Polyaniline-supported tungsten-catalyzed oxidative deoximation reaction with high catalyst turnover number
Wen Li,Feng Wang,Yaocheng Shi,Lei Yu
School of Chemistry and Chemical Engineering,Yangzhou University, Yangzhou 225002,China
Keywords:Deoximation Carbonyl Tungsten Free radical reaction Oxidation
ABSTRACT Polyaniline-supported tungsten (W@PANI) was easily prepared by immersing polyaniline (PANI) in the aqueous solution of Na2WO4.It was found to be an efficient catalyst for oxidative deoximation reaction,the very important transformation for pharmaceutical industry.Besides the green features,the method employed very few of catalytic tungsten (0.048 mol% vs.oxime substrates),resulting in the high turnover numbers (TONs) of the catalyst (ca.103 mol/mol) and the low metal residues in product (<0.1 ppm).The reaction is applicable for a variety of substrates,including those containing heterocycles,which are key intermediates in medicine synthesis.It has also been successfully magnified to kilogram scale production to afford the desired carbonyl products smoothly.
Deoximation reaction is an important transformation for pharmaceutical industry [1,2].Since oximes are usually stable crystals with relatively high melting points,the oximation-deoximation processes can be employed in protection,characterization and purification of the carbonyl-containing compounds.For example,the strategy has been successfully applied in the total synthesis of erythronolide A,a macro-cyclic antibiotic molecule bearing an endocyclic carbonyl [3].It is also applied to purify watermelon ketone in industrial production [4].Deoximation reaction can be employed to synthesize carbonyl-containing products from the non-carbonyl starting materials,and the conversion of limonene to carvone is a typical example [5–7].The reaction can be performed by using acidic promoters,but the employed stoichiometric/excess additives [8] or irritant reagents [9–11] may be hazardous to the environments.Catalytic oxidative deoximation reaction may afford alternatives occurring under neutral conditions [11–18].During the past five years,we have developed a series of catalytic oxidative deoximation reactions by using H2O2or molecular oxygen as the clean oxidants [19–25].In these reactions,organoselenium [19,20,25] or organotellurium [21,22] compounds,diaryl ketones [23] or free radical initiators such as azodiisobutyronitrile(AIBN) [24] were employed as the catalyst,while FeCl3[20] orNhydroxyphthalimide [25] were used as the additives to promote the catalyst activity.However,despite the high cost of organoselenium [19,20,25] or organotellurium [21,22] compounds,the employed catalyst dosage was high in these reactions [19–22,24,25]resulting in the low catalyst turnover numbers (TONs<100) unfavorable for industrial grade production.The visible light-driven autocatalytic oxidative deoximation reactions might not require additional catalyst,but the limitations of light absorption properties of substrates restricted its application scope of substrates [23].Thus,developing novel catalysts for the reactions that can run with high TONs and wide substrate scope is essential from the practical application viewpoint.
On the other hand,polyanilines (PANIs) have been found to be good supports for nano-metal catalysts [26].Although anilines may be toxic,their polymers are less toxic and safe to the environments [27,28].Since the aniline monomers are easily available and cheap chemicals,it is acceptable to use PANIs as the industrial catalyst supports from an economic point of view.In comparison with the traditional inorganic supports,PANIs are versatile materials and their electrical properties can be adjusted by introducing a series of functional groups into the aromatic rings of aniline monomers,which may exert significant influences on the catalytic activities of the prepared nano-metal catalysts [29,30].In our cases,we have successfully developed the organoseleniumcatalyzed green oxidative polymerization of anilines to prepare PANIs under mild and green conditions [31].The PANIs-supported nano-metal catalysts (M@PANIs) were then developed and have been successfully applied in Suzuki–Miyaura [32],Heck [33],Sonogashira [34],Buchwald-Hartwig [35] and Ullmann [29] coupling reactions.Notably,M@PANIs were found to be highly efficient and could catalyze the coupling reactions with very high catalyst turnover numbers (TONs) [29–35].Inspired by these findings,we began our project on M@PANIs-catalyzed oxidative deoximation reactions,which were different to the reported coupling reactions and were more challenging objectives.Tungsten-mediated deoximation has already been achieved [36].Although the method requiring stoichiometric/excess tungsten salt and zinc reductant is not environment friendly,it inspires us to design the W@PANI catalyst [37] and employ it in the oxidative deoximation reactions.Herein,we wish to report our findings.
PANI could be synthesizedviathe oxidative polymerization reaction of aniline by using H2O2as the oxidant [31].It was then immersed in aqueous Na2WO4to upload tungstenviathe coordination of the involved nitrogen with the metal.The prepared W@PANI was employed as catalyst for the oxidative deoximation reaction of (E)-1-(3-chlorophenyl)ethan-1-one oxime (1a).The reaction solvent was initially screened and the results were summarized in Table S1 (Supporting information).Heating the reaction mixtures in EtOH at 80 °C for 24 h,the desired (E)-1-(3-chlorophenyl)ethan-1-one (2a) could be obtained in only 42% yield(Table S1,entry 1).Ester solvents,such as EtOAc,dimethyl carbonate (DMC) and diethyl carbonate (DEC) were tested,but the deoximation reaction could hardly occur (Table S1,entries 2–4).The reactions in 1,4-dioxane orN,N-dimethylformamide (DMF) led to 2a in moderate yields (Table S1,entries 5 and 6).MeCN as a high polar organic solvent could well dissolve both of the substrate and H2O2oxidant.It was screened out to be a favorable solvent,affording 2a in 72% yield under the mild conditions (Table S1,entry 7).
The catalyst and H2O2dosages of the reaction were then optimizedviaa series of control reactions performed on the basis of the conditions described in Table S1,entry 7.It was found that,for the reaction of 0.5 mol of 1a,using 20 mg of W@PANI catalyst should be preferable,affording 2a in 77% yield (Fig.S1a in Supporting information).Reducing or enhancing the employed catalyst amount both resulted in the incomplete conversion of the substrate.In the cases using high loading catalyst,the catalytic metal might led to the H2O2decomposition,and this has been proved by the control experiment in which H2O2was added in batches.For example,in the reaction using 40 mg of W@PANI for 0.5 mmol of 1a,the product 2a was obtained in only 40% yield.However,if H2O2was introduced in four batches every 6 h,the yield of 2a could be significantly enhanced to 73%.The same technique did not work for the reaction using 20 mg of W@PANI for 0.5 mmol of 1a,for which the yield of 2a decreased to 75% contrarily when H2O2was introduced in four batches.The results in Fig.S1b (Supporting information) clearly indicated that H2O2was an essential oxidant for the reaction.The yield of 2a rose along with the increasing H2O2dosage and reached its peak when 100 mol% of H2O2vs.1a was employed,i.e.the theoretically required molar amount.Using excess H2O2resulted in the decreased 2a yield due to the generation of a series of over-oxidized by-products such as the ester and the carboxylic acid.
The reaction kinetics was then studiedviaa series of control experiments within different reaction times from 1 h to 24 h.The yields of 2a as well as the unconverted 1a recovery ratio data were recorded and illustrated by Fig.S2 (Supporting information).The yield of 2a increased sharply to 69% within the first 6 h,and then gradually increased to 77% after the 24 h reaction.Simultaneously,the unreacted 1a ratio decreased along with the increasing 2a yield.It was supposed that the exhaustion of H2O2might result in the significantly decreased reaction speed at 6 h,after which the oxygen in air could participate the reaction as the oxidant.This hypothesis could be verified by control experiments described and discussed in the mechanism study sectionvide infra.
The application scope of the reaction was examined by treating a series of oxime substrates 1 under the optimized reaction conditions (Table 1).Since ICP-MS analysis has indicated that the tungsten content in W@PANI was 0.22 wt%,it can be calculated that the reaction employed only 0.048 mol% of tungsten.Similarly,the catalyst turnover number (TON) of the reaction of 1a could be calculated to be 1.6×103accordingly (Table 1,entry 1),which was obviously higher than that of the reported works [19–22,24,25].Other electron-deficient or -sufficient methyl ketoximes such as 1b–1h were all fit for the reaction,affording the corresponding ketones 2b–2h in 62%–84% yields with 103grade TONs (Table 1,entries 2–8).It was notable that the ortho substituent of the substrate did not affect the reaction (Table 1,entries 3 and 5).The reaction of 0.5 mmol of 1-phenylethan-1-one oxime (1i) afforded 2i in 78% yield,and the product yield was enhanced to 89% in the magnified reaction using 200 mmol of 1i,in which the produced 2i was isolated by distillation other than chromatography separation to reduce the loss of weight caused by its volatility (Table 1,entry 9).The distillation residue containing catalytic species could be reused by adding fresh reactant and oxidant and heating,and it gave 2i in 86% yield (Table 1,entry 9).In comparison with 1i,the reaction of 1-phenylbutan-1-one oxime (1j) afforded 2j in decreased yield,indicating that the prolonged aliphatic chain might hinder the reaction due to its elevated steric hindrance (Table 1,entry 10).However,the reactivity of substrate 1k bearing bulky naphthyl was hardly influenced,and its reaction led to 2k in 84%yield (Table 1,entry 11).
Diaryl ketoximes,in regardless of their substituent electroproperties,were also favorable substrates,and their reactions occurred smoothly to produce the related ketones in moderate to good yields (Table 1,entries 12–18).Although the reactions were performed under oxidative conditions,it could still tolerate the reductive substituents such as aniline and phenol in substrates in certain degree (Table 1,entries 15 and 16).The W@PANI-catalyzed oxidative deoximation reaction could be successfully applied in the reactions of heterocycle-containing substrates,which might widely exist in pharmaceutical intermediates.In the reaction of(Z)-phenyl(thiophen-2-yl)methanone oxime (1s),the catalyst was not poisoned by the containing sulfur in substrate and it could afford the desired product 2s in 64% yield (Table 1,entry 19).The reaction of 0.5 mmol of (Z)-1-(furan-2-yl)ethan-1-one oxime (1t)led to 2t in 40% yield.Like the case of 1i,the reaction could be magnified to the scale using 200 mmol of 1t to produce 2t in 53%yield (Table 1,entry 20).Cyclic ketoximes such as 1u and 1v were also tested,and their reactions occurred smoothly to produce 2u and 2v in 81% and 77% yields respectively (Table 1,entries 21 and 22).The reaction of benzaldoxime 1w led to benzaldehyde(2w) in 42% yield,and the deeper oxidation and dehydration reaction by-products such as benzoic acid and benzonitrile were also obtained in 18% and 37% yields respectively (Table 1,entry 23).The catalyst has been successfully employed in our drug development project.For example,catalyzed by W@PANI,the oxidative deoximation of Cefixime derivative ethyl 2-(2-aminothiazol-4-yl)-2-(hydroxyimino)acetate (1x) could produce the related 2x in 71%yield (Table 1,entry 24).Notably,the tungsten residues in products were very low,making this protocol favorable for practical applications in pharmaceutical industry.
The mechanism of this W@PANI-catalyzed oxidative deoximation is our next concern and control experiments were conducted to get information for mechanism study (Table 2).The reaction could not occur without oxidant (Table 2,entry 1),and this result verified that it was an oxidative deoximation reaction other than the acid-promoted conversion.Without H2O2,heating 1a and the W@PANI in open air or O2could also produce 2a in 8% or 17% yield(Table 2,entries 2 and 3).Moreover,the product yield decreased when performing the reaction in N2atmosphere protection (Table 2,entries 5vs.4).Comparison of the above results indicated that the oxygen in air might also participate the reaction,in accordance with kinetic study results shown in Fig.S2 (Supporting information).Using Na2WO4as catalyst instead of W@PANI resulted in decreased product yield (Table 2,entries 6vs.4),showing that PANI support played significant roles in the catalytic system for dispersing the nano metal particles so that the catalytic sites could contact with the reactant sufficiently.Since the reaction could be obviously retarded by 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) or hydroquinone (HQ),the free radical scavengers,it was supposed that the processes might occurviaa free radical reaction route(Table 2,entries 7–10) [38].The reaction without any catalyst led to 2a in only 7% yield,showing that W@PANI catalyst was essential for the transformation (Table 2,entry 11).Moreover,it was found that althoughca.42% of W@PANI dissolved in MeCN,the leaked ratio of tungsten in solution during the process was very low(Table S2 in Supporting information),attesting that the reaction was catalyzed by PANI-supported tungsten,other than the leaked metal salt species.The strong coordination of nitrogen in PANI with the metal could well restrain the leaking during the reaction process.
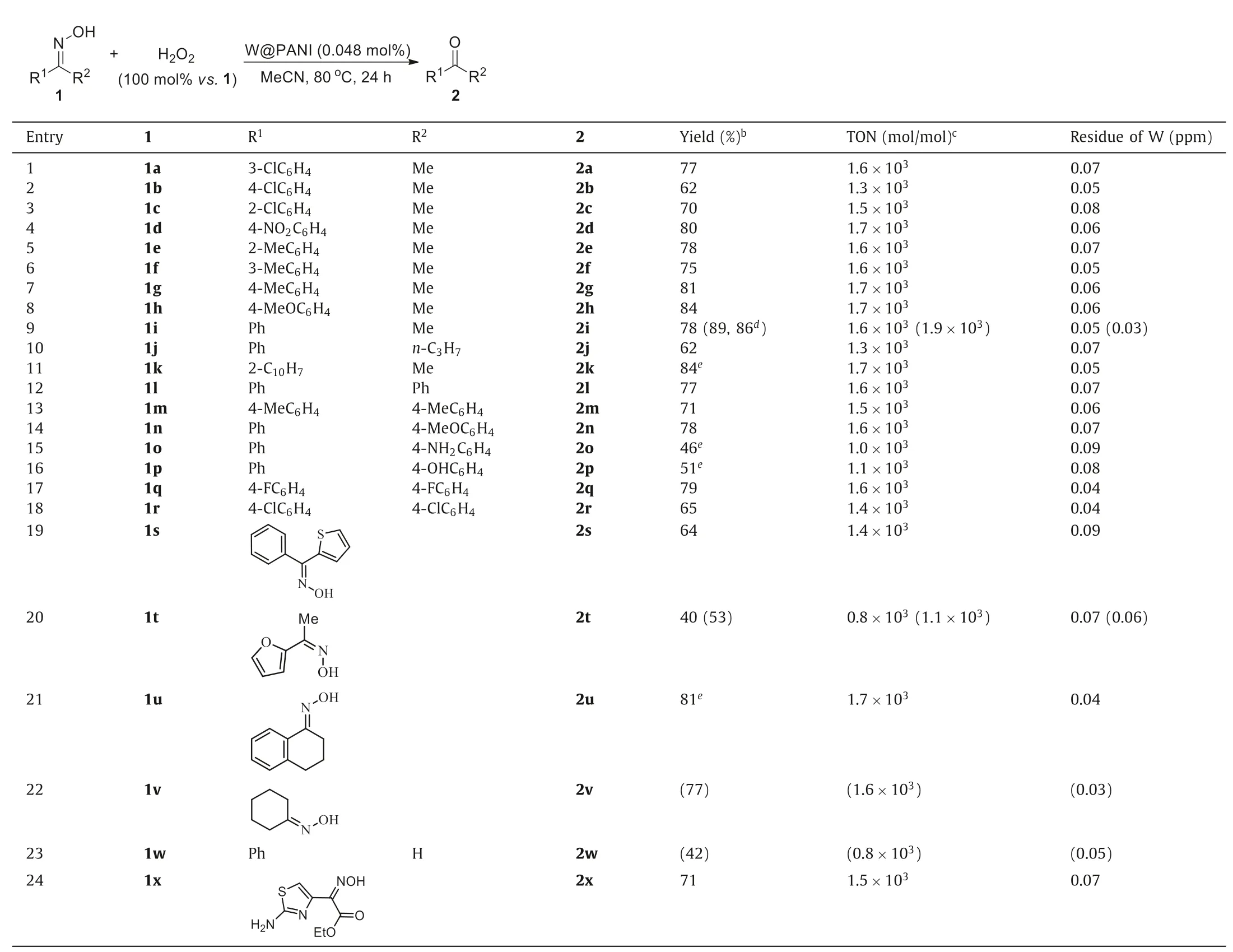
Table 1 Substrate extension for the W@PANI-catalyzed oxidative deoximation reactiona.
Thus,on the basis of the experimental results as well as reference reports,a plausible mechanism of the reaction could be supposed (Scheme 1).First,the thermo-promoted homogeneous cleavage of the peroxy bond in H2O2might lead to hydroxyl radicals[39],which reacted with the high valent tungstate species 3 in W@PANIviathe single electron transfer reaction to produce the active tungstate radicals 4 [40].Like the organotellurium-catalyzed oxidative deoximation reactions that also occurredviafree radical mechanism,the tungstate radicals 4 could react with oximes 1 to produce the intermediate 5,which was unstable and could soon decomposed,affording the tungsten species 6,products 2,and HNO [21,22].Oxidation of HNO led to nitrate,which was stable and could be detected by X-ray photoelectron spectroscopy(XPS) analysis [21].Oxidation of 6 could regenerate the catalytic species 4 and restart the catalysis circle [41].
In conclusion,W@PANI-catalyzed oxidative deoximation reaction could occur under mild and green conditions.It was of broadsubstrate scope involving the heterocycle-containing substrates,which might be useful pharmaceutical intermediates.In comparison with the reported catalytic oxidative deoximation reactions,the catalyst TON of the reaction was very high and this advantage could reduce the catalyst cost and metal residue in products.The work also demonstrates that the oxygen transfer features of metal nanoparticles anchored on PANI could be utilized in the green oxidation reactions with high efficiency [42].Continuous investigations are ongoing in our laboratory to apply the related technique in pharmaceutical intermediate production.
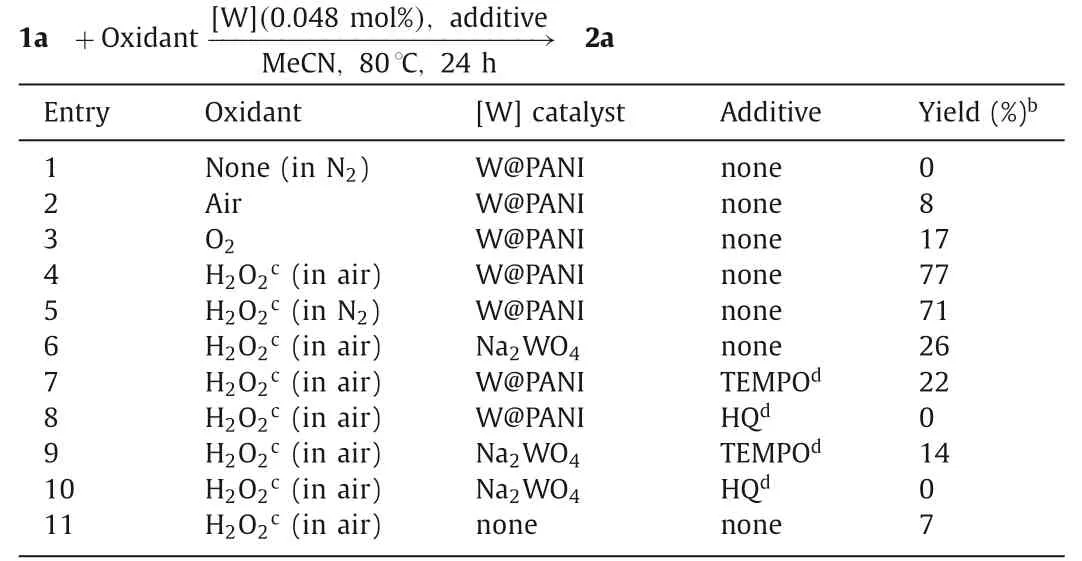
Table 2 Control experiments.a
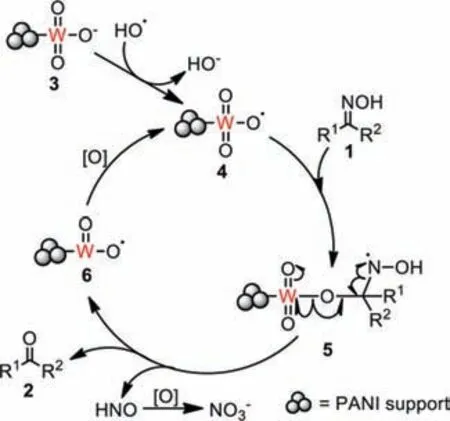
Scheme 1.Possible mechanisms of the reaction.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Jiangsu Provincial Six Talent Peaks Project (No.XCL-090),Natural Science Foundation of Jiangsu Province (No.BK20181449),and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) for support.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.05.019.
 Chinese Chemical Letters2023年1期
Chinese Chemical Letters2023年1期
- Chinese Chemical Letters的其它文章
- Diabetic wound healing activated by supramolecular cascade reaction
- MBenes: Two-dimensional transition-metal borides with ordered metal vacancies
- Wet-adhesive materials of oral and maxillofacial region: From design to application
- Diverse catalytic systems for nitrogen-heterocycle formation from O-acyl ketoximes
- Fluorine-containing drugs approved by the FDA in 2021
- The development and application of dual-comb spectroscopy in analytical chemistry
