Study on catalytic mechanisms of Fe3O4-rGOx in three typical advanced oxidation processes for tetracycline hydrochloride degradation
Heshn Zheng,Yunying Hou,Shuo Li,,**,Jun M,b,Jun Nn,Nnnn Wng
a College of Chemistry and Chemical Engineering,Qiqihar University,Qiqihar 161006,China
b Urban Water Resources Development and Northern National Engineering Research Center,Harbin 150090,China
c School of Environment,Harbin Institute of Technology,Harbin 150090,China
d Department of Environmental Engineering,Beijing Institute of Petrochemical Technology,Beijing 102617,China
Keywords:Advanced oxidation progresses Fe3O4 Graphene oxide Mechanism Reactive oxygen species
ABSTRACT This study explored the catalytic mechanism and performance impacted by the materials ratio of Fe3O4-GOx composites in three typical advanced oxidation processes (AOPs) of O3,peroxodisulfate (PDS) and photo-Fenton processes for tetracycline hydrochloride (TCH) degradation.The ratio of GO in the Fe3O4-GOx composites exhibited different trends of degradation capacity in each AOPs based on different mechanisms.Fe3O4-rGO20wt% exhibited the optimum catalytic performance which enhanced the ozone decomposition efficiency from 33.48% (ozone alone) to 51.83% with the major reactive oxygen species (ROS)of O2·-.In PDS and photo-Fenton processes,Fe3O4-rGO5wt% had the highest catalytic performance in PDS and H2O2 decomposition for SO4·–,and ·OH generation,respectively.Compared with using PDS alone,PDS decomposition rate and TCH degradation rate could be increased by 5.97 and 1.73 times under Fe3O4-rGO5wt% catalysis.In the photo-Fenton system,Fe3O4-rGO5wt% with the best catalyst performance in H2O2 decomposition,and TCH degradation rate increased by 2.02 times compared with blank group.Meantime,the catalytic mechanisms in those systems of that the ROS produced by conversion between Fe2+/Fe3+were also analyzed.
Advanced oxidation processes (AOPs) could produce reactive oxygen species (ROS) of hydroxyl radicals (·OH),superoxide radicals (O2·-),and sulfate radicals (SO4·–) that with strong oxidation capacity [1].·OH has great oxidation power to degrade recalcitrant organic contaminants,which has wide range of sources and can be produced by Fenton,Fenton-like,ozone,photocatalysis and sulfate radical-based AOPs (SR-AOPs) [2].O2·-is generated by ozone system,and SO4·–usually appears in persulfate (including peroxodisulfate (PDS) or peroxomonosulfate (PMS)) system [3].Various AOPs have been widely studied and applied in wastewater treatment for high effective degradation of toxic and biorefractory organic compounds [4].
To improve their efficiency and solve the drawbacks in practical application of low utilization rate of O3,high energy consumption for activation of SR-AOPs,and narrow pH limitation of Fenton,etc.,it is an urgent need to develop new catalysts.As the advantages of high abundance,non-toxicity,and good interface electron transfer ability of iron,iron-based materials have become commonly used heterogeneous catalyst in AOPs.Jinet al.synthesized Cu substituted magnetic Fe3O4@FeOOH to degrade ofloxacin in Fentonlike process [5].Fe3O4@hollow@mSiO2was used as catalyst to degrade sulfadiazine in ultrasound-assisted Fenton-persulfate system[6].Fe3O4@CuOxhollow ball activated PS can remove 95% of sulfadiazine [7].Composite materials such as Fe3O4nanoparticles are used in various advanced oxidations,which can quickly remove organic substances in wastewater.However,some catalysts have complex synthesis processes and large ion leaching concentrations,which are prone to metal agglomeration.Graphene oxide (GO) is highly oxidized structure of grapheme,comprises high density of oxygenated functional groups,which can effectively prevent the agglomeration and corrosion of metal oxides,making it commonly used as a support material in composite materials [8,9].Therefore,loading Fe3O4on GO to form a metal composite as a catalyst for AOPs can effectively degrade pollutants.rGO/Fe nanoparticles were used as adsorbents and Fenton-like catalysts to remove 93.6% of mitoxantrone from the wastewater [10].NGO/Fe3O4was used as a catalyst to activate potassium persulfate to degrade norfloxacin under the assistance of ultraviolet light [11].GO-Fe3O4nanocomposites was used as catalyst for degradation of ofloxacin by pulsed discharge plasma [12].
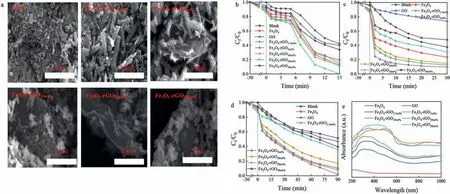
Fig.1.(a) SEM images of pure Fe3O4 and Fe3O4-rGOx samples.Catalytic degradation of TCH in (b) O3 system,(c) PDS system; (d) photo-Fenton progress.(e) UV-vis diffuse reflectance spectrum of pure Fe3O4 and Fe3O4-rGOx samples (Reaction conditions: [TCH] = 60 mg/L,[O3]=4.0 mg/min,[PDS]=0.1 g,the temperature of PDS system=60 °C,[H2O2]=0.2 mol/L,[UV]=200–400 nm,[catalysts]=0.5 g/L,pH 4.6).
Although it is reported that Fe3O4-GOxas a catalyst enhances the degradation of certain organic pollutants in AOPs,the mechanism of its catalytic degradation of pollutants is lack of in-depth analysis.Tetracycline hydrochloride (TCH) as a broad-spectrum antibiotic is widely used as an antibacterial agent and feed additive,thereby we chose it as the target pollutant.Therefore,we studied the catalytic mechanisms of Fe3O4-GOxsynergistically catalyzed oxidants decomposition and pollutant degradation in AOPs (including ozonation,persulfate catalysis and photo-Fenton progress) that based on different free radicals.In this paper,(i) the effect of GO loading ratio on the morphology of the composites was analyzed by characterization; (ii) the effects of different proportions of Fe3O4and GO on the degradation efficiency of TCH in three AOPs were systematically analyzed; (iii) the potential mechanism of TCH degradation of the composites were clarified by detecting the oxidant concentration in the solution,and utilizing X-ray photoelectron spectroscopy (XPS),quenching experiment and electron spin resonance (ESR).
According to the published paper,using solvothermal process synthesized Fe3O4and Fe3O4-rGOx(Text S1 in Supporting information) [12].The morphology of Fe3O4and Fe3O4-rGOxwere observed by SEM,which were shown in Fig.1a.Fe3O4exhibited irregular rob shape,after loading Fe3O4on the GO layer,the particle size of Fe3O4gradually decreased as the amount of GO increased.This may be that the GO surfaces contain negatively charged oxygen-containing groups which could be adsorbed on the broadside of Fe3O4crystals,and imposed a certain strain on them,thus limiting the growth of Fe3O4[13].Meantime,with the increase of GO content,the main crystal surface of Fe nanoparticles was affected,which made the larger crystal surface was replaced by the smaller crystal surface [12].Fig.S1a (Supporting information) was the X-ray diffraction (XRD) patterns of GO,Fe3O4and Fe3O4-rGOx.In the GO spectrum,an evident peak at 2θ= 11.2° was consistent with previous research [12,14].The peaks of Fe3O4and Fe3O4-rGOxlocated at 30.1°,35.5°,43.3°,53.6°,57.2° and 62.7° were accordance with the (220),(311),(400),(422),(511) and (440) crystal faces of Fe3O4[15].Moreover,the characteristic peaks of rGO were observed at 21.2° and 26° in Fe3O4-rGOxcomposites,indicating that GO had been reduced to rGO,which also demonstrated the successful synthesis of composites[16].Fig.S1b (Supporting information) displayed the FT-IR patterns of Fe3O4and Fe3O4-rGOx.The absorbed H2O molecule or surface hydroxyl were detected the range of 3000–3500 cm-1[17].The -CH2- was observed at 2800–2900 cm-1in Fe3O4-rGOx,and the peak shifted from 1633 to 1600 cm-1because of GO doping to form -COO- [3].Besides,all samples had an obvious absorption peak at 570 cm-1,which was the Fe-O-Fe stretching vibration [18].
Figs.1b–e depicted degradation rate of TCH with different ratio of Fe3O4to GOxin AOPs.All catalysts did not significantly adsorb TCH during 30 min,indicating that catalytic oxidation dominated the removal of TCH.The adsorption efficiency also increased with the increase of GO loading.However,when the GO loading increased to 30 wt%,the adsorption efficiency was only 3.6%.The reason was that GO folding each other lead to a reduction in the size and number of pores.The salient features and trends in TCH degradation in AOPs,as evident from the results were presented in Fig.1.In Fig.1b,the degradation rate was limited by the ozone dissolution in catalytic ozonation stages.After 5 min of treatment,the efficiency of O3to remove TCH alone was 9.31%,and it reached 57.93% after 15 min.After adding Fe3O4,the removal efficiency of TCH increased to 80.55%.Compared with Fe3O4,Fe3O4-rGOxenhanced the removal efficiency of TCH clearly.Because strong interaction between Fe3O4and GO could decompose O3to produce O2·-and H2O2,and then H2O2reacted with O3to produce·OH[19,20].Meantime,GO acted as a carrier to disperse the active sites on its surface and expanded the contact area between oxidants and contaminants.Fig.S2a (Supporting information) showed the transient photocurrent response of pure Fe3O4and Fe3O4-rGOx.Compared with Fe3O4,Fe3O4-rGOxcomposites exhibited a significantly enhanced photocurrent density,which indicated that the electron transfer was promoted between Fe3O4and rGO.Meantime,the higher photocurrent response of the sample was,the more effective separation of the photoelectron-hole pairs will be obtained,which evidenced that Fe3O4-rGO20wt%composite that with the most efficient separation were due to the strongest photocurrent response.Therefore,with the increased of GO content,the removal rate of TCH also increased.The degradation efficiency of TCH by the Fe3O4-rGO20wt%was 100% and the reaction rate constant (k2)was 0.017 g mg-1min-1and higher than that derived from other catalysts (thek2of other catalysts were 0.004,0.005,0.005,0.006 and 0.002 g mg-1min-1) (Text S2,Fig.S1c and Table S1 in Supporting information).
As illustrated in Fig.1c,the removal of TCH was observed about 52.17% in the blank group during 30 min reaction time,which may be attributed that PDS under the heating condition produced ROS to degrade TCH.Fe3O4and GO activating PDS systems had only 76.51% and 26.13% removal ratio of TCH,respectively.Under the support of GO,the catalytic performance of Fe3O4-rGOxwas remarkably improved.From Fig.S1d and Table S1 (Supporting information),TCH had a highest rate constant of 0.0052 g mg-1min-1(R2=0.954) in Fe3O4-rGO5wt%/PDS system,approximately 1.3 times than that of Fe3O4/PDS system.The raised degradation efficiency was obviously ascribed to the increase of available active sites for PDS decomposition.When GO loading was 2.5 wt%,10 wt%,20 wt%and 30 wt%,TCH removal rate andk2were both reduced.This was mainly attributable to the enhancement of free radicals scavenging effect of reactive sites on Fe3O4-rGOxsurface,meantime,acidic conditions will limite the produce of·OH [21].
In photo-Fenton system,as was displayed in Fig.1d,TCH degradation efficiency was less than 50% after 90 min without catalyst.However,the degradation rate of pure Fe3O4to TCH was 90.60%,which was close to that of Fe3O4-rGO5wt%to TCH (98.11%).Therefore,a pseudo-first-order model was used to compare the catalytic activity of the two catalysts.In Fig.S1e and Table S1 (Supporting information),the rate constant of Fe3O4-rGO5wt%was 1.5 times that of Fe3O4,so the catalytic activity of Fe3O4-rGO5wt%was better than Fe3O4.From the UV-vis diffuse reflectance spectrum of pure Fe3O4and GO,Fe3O4-rGOxcomposites,which can be seen that the optical properties of materials.Fig.1e showed that the Fe3O4-rGOxcomposites exhibited stronger absorption throughout visible light range than Fe3O4and GO.This may be attributed to the specific structure: GO sheets and Fe3O4particles allowed multiple reflections of light.According to the study of Moztahidaet al.[15],and the results of UV-vis diffuse reflectance spectrum and photocurrent (Fig.1e and Fig.S2a in Supporting information) also showed that the addition of rGO increased the light adsorption capacity of Fe3O4-rGOxcomposites and provided more electrons and holes(h+) for the production of ROS.However,by GO loading ratio from 5 wt% to 30 wt% increasing,its removal efficiency was diminished from 98.11% to 60.37%.Because the increase of GO loading affected the turbidity of solution,which lead the increase of the resistance of UV to penetrate it and shielded light adsorption [22],even affected charge separation [15].Hence,the Fe3O4-rGO5wt%had the optimum catalytic performance.
To investigate the potential for recycling of catalysts,the tetracycline oxidation testing was performed in 5 cycles in three AOPs,respectively.As could be seen from Fig.S2b (Supporting information),the removal efficiency of the TCH gradually decreased with the increase of the number of times of use.The removal rate of about 80% indicated that materials were stable and had a certain reusability.Compared with the 1strun,TCH degradation rate decreased slightly in the 5th,which might be due to the surface oxidation of the catalyst and leaching of metal ions during the catalytic process [20].The leaching rate of metal ions was shown in Table S2 (Supporting information).And the leaching amount of total iron and Fe2+was less than 0.1% with respect to the catalyst dose of 500 mg/L.However,the temperature condition of PDS system was 60 °C (O3and photo-Fenton systems were carried out at room temperature),which lead the increase of ion leaching rate of catalysts.In addition,the leached Fe concentration at the end of each round almost kept the same level,which satisfied the water environment discharge standards (2 mg/L) imposed by the European Union [23].
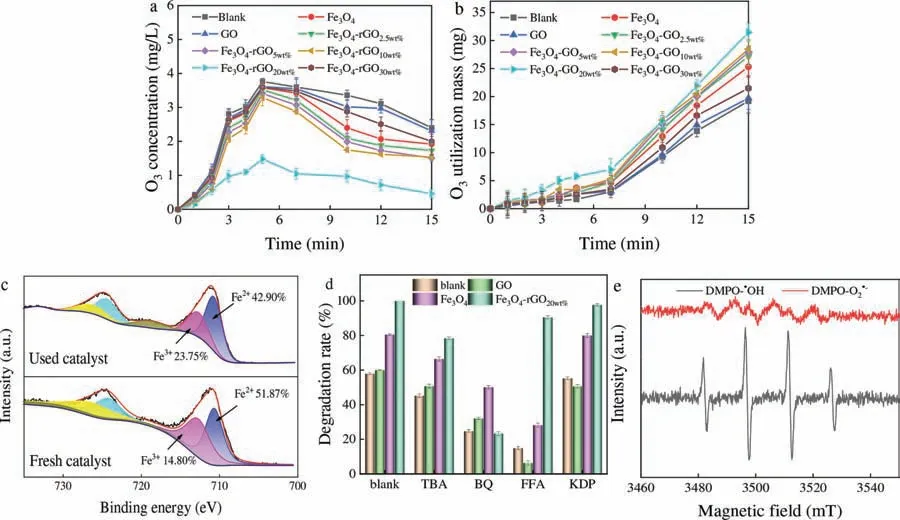
Fig.2.(a) Ozone concentration and (b) ozone utilization mass in solution.(c) XPS spectra for Fe 2p regions of fresh and used Fe3O4-rGO20wt% in O3 system.(d) Effects degradation efficiency of TCH with the presence of quenchers.(e) ESR spectra detected in Fe3O4-rGO20wt%/O3 systems for DMPO-·OH and DMPO-O2·- (Reaction conditions:[TCH]=60 mg/L,[O3]=4.0 mg/min,[catalysts]=0.5 g/L,pH 4.6).
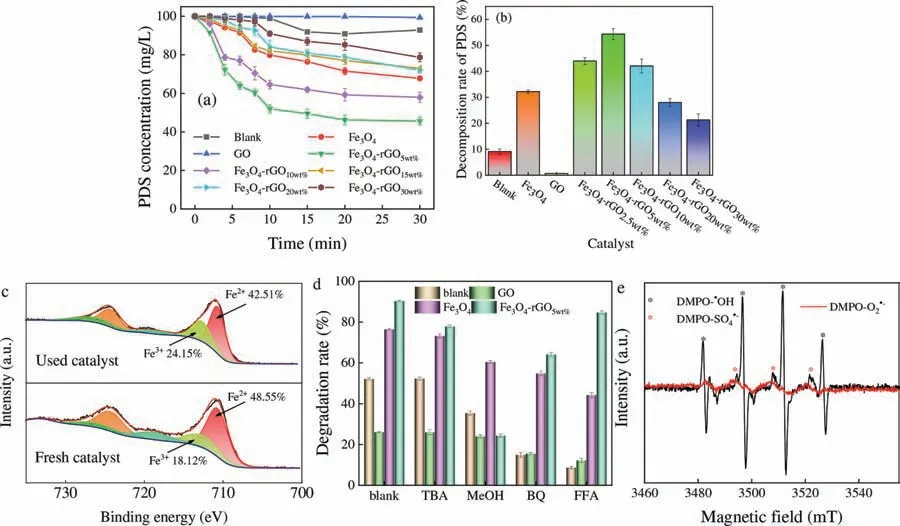
Fig.3.(a) PDS concentration in solution and (b) PDS decomposition rate.(c) XPS spectra for Fe 2p regions of fresh and used Fe3O4-rGO5wt% in PDS system.(d) Effects degradation efficiency of TCH with the presence of quenchers.(e) ESR spectra detected in Fe3O4-rGO5wt%/PDS system for DMPO-·OH,DMPO-SO4·– and DMPO-O2·- (Reaction conditions: [TCH]=60 mg/L,[PDS]=0.1 g,the temperature of PDS system=60 °C,[catalysts]=0.5 g/L,pH 4.6).
The proportion of GO in Fe3O4-rGOxaffected TCH degradation in the three AOPs.Therefore,we conducted a series of experiments to explore the effect of GO loading ratio on TCH degradation and the mechanism of catalysts in AOPs.Fig.1a showed that GOxaffected the morphology of catalyst,and further effected TCH degradation (Fig.1b).To verify that catalysts enhanced O3decomposition,this experiment measured the ozone concentration in liquid phase of each system,and the test results were shown in Fig.2a.The ozone concentration in the solution gradually rose within 5 min,because ozone undergo a mass transfer process from the gas phase to the liquid phase [24,25],and TCH degradation hardly changed within 5 min.The concentration of liquid phase ozone reached 3.76 mg in 5 min in blank group,and then remained stable; after adding Fe3O4,the ozone concentration reached 3.58 mg/L in 5 min,and Fe3O4-rGO20wt%/O3system reached 1.48 mg/L in 5 min (other catalysts system reached about 3.5 mg/L),and the solution ozone concentration finally reached 2.4,1.33,2.31,1.73,1.71,1.54,0.46 and 2.09 mg/L,respectively.It indicated that the catalyst can accelerate ozone decomposition,reduce solution ozone concentration,and thereby improve the utilization mass of ozone.Eq.S3 (Text S3 in Supporting information) was used to calculate the ozone utilization mass to better show the mechanism of Fe3O4-GOx/O3system [26].
The ozone utilization mass was shown in Fig.2b.According to Eq.S3,it under those systems was 19.19,25.31,19.70,27.2,27.92,28.46,31.45 and 21.47 mg,respectively.Compared with control groups,Fe3O4-rGOxenhanced TCH degradation and O3utilization mass clearly.The reason was that strong interaction between Fe3O4and GOxcould decompose O3to produce O2·-and H2O2,and then H2O2reacted with O3to produce·OH (Text S4 and Eqs.S4-S13 in Supporting information) [19,20].Fig.2c was XPS spectra of Fe 2p before and after Fe3O4-rGO20wt%,which corroborated the Fe2+/Fe3+species participated in the electron-transfer processes and contributed to the utilization of ozone (Fe2+changed from 51.87% to 42.90%).The fitting peaks at the binding energies of 710.7 and 724.3 eV belong to Fe2+,while the fitting peaks at the binding energies of 712.7 and 726.3 eV were Fe3+[27,28].To explore ROS in O3system,tert–butanol (TBA),benzoquinone (BQ),furfuryl alcohol(FFA) and phosphate (KDP) were added in solution to quench·OH,O2·-,1O2and surface of·OH,respectively Fig.2d displayed the major ROS in ozone system.After BQ injected,all the O2·-generated in Fe3O4-rGO20wt%/O3system was nearly quenched and resulting in a decrease in TCH removal from 100% (without quencher) to 23.35%.The addition of TBA,FFA and KDP reduced the TCH removal rate to 78.46%.,90.52%.and 97.31%.Therefore,it was deduced that O2·-was the main reactive oxygen species generated in the Fe3O4-rGO20wt%/O3system,·OH and1O2had no significant effects in the catalytic ozone system.However,1O2and O2·-were the major ROS in GO and Fe3O4system.ESR measurements were carried out to further accurately elucidate the existence of ROS in Fe3O4-rGO20wt%/O3system (Fig.2e).The O2·-and·OH signals can be observed in the Fe3O4-rGO20wt%/O3process in Fig.2e indicated that Fe3O4-rGO20wt%can decompose ozone to generate O2·-and·OH.
As shown in Fig.1c,PDS as the main oxidant participating of Fe3O4-rGOx/PDS process played an important role.In order to explore catalytic mechanism in system,PDS concentration and decomposition rate were measured and concluded in solution.It can be seen in Figs.3a and b,PDS decomposition increased with the decreased concentration of that in solution.Comparing with other catalysts,Fe3O4-rGO5wt%had the lowest PDS concentration (45.63 mg/L) and the highest PDS decomposition rate(54.37%).Therefore,Fe3O4-rGO5wt%had the highest TCH degradation in PDS system.As exhibited in Fig.3c,compared with fresh Fe3O4-rGO5wt%,it was found that the peak area of Fe2+decreases and Fe3+increased after reaction,which indicated that the transition between Fe2+and Fe3+during the reaction activated the decomposition of PDS to produce ROS.Therefore,to further explore the mechanism for Fe3O4-rGO5wt%,we analyzed the type of ROS in this system.It was reported that SO4·–and·OH could be produced by the metal-based catalysts activated PDS [29,30].As given in Fig.3d,the TCH degradation efficiency decreased from 90.4% to 24.38% and 57.3% with addition of methanol (MeOH) and TBA,respectively.And adding BQ,the removal rate of TCH decreased to 64.16%.This result clearly indicated that the degradation process was a pure radical-based oxidation process.Moreover,both SO4·–and·OH occurred in the Fe3O4-rGO5wt%/PDS system and the latter one played a much more important role.Similarly,Chenet al.[30] claimed that the sulfate radical was the dominating radical species responsible for the degradation of norfloxacin by CoFe2O4-GO/PMS system.However,Fig.3d results revealed that1O2,O2·-and SO4·–were generated in PDS,GO/PDS and Fe3O4/PDS system,in which1O2and O2·-were the dominant ROS [15].Therefore,PDS itself will produce a small amount of ROS to degrade TCH at 60 °C.Meantime,in a review about the treatment of wastewater by the sulfate-based AOPs,Fanget al.[31] have analyzed that SO4·–dominate the acidic pH conditions.According to study of Wanget al.[32] and Jinget al.[3],a possible mechanism for PDS activation by Fe3O4-rGO5wt%was proposed (Eqs.S14-S26 shown in Supporting information Text S5).ESR signals of·OH,SO4·–and O2·-were observed in the system (Fig.3e),the results confirmed the previous inference that SO4·–,O2·-and·OH existed in Fe3O4-rGO5wt%/PDS system.The increase of GO loading ratio expanded the reaction site and produced a large amount of ROS.However,excessive SO4·–will quench each other.Therefore,Fe3O4-rGO5wt%had the best catalytic performance compared with others.
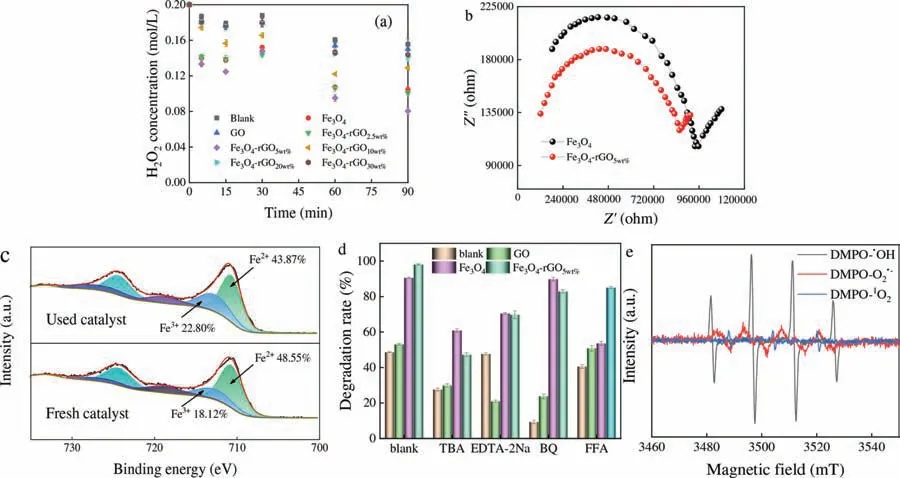
Fig.4.(a) H2O2 concentration in photo-Fenton system.(b) EIS Nyquist plots of Fe3O4 and Fe3O4-rGO5wt%.(c) XPS spectra for Fe 2p regions of fresh and used Fe3O4-rGO5wt% in PDS system.(d) Effects degradation efficiency of TCH with the presence of quenchers.(e) ESR spectra detected in Fe3O4-rGO5wt%/UV/H2O2 system for DMPO-·OH,DMPO-O2·-and DMPO-1O2 (Reaction conditions: [TCH]=60 mg/L,[H2O2]=0.2 mol/L,[UV]=200–400 nm,[catalysts]=0.5 g/L,pH 4.6).
About the difference of TCH degradation in photo-Fenton progress,it was speculated that increase of GO loading lead the turbidity of solution increased,which made high resistance for light to penetrate solution,decreased H2O2decomposition and further affected TCH degradation.To verify this hypothesis,the influences of those catalysts were tested on the concentration of H2O2.In Fig.4a,the H2O2concentration of blank,Fe3O4,GO and Fe3O4-rGOxwere 155.62,104.53,150.24,100.77,80.42,129.41,141.07 and 143.85 mg/L,which suggested that GO loading ratio affected H2O2decomposition and TCH degradation.Compared with other systems,Fe3O4-rGO5wt%/UV-H2O2system had the lowest H2O2concentration.Moreover,using Fig.4b analyzed the interfacial charge transfer capabilities of Fe3O4and Fe3O4-rGO5wt%.The arc radius of the Nyquist curve for Fe3O4-rGO5wt%was significantly smaller than that of Fe3O4,which implied that the charge of the Fe3O4-rGO5wt%at the interface migration resistance decreased and the e-mobility of the composite was enhanced.As shown in Fig.4c,supported by the fitting parameters,that of Fe2+and Fe3+for the pristine Fe3O4-rGO5wt%were 48.55% and 18.12%,respectively.After the degradation reactions,the peak area of Fe2+slumped to 43.87%,while the peak area ratio of Fe3+went up to 22.80%,which suggested that Fe2+/Fe3+were involved in electron transfer processes.And Fig.4d illustrated·OH and h+were the main ROS in Fe3O4-rGO5wt%/UV/H2O2system (ethylenediamine tetraacetic acid disodium (EDTA-2Na) quenched h+),O2·-,·OH and h+existed in GO and Fe3O4system,and the major ROS of blank group were O2·-and·OH (corresponding degradation mechanism was shown in Eqs.S27-S34 and Text S6 in Supporting information) [15,33].As shown in Fig.4e,characteristic ESR signals of·OH,O2·-and1O2can be observed in the catalytic system of Fe3O4-rGO5wt%/UV/H2O2,thus·OH,O2·-and1O2were existed in this system.Meantime,Huanget al.[35] observed the similar conclusion in the removal of sulfamethoxazole in the Fe3S4derived from MIL-100(Fe)/Vis/H2O2system.
In order to understand the source of O2·-in three AOPs,nitrogen bubble was blown into the solution to further verify and revealed degradation mechanism.In Fig.S3 (Supporting information),the degradation rate of TCH in PDS system was 72.96%.Compared with no N2,that of TCH decreased by 17.44%.However,in Fig.3d,the inhibition rate of TCH after adding BQ was 26.24%,which indicated that the formation of O2·-in PDS system was attributed in dissolved oxygen in solution and the decomposition of water molecules.However,none of the other systems were affected,which indicated that O2·-all came from the reaction between catalyst and substances in systems.And among these three AOPs,O3system completely removed TCH within 15 min,which is far superior to the PDS and photo-Fenton systems.Therefore,O3system is an effective technology to treat TCH wastewater.
In conclusion,the content of GO in Fe3O4-rGOxcomposites greatly affected the performance and mechanism of TCH degradation in three typical AOPs.In O3system,Fe3O4-rGO20wt%exhibited the best catalytic efficiency which could increase the degradation rate of TCH from 57.93% (only O3) to 100%.The results showed that the strong force between Fe3O4and rGO also improved the decomposition rate of O3by Fe3O4-rGO20wt%,and the conversion between Fe2+and Fe3+decomposed oxidant and produced O2·-and·OH to degrade TCH that detected by ESR spectra.For PDS oxidation system,Fe3O4-rGO5wt%showed higher catalytic activity compared with others By detecting the concentration of PDS in the solution,quenching experiment and ESR analysis found that Fe3O4-rGO5wt%can effectively decompose PDS and produce SO4·–and O2·-,and PDS decomposition rate and TCH degradation rate were 5.97 and 1.73 times higher than those of the blank group.In the photo-Fenton system,the rate of H2O2decomposition by Fe3O4-rGO5wt%was higher than that of others.Meantime,the addition of rGO enhanced the corresponding light region of the composite and separation of photogenerated electron-holes.However,increased GO ratio also enhanced the turbidity of the solution and shielded UV.Therefore,though Fe3O4-rGO10wt%-Fe3O4-rGO30wt%with higher photocurrent density,Fe3O4-rGO5wt%exhibited a higher rate of TCH degradation in UV-H2O2system.In this system,multiple reactive species including·OH,O2·-and1O2were detected that responsible for the TCH degradation.
Declaration of competing interest
All authors declared that they do not have any commercial and associative interests that represent a conflict of interest in connection with the other work submitted.
Acknowledgments
The work was supported by the National Natural Science Foundation of China (Nos.21906088,51902169,52170039),the National Science Foundation for Post-doctoral Scientists of China(No.2021T140165),the Natural Science Foundation of Heilongjiang Province,China (No.LH2020B023),Department of Education Heilongjiang Province (No.135309338),University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province(Nos.UNPYSCT-2020068,UNPYSCT-2020067),the authors also gratefully acknowledge the financial support by the Heilongjiang Provincial Key Laboratory of Surface Active Agent and Auxiliary(No.BMHXJKF009).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.02.058.
 Chinese Chemical Letters2023年1期
Chinese Chemical Letters2023年1期
- Chinese Chemical Letters的其它文章
- Diabetic wound healing activated by supramolecular cascade reaction
- MBenes: Two-dimensional transition-metal borides with ordered metal vacancies
- Wet-adhesive materials of oral and maxillofacial region: From design to application
- Diverse catalytic systems for nitrogen-heterocycle formation from O-acyl ketoximes
- Fluorine-containing drugs approved by the FDA in 2021
- The development and application of dual-comb spectroscopy in analytical chemistry
