patients[141,142]. The degree of glycemic control might be an important confounding variable. More specifically, a 2015 metanalysis concluded that AF ablation has similar safety and efficacy for diabetic patients as for the general population, especially for younger patients with efficient glycemic control; however, it was shown that higher basal glycated hemoglobin levels were associated with a higher incidence of AF recurrence after catheter ablation[143]. Although the literature has not yet concluded, insufficiently managed T2DM may be a risk factor for AF recurrence following catheter ablation[144]. T2DM has also been correlated with a higher risk of cardioversion failure for early AF recurrence (≤ 7 d) after ablation[115].
Antidiabetic drugs may alter the efficacy of AF ablation in individuals with T2DM. Metformin was recently shown to be independently associated with a lower risk of AF recurrence in T2DM patients after catheter ablation[145]. A randomized trial contemplating the effect of SGLT2 inhibitors on AF following ablation concluded that tofogliflozin exhibited a better profile and less AF recurrence when compared to anagliptin[146]. Previously, dapagliflozin was an independent predictor of longer arrhythmia-free intervals in patients with T2DM undergoing radiofrequency catheter ablation after a mean follow-up of 15.5 mo[147].
CONCLUSION
DM and AF are widely affiliated entities. DM has been closely associated with the risk of AF development, identified as an independent risk factor for AF. Regarding cardiovascular risk and mortality, the presence of both conditions has been linked with high mortality. Even though the pathophysiology is still not fully determined, structural, electrical, and autonomic pathways have been identified as underlying mechanisms. Regarding therapy, novel antidiabetic agents and revolutionary antiarrhythmic and antithrombotic strategies are being examined concerning the optimal therapeutic plan for diabetic patients with AF.
FOOTNOTES
Author contributions:Tousoulis D contributed to the conceptualization; Leopoulou M and Theofilis P contributed to the investigation; Kordalis A, Papageorgiou N, Oikonomou E, Tousoulis D contributed to the supervision; Leopoulou M and Theofilis P wrote the original draft; Kordalis A, Papageorgiou N, Sagris M, Oikonomou E, and Tousoulis D edited the original draft; all authors have read and agree to the published version of the manuscript.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Greece
ORCID number:Marianna Leopoulou 0000-0001-9495-059X; Panagiotis Theofilis 0000-0001-9260-6306; Athanasios Kordalis 0000-0003-4093-4601; Marios Sagris 0000-0002-3473-1368; Evangelos Oikonomou 0000-0001-8079-0599; Dimitris Tousoulis 0000-0001-7492-4984.
S-Editor:Fan JR
L-Editor:A
P-Editor:Fan JR
 World Journal of Diabetes2023年5期
World Journal of Diabetes2023年5期
- World Journal of Diabetes的其它文章
- Early diabetic kidney disease: Focus on the glycocalyx
- Inter-relationships between gastric emptying and glycaemia:Implications for clinical practice
- Cardiometabolic effects of breastfeeding on infants of diabetic mothers
- Efficacy of multigrain supplementation in type 2 diabetes mellitus: A pilot study protocol for a randomized intervention trial
- Association of bone turnover biomarkers with severe intracranial and extracranial artery stenosis in type 2 diabetes mellitus patients
- Association between metformin and vitamin B12 deficiency in patients with type 2 diabetes
