钆塞酸二钠增强MRI T1 mapping评价肝细胞癌病理分级的价值
吕清清 徐兵 陈静静

[摘要] 目的 探討钆塞酸二钠(Gd-EOB-DTPA)增强MRI增强前后的T1值评价肝细胞癌(hepatocelluar carcinoma,HCC)病理分级的价值。方法 回顾性分析2019年3月—2020年6月青岛大学附属医院收治的75例HCC患者(共75枚病灶)的平扫及Gd-EOB-DTPA增强MRI图像,根据Edmondson-Steiner分级将病灶分为低级别组(Ⅰ~Ⅱ级)和高级别组(Ⅲ~Ⅳ级)。采用T1 mapping技术,分别测量两组病灶增强前T1值(T1pre)及肝胆特异期T1值(T1-HBP),并计算T1减少值(△T1)和T1值减少率(△T1%),比较不同病理分级间T1pre、T1-HBP、△T1、△T1%的差异,并分析各定量指标与病理分级的相关性,对有显著性的指标采用受试者工作特征(ROC)曲线及曲线下面积(AUC)分析其检验效能。结果 低级别组与高级别组HCC患者T1pre、T1-HBP、△T1%比较差异有显著性(t=-3.725~2.551,P<0.05),△T1比较差异无显著性(P>0.05);相关性分析显示T1pre、T1-HBP与HCC病理分级呈正相关(r=0.293、0.472,P<0.05),△T1%与HCC病理分级呈负相关(r=-0.254,P<0.05),△T1与HCC病理分级无显著相关性(P>0.05);T1pre、T1-HBP、△T1%区分低级别组与高级别组HCC的AUC分别为0.676(95%CI=0.542~0.810,P<0.05)、0.784(95%CI=0.671~0.897,P<0.05)、0.654(95%CI=0.531~0.775,P<0.05),最佳截断值分别为1 253.5 ms、875.5 ms与40.5%,相应的灵敏度分别为72.9%、83.3%、39.6%,特异度分别为63.0%、74.1%、92.6%。结论 Gd-EOB-DTPA增强MRI T1pre、T1-HBP及△T1%对术前HCC病理分级具有一定的预测价值。
[关键词] 癌,肝细胞;磁共振成像;钆DTPA;T1 mapping;肿瘤分级;诊断
[中图分类号] R445.2;R735.7 [文献标志码] A
[ABSTRACT] Objective To investigate the value of T1 value before and after Gd-EOB-DTPA-enhanced MRI in evaluating the pathological grade of hepatocellular carcinoma (HCC). Methods A retrospective analysis was performed for the plain scan and Gd-EOB-DTPA-enhanced MRI images of 75 HCC patients (75 lesions in total) who were admitted to The Affiliated Hospital of Qingdao University from March 2019 to June 2020. According to Edmondson-Steiner grading, lesions were divided into low-grade group (grade Ⅰ-Ⅱ) and high-grade group (grade Ⅲ-Ⅳ). The T1 mapping technique was used to measure pre-enhanced T1 value (T1pre) and hepatobiliary-specific T1 value (T1-HBP), and the reduction in T1 value (△T1) and the reduction rate of T1 value (△T1%) were calculated. T1pre, T1-HBP, △T1, and △T1% were compared between the lesions with different pathological grades, and the correlation between each quantitative index and pathological grade was analyzed. The receiver operating characteristic (ROC) curve and the area under the ROC curve (AUC) were used to analyze the efficiency of each statistically significant index. Results There were significant differences in T1pre, T1-HBP, and △T1% between the low-grade group and the high-grade group (t=-3.725-2.551,P<0.05), while there was no significant difference in △T1 between the two groups (P>0.05). Correlation analysis showed that T1pre and T1-HBP were positively correlated with the pathological grade of HCC (r=0.293,0.472,P<0.05), and △T1% was negatively correlated with the pathological grade of HCC (r=-0.254,P<0.05); there was no significant correlation between △T1 and the pathological grade of HCC. T1pre, T1-HBP, and △T1% had an AUC of 0.676 (95%CI=0.542-0.810,P<0.05), 0.784 (95%CI=0.671-0.897,P<0.05), and 0.654 (95%CI=0.531-0.775,P<0.05), respectively, in differentiating between low-grade HCC and high-grade HCC, with a sensitivity of 72.9%, 83.3%, and 39.6%, respectively, and a specificity of 63.0%, 74.1%, and 92.6%, respectively, at the optimal cut-off values of 1 253.5 ms, 875.5 ms, and 40.5%, respectively. Conclusion T1pre, T1-HBP, and △T1% of Gd-EOB-DTPA-enhanced MRI have a certain value in predicting the pathological grade of HCC.
[KEY WORDS] Carcinoma, hepatocellular; Magnetic resonance imaging; Gadolinium DTPA; T1 mapping; Neoplasm gra-ding; Diagnosis
肝细胞癌(hepatocellular carcinoma,HCC)是全球发病率第6位恶性肿瘤,也是导致癌症相关死亡的第4大原因[1]。我国是肝癌的高发国家,手术切除是目前HCC最有效的治疗方法,但手术预后往往并不理想,5年复发率高达80%[2]。研究表明,Edmondson-Steiner分级是影响HCC患者术后复发的独立因素[3-5]。因此术前明确HCC组织病理学分级,有助于指导临床选择有效的治疗方案,以提高手术治疗效果,改善患者预后。既往有很多研究以MRI病变组织信号强度的变化来预测HCC病理分级,但这种方法有很大的主观性,导致预测的病理分级结果不相同[6-7]。T1 mapping作为一种测量组织T1值的MRI定量技术,测量的T1值更加客观、可靠。目前,国内利用T1 mapping评价HCC病理分级的研究报道比较少。本研究采用钆塞酸二钠(Gd-EOB-DTPA)进行增强MRI检查,利用T1 mapping通过测量增强前后的T1值,探讨各定量指标在术前评价HCC病理分级的价值。
1 资料与方法
1.1 一般资料
选取我院2019年3月—2020年6月经病理证实的HCC患者。纳入标准:①经手术或穿刺病理检查证实为HCC,并且有组织分化程度的评估者;②患者术前2周内行MRI平扫及Gd-EOB-DTPA增强MRI扫描,且有T1 mapping序列者;③图像质量较优者。排除标准:①患者影像学资料不完整;②患者术前接受过其他治疗,如射频或微波消融、肝动脉化疗栓塞、放射治疗等;③图像质量较差者。根据以上标准,最终纳入了HCC患者共计75例(75枚病灶)。
1.2 图像分析及T1值的测量
所有图像均传输至图像存储与传输系统进行分析,由2位腹部影像诊断医师对图像能否用于测量和计算进行判断。T1值的测定在增强前及肝胆特异期的T1 mapping序列上进行,于相同部位分别勾画圆形或椭圆形感兴趣区(ROI),测量同一位置的T1值,病灶的ROI放置在病灶最大层面上,并参考T2WI图像和增强图像,ROI的选择应避免包括坏死组织、大的血管、胆管和伪影区域。增强前T1值(T1pre)以及肝胆特异期T1值(T1-HBP)分别测量3次,取平均值,并计算病灶T1减少值(△T1)和T1值减少率(△T1%),其中△T1=T1pre-T1-HBP;△T1%=(T1pre-T1-HBP)/T1pre×100%。
1.3 病理学诊断
根据Edmondson-Steiner病理分级,所有病灶分为Ⅰ~Ⅳ级[8]。本研究将Ⅰ~Ⅱ级病灶纳入低级别组,Ⅲ~Ⅳ级病灶纳入高级别组。
1.4 统计学方法
利用SPSS 19.0软件进行统计学处理。对所有数据进行正态性检验,正态分布的计量资料以±s表示,计数资料用率表示。正态分布计量资料的组间比较采用独立样本t检验,计数资料的组间比较采用χ2检验或Fisher确切概率法。相关性分析采用Spearman相关分析方法。使用受试者工作特征(ROC)曲线及曲线下面积(AUC)分析有显著性指标的诊断效能。以P<0.05为差异有显著性。
2 结 果
75例HCC患者(共75枚病灶)中,男63例,女12例;年龄41~75岁,平均(56.43±7.57)岁;75例患者均有慢性肝炎病史,44例伴有肝硬化;病灶位于肝右叶57例,位于肝左叶18例;病灶最大径10~70 mm,平均(31.04±13.25)mm;根据Edmondson-Steiner病理分级:Ⅰ级6例,Ⅱ级42例,Ⅲ级26例,Ⅳ级1例,低级别组48枚病灶,高级别组27枚病灶;两组HCC患者年龄、性别、慢性肝炎情况、肝硬化情况、病灶部位及病灶大小方面比较差异无显著性(P>0.05)。见表1。
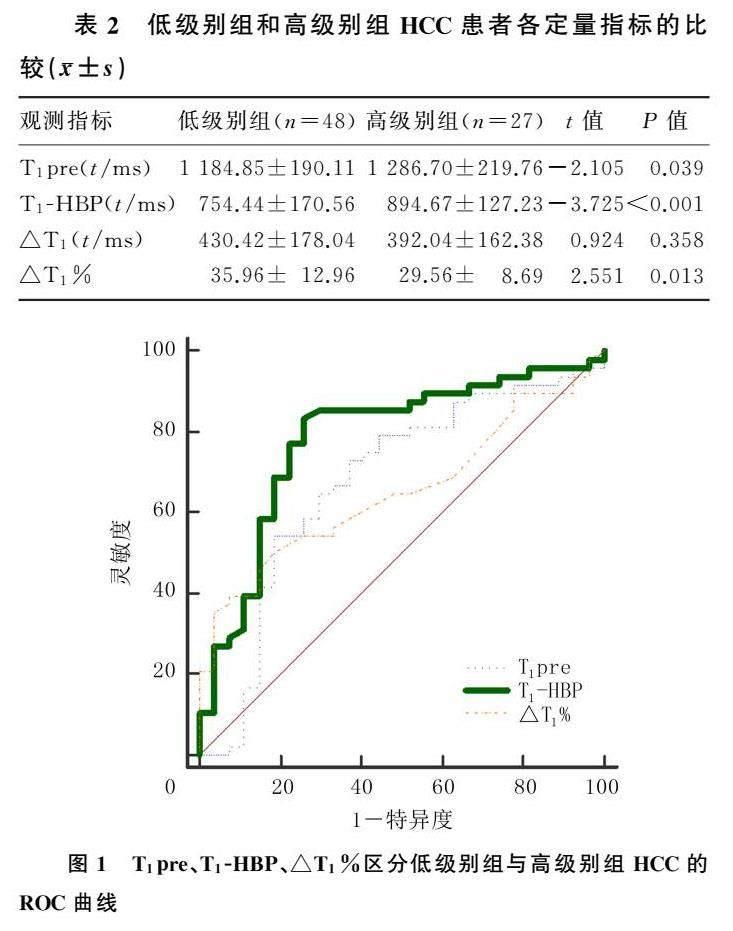
低级别组与高级别组HCC患者的T1pre、T1-HBP、△T1%比较差异具有显著意义(t=-3.725~2.551,P<0.05),△T1比较差异无显著意义(P>0.05),见表2。相关性分析显示,T1pre、T1-HBP与HCC的病理分级呈正相关(r=0.293、0.472,P<0.05),△T1%与HCC病理分级呈明显的负相关关系(r=-0.254,P<0.05),△T1与HCC病理分级则没有显著相关性(P>0.05)。T1pre、T1-HBP、△T1%区分低级别组以及高级别组HCC的AUC分别为0.676(95%CI=0.542~0.810,P<0.05),0.784(95%CI=0.671~0.897,P<0.05)以及0.654(95%CI=0.531~0.775,P<0.05),最佳截斷值分别为1 253.5 ms、875.5 ms、40.5%,所对应灵敏度分别为72.9%、83.3%、39.6%,特异度分别为63.0%、74.1%、92.6%。见图1。
3 讨 论
Gd-EOB-DTPA是一种新型的肝细胞特异性对比剂,其化学结构决定了其特有的双重生物学行为,一方面可用作非特异性细胞外间隙对比剂,并通过肾脏排泄;另一方面,可通过肝细胞膜上的有机阴离子转运体(OATP)1B3被肝细胞选择性吸收,再通过肝细胞膜胆系面上的多耐药性蛋白由胆管系统排泄[9]。Gd-EOB-DTPA经静脉注入体内后,约50%被功能正常的肝细胞摄取,正常肝实质细胞强化,大约20 min后肝实质达到最大强化程度,即肝胆特异期,持续约2 h[10]。Gd-EOB-DTPA在肝胆特异期的图像使得病变与正常肝实质的对比更明显,从而可以提高肝脏病变影像学检测和分类的灵敏度及特异度[11-13],Gd-EOB-DTPA增强MRI还被认为是诊断早期肝癌灵敏度最高的检查方法[14-15]。
既往的研究中,常用的方法是通过分析病变组织的相关检查信号强度变化来预测HCC的病理分级。如TONG等[16]通过研究分析Gd-EOB-DTPA增强MRI平扫以及肝胆特异期的HCC信号强度变化,得出肿瘤与正常肝脏组织对比增强率,以此来预测较小的且内部无坏死的HCC的病理分级。而SCHELHORN等[17]则通过观测动脉期、门脉期、延迟期及肝胆特异期HCC信号强度,发现其信号强度与病理分级没有明显相关性。通过测量信号强度变化预测HCC病理分级存在一定的局限性,因为这种方法有很大的主观性,且信号强度受很多因素影响。T1 mapping技术弥补了这一不足,它是一种测量组织T1值的MRI定量技术,测量的T1值反映了组织弛豫时间的变化,弛豫时间是组织固有性质,不会受到技术因素及成像参数等的影响。Gd-EOB-DTPA在体内能被肝细胞特异性摄取而缩短其T1值,不同的病变因对Gd-EOB-DTPA的攝取、分布不同而会有不同的T1值,因此,通过测量肝内局灶性病变在增强前后的T1值,可以了解病变T1值的变化规律,为诊断和鉴别诊断提供定量指标[18-19]。
本研究采用肝细胞特异性对比剂Gd-EOB-DTPA进行增强MRI,结合T1 mapping技术,结果显示,利用T1pre、T1-HBP及△T1%可以区分低级别组HCC与高级别组HCC,随着病灶级别增高,T1pre、T1-HBP增高,而△T1%降低。本研究结果显示,T1pre、T1-HBP及△T1%与HCC病理分级均具有相关性,T1-HBP的相关性最好。通过T1pre、T1-HBP以及△T1%区分低级别组与高级别组HCC的AUC分别为0.676、0.784、0.654,与T1pre及△T1%相比,T1-HBP对区分低级别组与高级别组HCC有较好的诊断价值。根据最大约登指数相对应截断值的灵敏度及特异度中,T1-HBP较T1pre的灵敏度、特异度更高,具有更好的诊断效能。当△T1%取值为40.5%时,特异度高达90%以上,但随着特异度的增高,其灵敏度降低。本研究中,低级别组与高级别组HCC的T1-HBP较T1pre均减低,但其对应的△T1在两组间的差异无显著性,且与病理分级无显著相关关系。
本研究的结果提示,HCC病灶可以摄取Gd-EOB-DTPA,随着HCC分级的增加,Gd-EOB-DTPA的摄取量减少。HCC的肿瘤细胞仍保存着部分肝细胞的功能,其超微结构观察亦显示高、中分化癌在细胞形态、细胞核、细胞器、细胞接触面等更接近于正常肝细胞,因而具有部分正常肝细胞功能,能摄取Gd-EOB-DTPA。HCC对Gd-EOB-DTPA的摄取是由OATP1B3的表达决定的[20]。本研究中,T1pre、T1-HBP、△T1%可以区分低级别组与高级别组HCC的机制与OATP1B3的表达是否有关有待进一步研究。
近年来,T1 mapping技术的应用得到了简化,在MRI扫描过程中,可以自动地获取对比前后T1mapping。基于Gd-EOB-DTPA增强MRI的T1 mapping技术主要应用在肝脏弥漫性及局灶性病变的诊断研究[21-26]。CHEN等[27]通过对45例HCC患者的研究表明,Gd-EOB-DTPA增强MRI的平扫T1值及T1-HBP对预测HCC的病理分级具有一定的参考价值,这与本研究结果相符。然而,CHEN等[27]研究中△T1%与HCC病理分级无相关性,与本研究结果并不一致,造成这种差异的原因,可能是样本选择偏差的结果。PENG等[28]研究结果表明,不同病理分级的HCC在Gd-EOB-DTPA增强前后T1降低的百分率不同,本研究结果与此相似。
本研究的局限性主要有:①本研究属于回顾性研究,需要今后更进一步的临床实践和前瞻性研究来丰富和确认相关的研究结果;②本研究入组病例不够多,Edmondson-SteinerⅠ级、Ⅳ级的HCC分别只有6例和1例,对整体数据可能会有影响,还需要后续进一步研究来证实T1值在预测HCC病理分级中的作用。
综上所述,本研究中Gd-EOB-DTPA增强MRI T1pre、T1-HBP及△T1%在术前预测HCC病理分级具有一定的可行性,其准确结论还需大样本研究以进一步验证。
伦理批准和知情同意:本研究涉及的所有试验均已通过青岛大学附属医院医学伦理委员会的审核批准(文件号QYFYWZLL26909)。所有试验过程均遵照《人体医学研究的伦理准则》 的条例进行。受试对象或其亲属已经签署知情同意书。
作者声明:吕清清、陈静静参与了研究设计;吕清清、徐兵、陈静静参与了论文的写作和修改。所有作者均阅读并同意发表该论文。所有作者均声明不存在利益冲突。
[参考文献]
[1] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018,68(6):394-424.
[2] BRUIX J, TAKAYAMA T, MAZZAFERRO V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM):A phase 3, randomised, double-blind, placebo-controlled trial[J]. Lancet Oncol, 2015,16(13):1344-1354.
[3] HUANG J, LIU F C, LI L, et al. Nomograms to predict the long-time prognosis in patients with alpha-fetoprotein negative hepatocellular carcinoma following radical resection[J]. Cancer Med, 2020,9(8):2791-2802.
[4] ZHOU L, WANG S B, CHEN S G, et al. Risk factors of recurrence and poor survival in curatively resected hepatocellular carcinoma with microvascular invasion[J]. Adv Clin Exp Med, 2020, 29(7):887-892.
[5] QIN X L, YANG T F, HUANG Z K, et al. Hepatocellular carcinoma grading and recurrence prediction using T1 mapping on gadolinium-ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging[J]. Oncol Lett, 2019,18(3):2322-2329.
[6] 胡夢洁,郁义星,范艳芬,等. 钆塞酸二钠增强磁共振影像学特征联合定量分析在预测肝细胞癌病理分级中的价值[J]. 中华医学杂志, 2020,100(17):1299-1304.
[7] IWASA Y, KITAZUME Y, TATEISHI U, et al. Hepatocellular carcinoma histological grade prediction: A quantitative comparison of diffusion-weighted, T2-weighted, and hepatobiliary-phase magnetic resonance imaging[J]. J Comput Assist Tomogr, 2016,40(3):463-470.
[8] EDMONDSON H A, STEINER P E. Primary carcinoma of the liver: A study of 100 cases among 48, 900 necropsies[J]. Cancer, 1954,7(3):462-503.
[9] CHOI Y, HUH J, WOO D C, et al. Use of gadoxetate diso-dium for functional MRI based on its unique molecular mechanism[J]. Br J Radiol, 2016,89(1058):20150666.
[10] LAGADEC M, DOBLAS S, GIRAUDEAU C, et al. Advanced fibrosis: Correlation between pharmacokinetic parameters at dynamic gadoxetate-enhanced MR imaging and hepatocyte organic anion transporter expression in rat liver[J]. Ra-diology, 2015, 274(2):379-386.
[11] AN C, LEE C H, BYUN J H, et al. Intraindividual comparison between gadoxetate-enhanced magnetic resonance imaging and dynamic computed tomography for characterizing focal hepatic lesions: A multicenter, multireader study[J]. Korean J Radiol, 2019, 20(12):1616-1626.
[12] ORLACCHIO A, CHEGAI F, FABIANO S, et al. Role of MRI with hepatospecific contrast agent in the identification and characterization of focal liver lesions: Pathological correlation in explanted livers[J]. Radiol Med, 2016,121(7):588-596.
[13] AGNELLO F, DIOGUARDI BURGIO M, PICONE D, et al. Magnetic resonance imaging of the cirrhotic liver in the era of gadoxetic acid[J]. World J Gastroenterol, 2016, 22(1):103-111.
[14] OMATA M, CHENG A L, KOKUDO N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update[J]. Hepatol Int, 2017,11(4):317-370.
[15] LEE S M, LEE J M, AHN S J, et al. LI-RADS version 2017 versus version 2018:Diagnosis of hepatocellular carcinoma on gadoxetate disodium-enhanced MRI[J]. Radiology, 2019, 292(3):655-663.
[16] TONG H F, LIANG H B, MO Z K, et al. Quantitative analysis of gadoxetic acid-enhanced magnetic resonance imaging predicts histological grade of hepatocellular carcinoma[J]. Clin Imaging, 2017,43:9-14.
[17] SCHELHORN J, BEST J, DECHNE A, et al. Evaluation of combined Gd-EOB-DTPA and gadobutrol magnetic resonance imaging for the prediction of hepatocellular carcinoma grading[J]. Acta Radiol, 2016,57(8):932-938.
[18] KATSUBE T, OKADA M, KUMANO S, et al. Estimation of liver function using T1 mapping on Gd-EOB-DTPA-enhanced magnetic resonance imaging[J]. Invest Radiol, 2011,46(4):277-283.
[19] YOON J H, LEE J M, KANG H J, et al. Quantitative assessment of liver function by using gadoxetic acid-enhanced MRI: Hepatocyte uptake ratio[J]. Radiology, 2019, 290(1):125-133.
[20] UENO A, MASUGI Y, YAMAZAKI K, et al. OATP1B3 expression is strongly associated with Wnt/β-catenin signalling and represents the transporter of gadoxetic acid in hepatocellular carcinoma[J]. J Hepatol, 2014,61(5):1080-1087.
[21] 蒋宇,邱维加,周智鹏. Gd-EOB-DTPA增强MRI T1 mapping技术在肝脏疾病的应用进展[J]. 国际医学放射学杂志, 2019,42(2):208-211.
[22] FERNANDES J L, ROCHITTE C E. T1 mapping: Technique and applications[J]. Magn Reson Imaging Clin N Am, 2015, 23(1):25-34.
[23] DUAN T, JIANG H Y, XIA C C, et al. Assessing liver function in liver tumors patients: The performance of T1 mapping and residual liver volume on Gd-EOBDTPA-enhanced MRI[J]. Front Med (Lausanne), 2020,7:215.
[24] PAN S, WANG X Q, GUO Q Y. Quantitative assessment of hepatic fibrosis in chronic hepatitis B and C: T1 mapping on Gd-EOB-DTPA-enhanced liver magnetic resonance imaging[J]. World J Gastroenterol, 2018, 24(18):2024-2035.
[25] PENG Z P, LI C, CHAN T, et al. Quantitative evaluation of Gd-EOB-DTPA uptake in focal liver lesions by using T1 mapping: Differences between hepatocellular carcinoma, hepatic focal nodular hyperplasia and cavernous hemangioma[J]. Oncotarget, 2017,8(39):65435-65444.
[26] 徐萍,黄梦琪,廖冰,等. Gd-EOB-DTPA MRI动态增强预测孤立性肝细胞癌微血管侵犯的单因素及多因素回归分析[J]. 影像诊断与介入放射学, 2017,26(1):31-36.
[27] CHEN C Y, CHEN J, XIA C C, et al. T1 mapping combined with Gd-EOB-DTPA-enhanced magnetic resonance imaging in predicting the pathologic grading of hepatocellular carcinoma[J]. J Biol Regul Homeost Agents, 2017,31(4):1029-1036.
[28] PENG Z P, JIANG M J, CAI H S, et al. Gd-EOB-DTPA-enhanced magnetic resonance imaging combined with T1 mapping predicts the degree of differentiation in hepatocellular carcinoma[J]. BMC Cancer, 2016,16:625.
(本文編辑 耿波 厉建强)

