Anhydride type film-forming electrolyte additives for high-temperature LiNi0.6Co0.2Mn0.2O2//graphite pouch cells
Anwei Zhng, Chengyun Wng,b,**, Weizhen Fn, Jingwei Zho, Ynping Huo,Xijun Xub,,*
a GAC Automotive Research &Development Center, Guangzhou, 511434, China
b Guangdong Provincial Key Laboratory of Advanced Energy Storage Materials, School of Materials Science and Engineering, South China University of Technology,Guangzhou, 510641, PR China
c College of Chemical Engineering and Light Industry, Guangdong University of Technology, Guangzhou, 510006, PR China
d Research and Development Center, Guangzhou Tinci Materials Technology Co., Ltd., Guangzhou, 510765, China
Keywords:Anhydride-based Electrolyte additives Film-forming LiNi0.6Co0.2Mn0.2O2//graphite High-temperature performance
A B S T R A C T LiNi0.6Co0.2Mn0.2O2//graphite full cell,due to its high energy density as the most promising candidate for power batteries received increased attention, but the severe capacity loss under high-temperature conditions prevents their large-scale application.Here, glutaric anhydride (GA) and succinic anhydride (SA) as electrolyte additives are investigated to overcome the high-temperature problem of LiNi0.6Co0.2Mn0.2O2//graphite cells.Due to the unique film-forming features and high-temperature stability,the cells containing 0.5%GA and 0.5%SA can retain capacity retention of 91.2%and 92.4%stored at 60 °C for 15 days,corresponding to a negligible capacity loss of 4.6 and 0.8 mAh g-1 after 200 cycles, respectively.In addition, the lifespans of cells under 25 °C are also significantly extended by introducing GA and SA into the electrolytes.Electrochemical and spectroscopic techniques results indicate the full cell capacity loss is predominantly caused by the unstable anode solid electrolyte interphase(SEI)layer and a stable SEI film derived from GA and SA is formed on the graphite electrode surfaces.The SEI film can effectively inhibit the consumption of electrolytes and enhance the high-temperature performance of cells.This work paves a way for moderating high energy density cells' work under high-temperature operation by developing special anodic film-forming electrolyte additives.
1.Introduction
Lithium-ion batteries(LIBs)have been widely applied in our daily life due to their high energy density and output voltage [1].However, the limited lithium salts and security of LIBs seriously threaten its application.Some researchers paid attention to exploring new battery systems such as sodium-ion batteries (SIBs), and solid oxide fuel cells (SOFCs)[2-4].At the same time, a series of technologies for LIBs including electrodes, separators, and technologies have been explored for LIBs,such as MnS anode, Li3N film-modified separator, and life prediction[5-8].Nevertheless,these new battery systems and technologies are still far from commercial applications.Traditional LIBs still have occupied the energy conversion and storage field for portable electronic devices and electric vehicles (EVs), owing to their high energy density beyond 300 Wh kg-1and other excellent electrochemical characteristics [9-13].Furthermore, LIBs are ubiquitous in suites of smartphone and notebook computers because of their good performance at room temperature[14-18].
The performances of LIBs, such as cycling stability, rate capability,and safety et al.,are deeply dependent on the stability of the solid electrolyte interphase (SEI) layer [19,20].Nevertheless, the SEI deserved from the traditional electrolyte composition decomposition on the graphite electrode surface begins to disintegrate above 65°C, the unstable SEI layer causing irreversible side reactions and continuous electrolyte decomposition and finally brings a rapid degradation under the high-temperature working condition [21-23].Additionally, the traditional electrolyte starts to decompose above 55°C, the high operating temperature brings an increase in the internal temperature and even thermal runaway[24,25].In large-scale applications,the risk of thermal runaway is not to be underestimated, which is generally considered the reason for cell combustion and explosion [25,26].Consequently, to mitigate the high-temperature issues, extensive efforts have been devoted to controlling the mechanical chemical, and morphological properties of the surface film.Among the various methods,the formation of a stable protective film on the anode surface by electrolyte additive has been explored as an economical and effective solution [27-29].It is commonly believed that the electrolyte decomposition on the electrode’s surface from the solid electrolyte interphase (SEI) formed can stabilize storage performance for LIBs [30-32].This viewpoint has contributed significantly to improving the performance of LIBs and has become a consensus over the past two decades, which may be called the SEI era[30-35].Based on this understanding, engineering a robust SEI by regulating the electrolyte compositions (i.e., lithium salt, solvent, additives, etc.) has become a principle of electrolyte design [36-38].However,it remains a challenge to elucidate appropriate additives to generate a stable SEI layer on the high-voltage Ni-rich cathode with excellent high-temperature storage performance [39-42].The chemical,morphological and mechanical properties of such interphases predetermine a series of key properties of LIBs, which include accessibility,throughout the battery life[43,44].
Recently, some anhydride-type molecules have been discovered as appropriate electrolyte additives for LIBs, which can participate in the film-forming process and protect the electrode material from the erosion of the electrolyte [45-47].Wang’s group explored butyric anhydride(BA) as an electrolyte additive and verified that this additive enhanced the cycling performance and rate capability of LiNi0.5Mn1.5O4cathode[48].In the work of Ouyang’s team, citraconic anhydride (CA) was explored as an electrolyte additive to improve the electrochemical performance of LiNi0.6Co0.2Mn0.2O2(NCM622)/graphite pouch cells [49].Though, the SEI has been widely investigated, repeated consumption of cyclic Li+, which deterioration the performance of LIBs at high temperatures suffers severely from cycle instability[50-52].High-temperature stability is a critical issue for lithium-ion batteries(LIBs),because of the large capacity loss and impedance rise during storage, thus causing severe electrochemical performance degradation [53,54].Therefore,adequate high-temperature performance is an urgent necessity for LIBs,especially in the field of electric vehicle practical applications [49,55].Therefore, the development of new and intrinsically more stable electrolytes has become a priority for LIB researchers [56,57].Huo’s work discovered phenyl 4-fluorobenzene sulfonate (PFBS) molecule as a multifunctional film-forming additive,which can preferentially occur in electrochemical reactions than the electrolyte solvents on both cathode and anode to form stabilized SEI, and thus achieving excellent cycling performance in a wide temperature range[58].Sun’s group explored 1,3-propane sultone (PS) as a functional electrolyte additive constructed robust SEI for high-voltage Li//LiNi0.8Co0.1Mn0.1O2batteries and achieved superior cycling stability at elevated temperature of 45°C [59].However, for these weather-sensitive applications, more feasible electrolyte additives must be explored to overcome the drastic for high-temperature LIBs.
Here,two anhydride derivatives glutaric anhydride(GA)and succinic anhydride (SA) were discovered as electrolyte additives to adequate high-temperature performance of LiNi0.6Co0.2Mn0.2O2//graphite cells.Electrochemical and spectroscopic techniques results indicate the full cell is significantly extended by introducing GA and SA into the electrolytes.A stable SEI film derived from GA and SA is formed on the graphite electrode surfaces, which can effectively inhibit the consumption of electrolytes and enhance the high-temperature performance of cells.This work shed light on boosting high energy density cells under hightemperature operation by developing special anodic film-forming electrolyte additives.
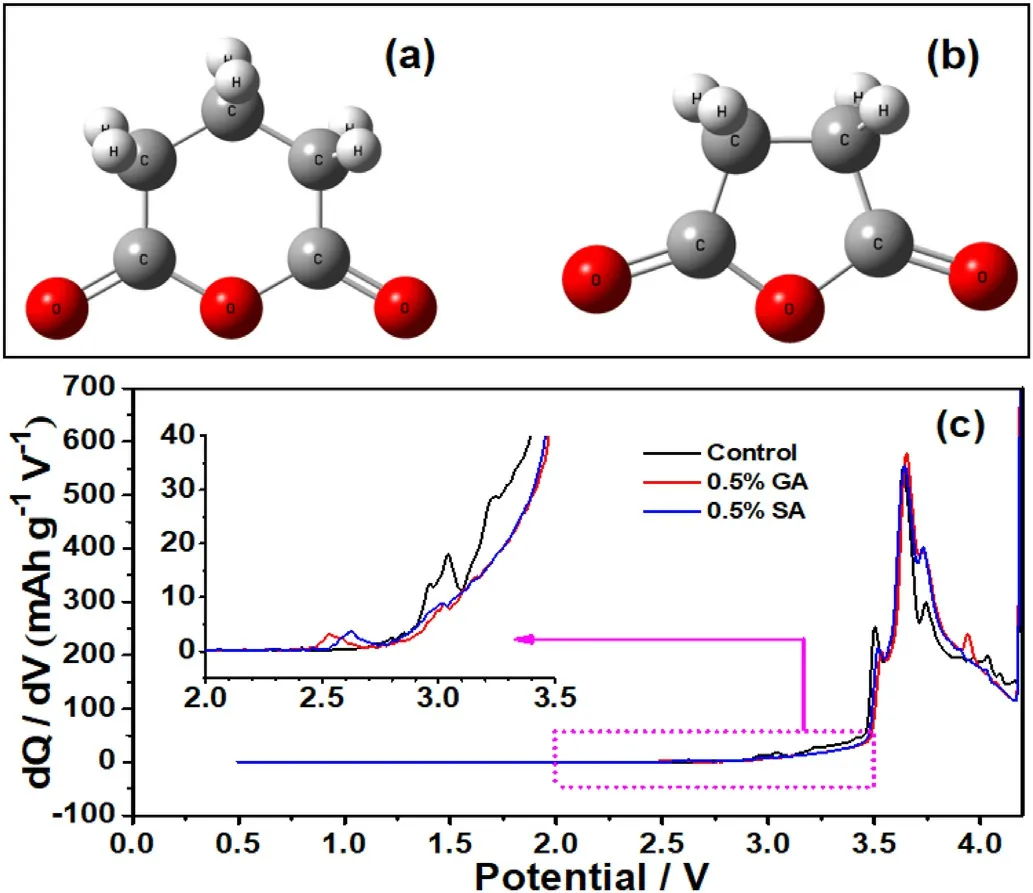
Fig.1.The molecular structures of a) GA and b) SA, c) differential capacity(dQ/dV) vs.potential (V) of LiNi0.6Co0.2Mn0.2O2/graphite pouch cells using different electrolyte additives at 0.1C in the initial charge process.
2.Experimental section
The NCM622 cathode was purchased from Ningbo Jinhe New Materials Co., Ltd., (Ningbo, China), and the graphite anode material was purchased from Shenzhen BTR New Energy Materials Co.,LTD.Carl Zeiss Supra type 40 field emission scanning electron microscope(FESEM)was used for morphology characterization.The X-ray photoelectron spectrometer(XPS)was elucidated by using a Thermo Fisher(USA)scientific instrument to obtain a spectrum with an Al Κα(1486.8 eV)X-ray source with a voltage of 15 kV and a current of 10 mA.The separator used model SD216202 is purchased from Shenzhen Star Source Material Technology Co.,LTD.The active material load and thickness of the NCM622 cathode were 35.04 mg cm-2and 126 μm, respectively.The corresponding graphite anodes are 16.95 mg cm-2and 121 μm, respectively.The NCM622//Graphite pouch cell with a designed capacity of 1800 mAh under the charging cutoff voltage of 4.2V.1.0 M LiPF6- ethylene carbonate (EC)/diethyl carbonate (DEC)/ethyl methyl carbonate (EMC)(2:3:3, mass ratio) was used as the basic electrolyte, and GA, SA of different mass concentrations as the electrolyte additive.The injection volume of each NCM622//graphite pouch cell was 6.00 ± 0.05 g NCM622 and graphite electrodes were disassembled in a glove box and washed with dimethyl carbonate (DMC) 3 times and dried in vacuum condition.The AC impedance of the NCM622//Graphite pouch cell was measured by Solartron1455A multi-channel charge-discharge tester with a frequency region of 0.04 Hz-100.0 kHz,and the disturbance amplitude was 10 mV.DF411SC/611SC(Japan Yamato)Explosion-proof precision constant-temperature incubator is used for 60°C storage at 100%stateof-charge (SOC).DHG-9053BS/S-III (Shanghai Xinmiao medical equipment) constant temperature air dry oven for cycling performance.The battery was tested by Neware charge-discharge test device for capacity recovery.
3.Results and discussion
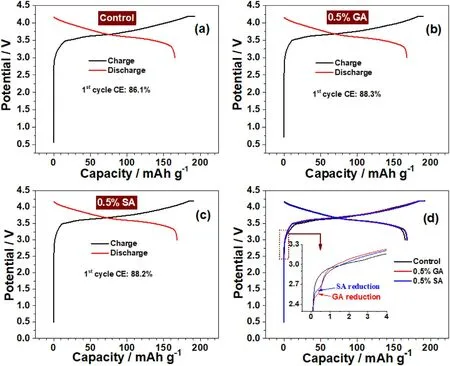
Fig.2.Voltage profiles and coulombic efficiency during the first cycling at 0.1C between 3.0 and 4.2 V of LiNi0.6Co0.2Mn0.2O2//graphite pouch cells with a) no additive, b) 0.5% GA, c) 0.5% SA, d) comparison of the first voltage profiles of the cells using different additives.
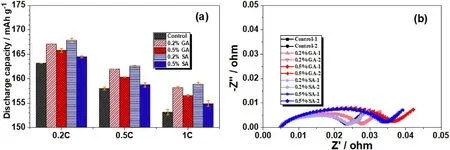
Fig.3.(a)Discharge capacities of LiNi0.6Co0.2Mn0.2O2//graphite pouch cells with different amounts of GA and SA at 0.2C,0.5C,and 1.0C between 3.0 and 4.2 V for the initial three cycles; (b) EIS of the cells using different additives.
In this work,two anhydride derivatives glutaric anhydride(GA)and succinic anhydride(SA)were investigated,and corresponding molecular structures were displayed in Fig.1a and 1b.These two molecules with similar structures, the GA molecule possesses one more carbon than the SA molecule.According to previous reports, anhydride substances possess film-forming properties on the anode surface to a certain degree[45,46].To further explore the electrochemical behavior of the above two substances on the anode interface,the pouch cells were precharged with 0.1C, respectively, and the initial charging curve was analyzed.As shown in Fig.1c, the pre-charge curves of batteries with different electrolyte batteries emerged with some differences in the 2.0~3.5 V voltage range.The battery without additive exist a distinct reaction peak around~3.0 V,which mainly corresponds to the reduction of EC molecules[59].When a certain amount of GA and SA components is added to the electrolyte, the peak strength of EC decomposition becomes weakened and two peaks appear at ~2.5 V and 2.6 V respectively.While the pre-charging curve at these two potentials remains flat, and there is no peak appears for the without additive one.This indicates that the two peaks occurring at 2.5 V and 2.6 V are caused by the reaction of GA and SA on the graphite anode, respectively, which further confirms that the anhydride additives GA and SA have good anode film-forming properties.
Fig.2 shows the initial charge/discharge curves of LiNi0.6-Co0.2Mn0.2O2//graphite pouch cells with different electrolytes at a 0.1C rate.It can be observed that these pouch cells both with a higher initial charge capacity than discharge, which is mainly related to the decomposition of the electrolyte on the surface of the electrode during the charging process.As can be observed from Fig.2a,the initial coulombic efficiency (ICE) of the LiNi0.6Co0.2Mn0.2O2//graphite cell without the additive is 86.1%, while the initial coulombic efficiency of the LiNi0.6-Co0.2Mn0.2O2//graphite cells with 0.5%GA and SA is 88.3%and 88.2%,respectively(Figures 2b and 2c).This phenomenon indicates that adding 0.5 wt%anhydride additive to the electrolyte can improve the ICE of the LiNi0.6Co0.2Mn0.2O2//graphite cells.The charging/discharging curves of LiNi0.6Co0.2Mn0.2O2//graphite cells have an additional voltage platform in the 2.5-2.7 V voltage region (Fig.2d) when containing 0.5% GA and SA, but for the additive-free without this phenomenon.It can be concluded that GA and SA additives participated in the film-forming reaction on the graphite surface in the initial charging process.
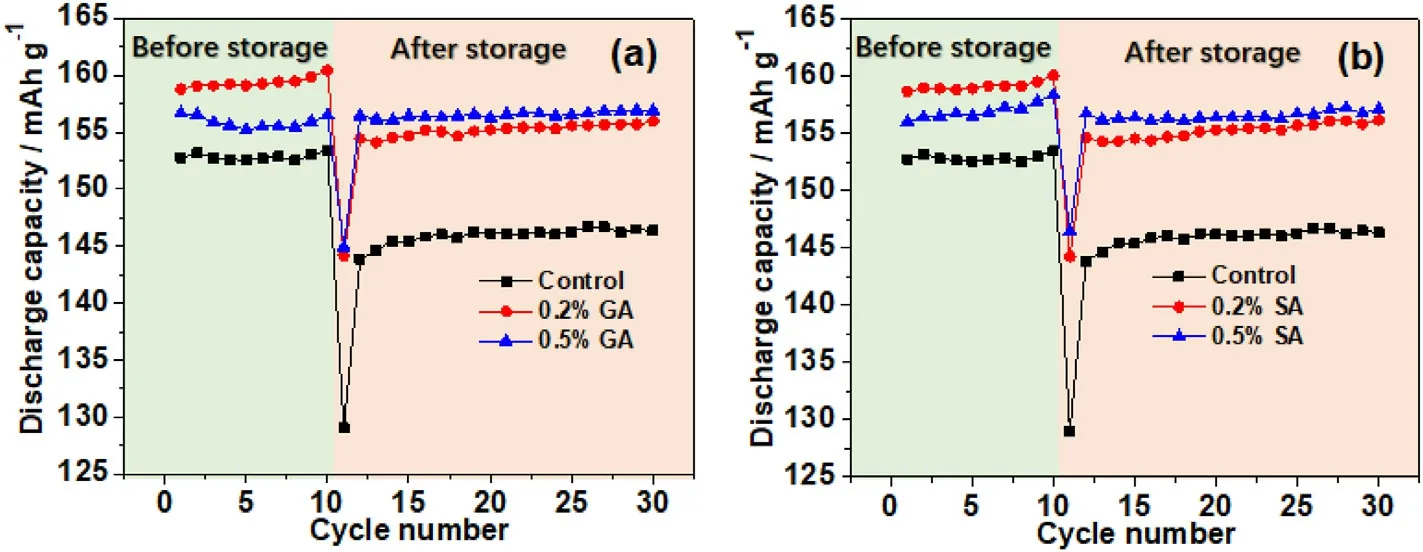
Fig.4.The cycling performance of NCM622//graphite pouch cells with different electrolytes before and after 60 °C storage for 15 days:(a)the electrolytes containing various concentrations of GA and (b) the electrolytes containing various concentrations of SA.
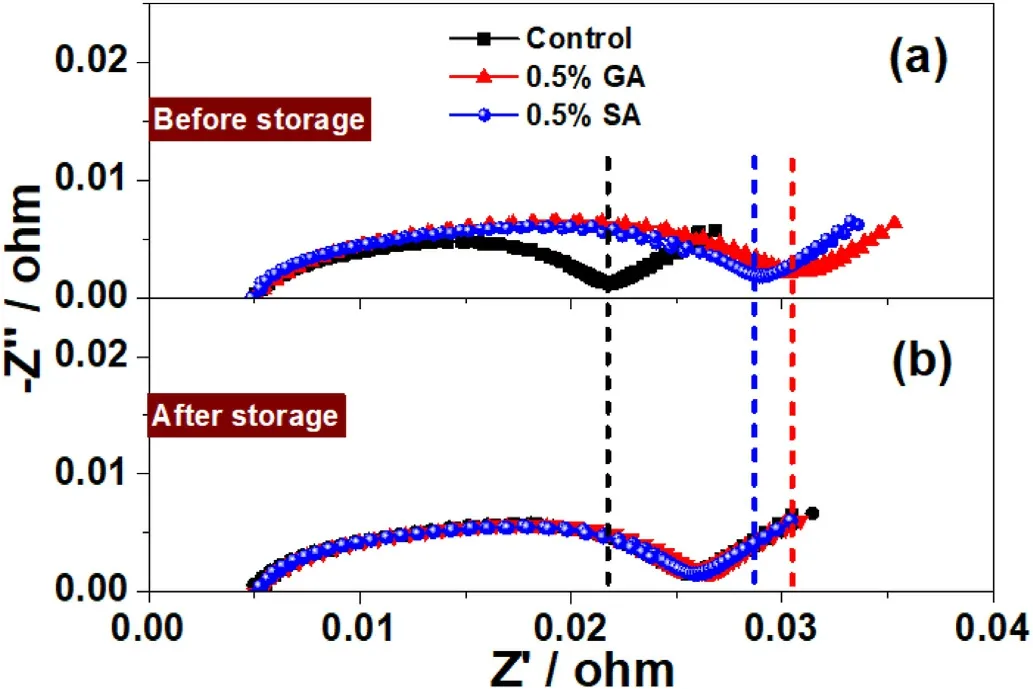
Fig.5.EIS patterns of NCM622//graphite pouch cells using different electrolytes (a) before and (b) after storage 60 °C for 15 days (full charge state of ~4.2 V).
To explore the influence of GA and SA on the LiNi0.6Co0.2Mn0.2O2//graphite pouch cells,the above pre-charged batteries were tested at 0.2C,0.5C,and 1C,respectively.Fig.3 shows the comparison of the discharge capacity of LiNi0.6Co0.2Mn0.2O2//graphite cells using different electrolytes at different current densities,respectively.It can be seen that adding GA and SA to the electrolyte can increase the discharge capacity of the battery to a certain degree,which is consistent with the improvement of 0.5% GA and SA of the battery described in Fig.2.According to the comparison results, the discharge capacity of LiNi0.6Co0.2Mn0.2O2//graphite cells with 0.2% GA and SA is higher than that of 0.5% at the same discharge current,and not the higher the additive content the more beneficial to the specific capacity.Fig.3b shows the electrochemical impedance spectroscopy (EIS) of the LiNi0.6Co0.2Mn0.2O2//graphite pouch cells after grading,respectively.It can be seen that the impedance of the cells containing 0.2%GA or SA battery is slightly higher than the one without additive.As increase the amount of additive,the impedance difference becomes more obvious, indicating that GA and SA additives with large anode film-forming impedance, need to strictly control the additional amount in the electrolyte in practical applications.
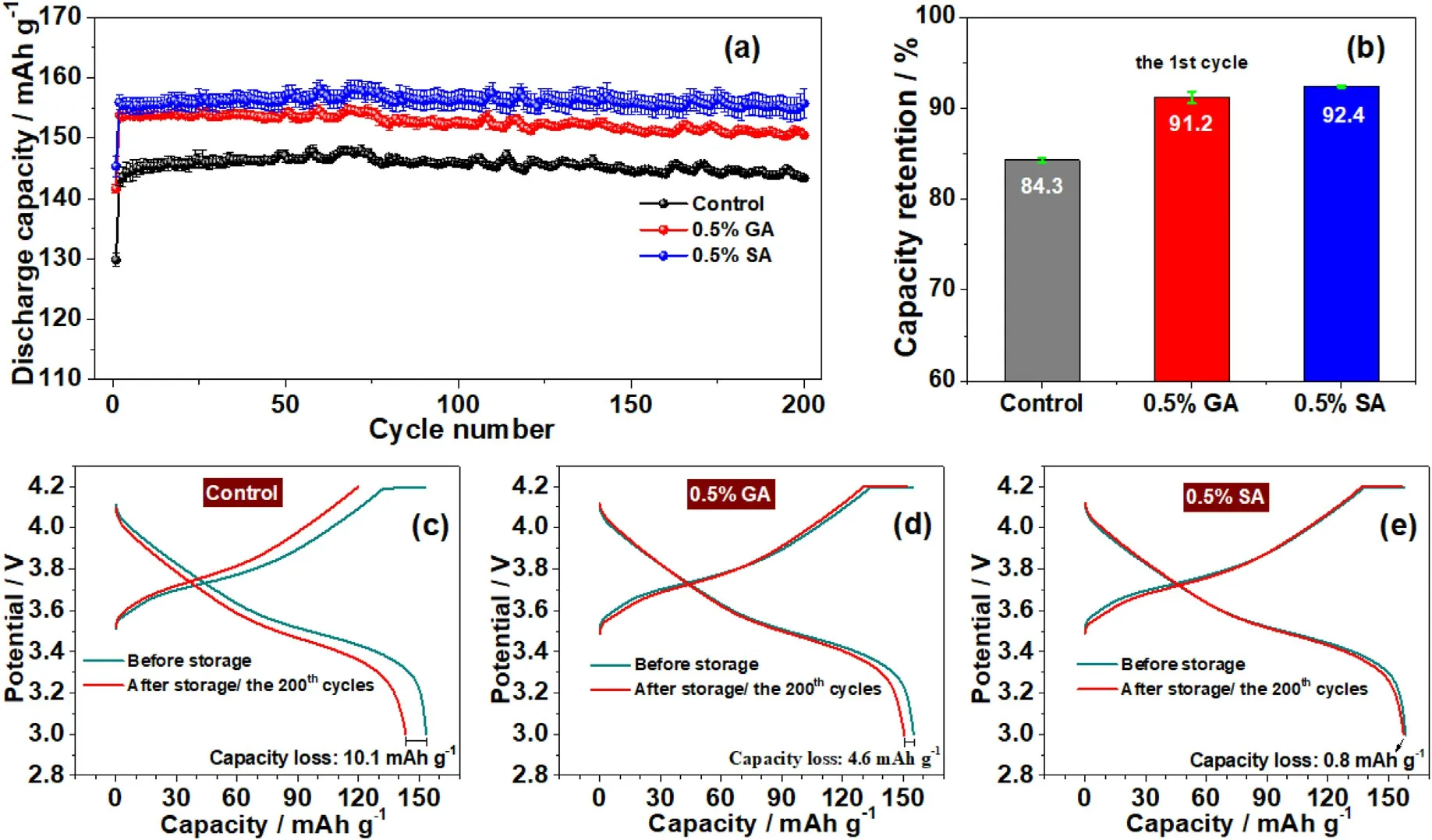
Fig.6.(a) Cycling performance of NCM622//graphite pouch cells using different electrolytes after storage; (b) the capacity retention of the first cycle; (c-e) comparison of the charge/discharge profiles obtained from different cells: (c) without additive, (d) with 0.5% GA, and (e) with 0.5% SA.
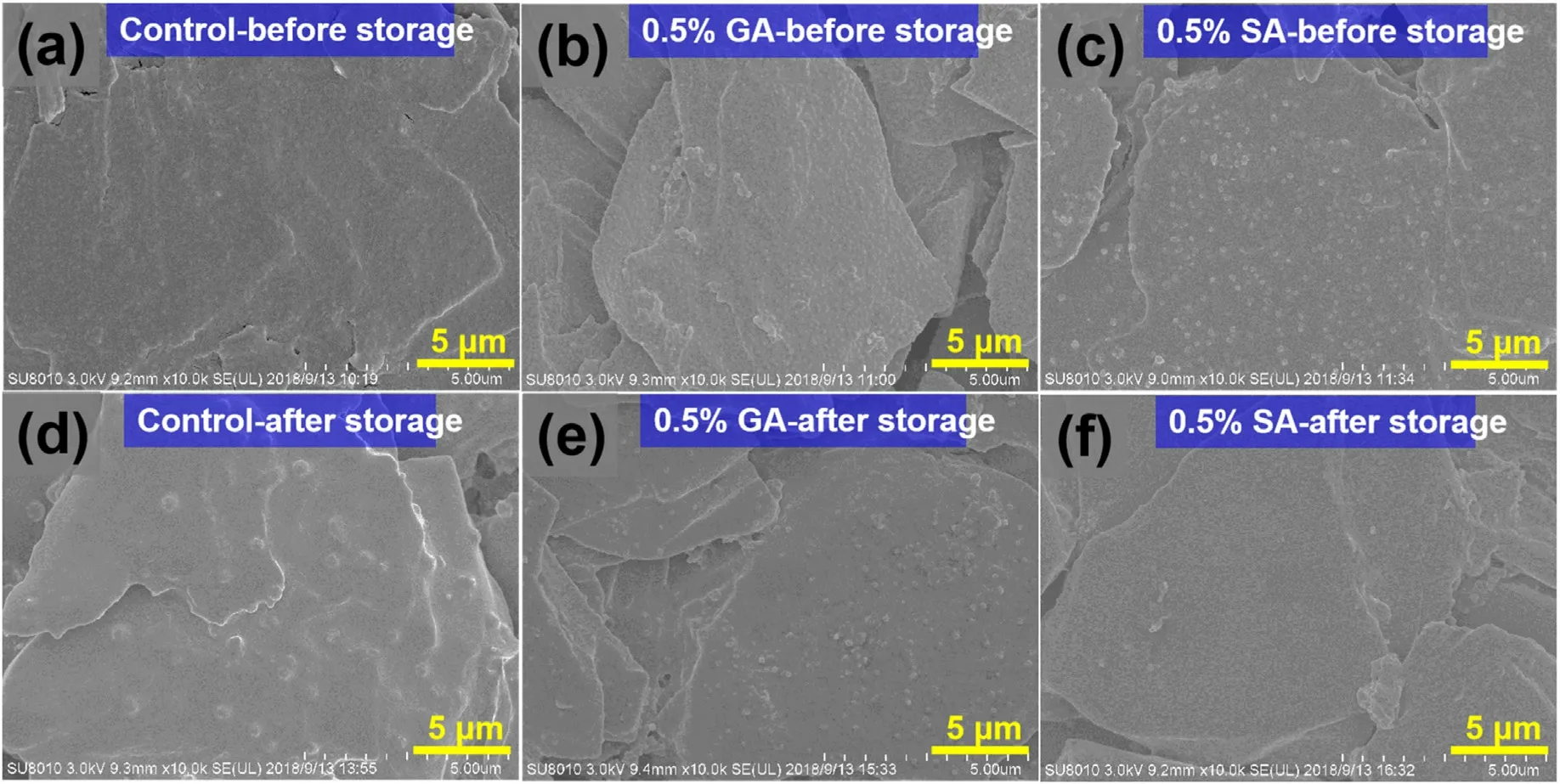
Fig.7.SEM images of graphite anodes cycled in different electrolytes before high-temperature storage:(a)without additive,(b)with 0.5%GA,(c)with 0.5%SA;after high-temperature storage: (d) without additive, (e) with 0.5% GA, (f) with 0.5% SA.
Fig.4 shows the influence of GA and SA with different amounts on the cycling performance at high-temperature conditions.As can be observed from Fig.4a, the LiNi0.6Co0.2Mn0.2O2//graphite containing 0.2% GA shows the highest discharge capacity than the one with 0.5%GA and GAfree before high-temperature storage, which is consistent with the test results discussed in Fig.3a.These results indicate that adding a certain amount of GA components to the electrolyte is beneficial to moderate the specific capacity of the LiNi0.6Co0.2Mn0.2O2.After being stored at 60°C for 15 days, the cycling performance of NCM622//graphite pouch cells containing GA additives and without GA was measured and displayed in Fig.4a.It can be concluded that both the NCM622//graphite with and without GA additives undergo capacity decay to a certain degree, but evidently that the NCM622//graphite cell with 0.5% GA additive inherits the highest specific capacity and without obvious capacity loss.This result means that the GA additive significantly improved the hightemperature storage performance and the electrolyte containing 0.5%GA possesses the best performance.As shown in Fig.4b, the SA shows similar high-temperature storage properties with GA, that is, the cells containing 0.2%of SA show the highest discharge capacity before hightemperature storage,and 0.5%of SA cells attained the least capacity loss stored at 60°C for 15 days.Therefore,considering the effect of different amounts of GA and SA additives on the capacity retention and ESI of the NCM622//graphite pouch cells, the electrolyte with a 0.5% addition amount of GA and SA was taken as the research object.
To in-depth reveal the influence of GA and SA on the electrode/electrolyte interface stability the EIS test of the NCM622//graphite pouch cells before and after high-temperature storage was analyzed.As shown in Fig.5a,the EIS resistance of the NCM622//graphite pouch cells added 0.5%GA and SA is evidently higher than that of the blank one,and the impedance of the GA one is slightly higher than that of the SA,indicating that the initial film-forming impedance of both GA and SA on the graphite is large.After being stored at 60°C for 15 days(Fig.5b),the impedance of the black NCM622//graphite pouch cells is significantly increased, indicating that the interface impedance is constantly increasing during the high-temperature storage process[56].But for the batteries using GA and SA components as additives, both of the impedance have a certain reduction trend after high-temperature storage compared with that before high-temperature storage.This phenomenon can be ascribed to the anhydride additives (GA, SA) involved in the modification of the graphite interface layer under high-temperature conditions, and such anhydride components may reduce the side reaction or generate some intermediate that can maintain the stability of SEI.
Furthermore, the cycling of NCM622//graphite pouch cells containing 0.5 wt% GA and SA components after the high-temperature storage was analyzed.Fig.6a displays the cycling properties of NCM622//graphite pouch cells in the electrolyte containing 0.5 wt% SA, 0.5 wt%GA,and additive-free,respectively.It can be attained that the NCM622//graphite cell with the electrolyte containing 0.5 wt% SA exhibit the highest capacity and both the cells in the electrolyte containing 0.5 wt%SA and 0.5 wt% GA demonstrate a significant improvement in the specific capacity.Fig.6b shows the capacity retention of NCM622//graphite pouch cells after high-temperature storage compare to the initial cycle with these three different electrolytes.It is apparent that the NCM622//graphite batteries containing 0.5 wt% SA and GA exhibit high capacity retention of 92.4% and 91.2%, respectively.Fig.6c-e depict the initial and 200th charge/discharge profiles of NCM622//graphite batteries in these three electrolytes.It can be clearly seen from the figure that the NCM622//graphite batteries undergo a capacity loss of ~10.1 mAh g-1after high-temperature storage.While for the pouch cells containing 0.5%GA and SA,the capacity decay after 200 cycles is only 4.6 mAh g-1and 0.8 mAh g-1,respectively,indicating that these anhydride additives can effectively alleviate the problem of capacity loss during hightemperature storage.The cycling stability of these three types of pouch cells was also measured at room temperature and the results are depicted in Fig.S1.Obviously, the cells with 0.5% GA and SA as electrolyte additives attained a higher specific capacity than the ones without additives.The LiNi0.6Co0.2Mn0.2O2//graphite pouch cells the containing 0.5% GA and 0.5% SA achieved high capacity retention of 98.4% and 98.7% at room temperature (Table S1), respectively, while the one without additive is only 94.1%.These results confirmed that the GA and SA have properties that improved the capacity retention of LiNi0.6-Co0.2Mn0.2O2//graphite pouch cells at both room temperature and after 60°C storage.
To reveal the influence of GA and SA additives on the graphite anode,the scanning electron microscopy (SEM) characterization of NCM622//graphite batteries before and after high-temperature storage was carried out.As shown in Fig.7a-c,no matter what electrolyte the battery cycled,the anode's surface is covered by a layer of SEI, and the state of the original graphite sheet cannot be clearly observed.The covered SEI film is formed during the initial cycle procedure.Compared with the SEM images of graphite before high-temperature storage, there are some unknown particles on the anode interface of these three electrolytes,especially for the 0.5%GA-containing one with numerous small particles distribute on the graphite surface, while the SA samples are relatively sparse, which may ascribe to the SEI with different electrolytedecomposition components.The SEM image of graphite anode in these three electrolytes after being stored at 60°C for 15 days was also carried out.As shown in Fig.7d, in the anode without additives, the small particles on the graphite surface become larger compared to those before high-temperature storage (Fig.7a) and the surface is uneven and thick.For the anode cycled in the electrolyte with 0.5% GA and SA additives,the graphite surface becomes smooth and has fewer small particles after high-temperature storage,especially for SA one without obvious particle accumulation and distribution.This phenomenon can be attributed to the high temperature accelerated the stable SEI layer form and is also consistent with the EIS results(Fig.5).On account of the above analysis,it can be concluded that the SA and GA additives participated in the filmforming procedure and resulting in a smooth and stable SEI layer after the high-temperature storage, thus avoiding the continuous side effects between electrolyte and electrode and thereby achieving stable cycling performance and high capacity retention.
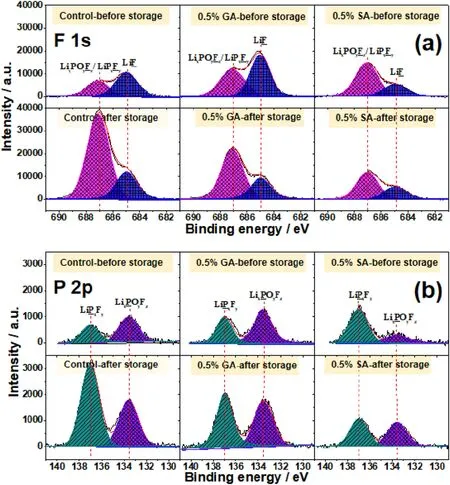
Fig.8.XPS spectra of (a) F1s and (b) P2p for the graphite anodes obtained from LiNi0.6Co0.2Mn0.2O2//graphite pouch cells with different electrolytes (without additive, with 0.5% GA and 0.5% SA) before and after high-temperature storage.
To further confirm the influence of GA and SA on the graphite/electrolyte interface,the XPS technique was applied to analyze the chemical components of SEI before and after the high-temperature storage in different electrolytes.As shown in Fig.8a, the high-resolution F 1s spectrum mainly consists of two peaks at ~687 eV and ~685 eV,which can be assigned to the LixPOyFz/LixPFyand LiF,respectively[48,56].It is well known that LiF is a main component of the SEI layer and insulates Li+and electrons,the higher the proportion of this component,the larger impedance of the battery.The LixPOyFzand LixPFymainly originated from the decomposition of LiPF6in the electrolyte.It can be seen that the peak intensity of LixPOyFz/LixPFyfor the graphite/electrolyte interface with the GA and SA is stronger than the additive-free one[43,50].After being stored at 60°C for 15 days,the strength of LixPOyFz/LixPFypeaks for the graphite/electrolyte interface is significantly enhanced for the one without additive,while the change of the peak intensity for the one with GA and SA is less, indicating that the anhydride derivatives suppressed the continuous decomposition of lithium salt on the graphite surface in the electrolyte[43,50,58].Combined with the EIS results(Fig.5),it can be speculated that the increase in impedance is directly related to the decomposition degree of LiPF6.To further understand the decomposition of LiPF6, P2p spectra were also measured and presented in Fig.8b.The peaks located at ~133.5 eV and ~137 eV were mainly related to the LixPOyFzand LixPFy, respectively [43,58].From the P 2p spectrum, the proportion of LixPFyincreased dramatically for blank one after high-temperature storage, indicating a lot of LiPF6decomposition at 60°C, which is consistent with the change of resistance before and after high-temperature storage.Therefore, the P 2p spectrum further confirmed that the LiPF6was greatly decomposed on the graphite surface during the high-temperature process,and the irreversible consumption of the LiPF6caused a large capacity loss of the LiNi0.6Co0.2Mn0.2O2//graphite pouch cells after storage at the high temperature.The electrochemical performance of current GA and SA electrolyte additives for LIBs with reported anhydride-type electrolyte additives were summarized in Table S2.It can be observed that these GA and SA electrolyte additives show advantages in prolonging the cycling performance of NCM622//graphite cells and effectively improving capacity retention.
4.Conclusion
In this work,GA and SA were discovered as electrolyte additives for LiNi0.6Co0.2Mn0.2O2//graphite pouch cells.Both the GA and SA can improve the initial specific capacity and the coulombic efficiency to a certain degree.After being stored at 60°C for 15 days, the initial discharge capacity retention is 84.3%, 91.2%, and 92.4% for the blank and containing 0.5% GA and SA additives pouch-cells, respectively.Benefiting from the unique film-forming function of GA and SA, the LiNi0.6Co0.2Mn0.2O2//graphite pouch cells with the electrolyte containing 0.5%GA and SA after 200 cycles only undergo 4.6 mAh g-1and 0.8 mAh g-1capacity loss, respectively.Through the XPS analysis, the GA and SA additive can effectively suppress the irreversible consumption of LiPF6on graphite surfaces under high-temperature conditions.A certain amount of GA and SA components in the electrolyte can effectively improve the thermal stability of the SEI on the graphite anode and isolate the direct contact between the electrolyte and graphite inhibiting the irreversible consumption of electrolyte and LiPF6under the hightemperature condition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the R&D Program in Key Areas of Guangdong Province (no.2020B0101030005), Guangdong Basic and Applied Basic Research Foundation (nos.2021A1515010332), China Postdoctoral Science Foundation (no.2020M672622) and Ph.D.Innovation Fund of GAC Automotive Research &Development Center (no.XVY-FD-3).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://do i.org/10.1016/j.pnsc.2023.08.002.
 Progress in Natural Science:Materials International2023年3期
Progress in Natural Science:Materials International2023年3期
- Progress in Natural Science:Materials International的其它文章
- Effect of the withdrawal rate on the microstructure and creep behavior of the directionally solidified nickel-based superalloy
- Simultaneous high-performance detection and removal of tetracycline with a polyoxomolybdate-based coordination polymer
- Strong interaction and confine effect of manganese oxide-cobalt embedded in self-templated nitrogen-doped carbon enable ultra-stable zinc-air batteries
- Countering microplastics pollution with photocatalysis: Challenge and prospects
- Research advances on electrode materials for solid oxide electrolysis cells
- Towards high-efficiency of hydrogen purification in metal hydride
