Construction of oxygen defective ZnO/ZnFe2O4 yolk-shell composite with photothermal effect for tetracycline degradation: Performance and mechanism insight
Ke Zhang,Hongyang Cao,Afzal Dar,Danqing Li,Lina Zhou,Chuanyi Wang
School of Environmental Science and Engineering,Shaanxi University of Science and Technology,Xi’an 710021,China
Keywords:Photocatalysis Photothermal Oxygen vacancy Yolk-shell Tetracycline
ABSTRACT Oxygen vacancy induced photothermal effect is of great significant but lack of adequate attentions for environmental remediation.In this paper,green recyclable ZnO/ZnFe2O4 with oxygen vacancy was prepared by a solvothermal-calcination method.The UV-vis light capture ability of ZnO/ZnFe2O4 microspheres is improved with the multiple light reflections due to the yolk-shell structure,and the oxygen vacancy expands the absorption range of photocatalyst and enhances photothermal conversion.The optimized photocatalyst can heat the solution from room temperature to 70 °C within 60 min of visible light illumination,and the light to heat conversion efficiency is achieved by 61.3%.Compared with the degradation efficiency at 20 °C,photothermal catalysis achieves a stable degradation in 80 min,and the degradation efficiency is increased by 41.5%.This can be attributed to the fact that light induced thermal energy accelerates the migration of electrons and holes,and promotes the diffusion of free radicals by heating active centers in situ.The active species contributing to the degradation,in order of importance,are the superoxide radical,hydroxyl radical,hole and electron.The light-to-thermal assisted photocatalysis with ZnO/ZnFe2O4 provides a new sight for the pollution control in the future practical applications.
As an effective antibiotic,tetracycline (TC) has been widely used in recent decades,which can interfere with cells and hinder their normal function [1,2].Due to its stability in aqueous solution,TC is difficult to be eliminated by conventional treatment or biodegradation process,thus giving rise to significant potential hazards to human health and ecological balance.In recent years,many technologies,such as photocatalysis,adsorption and Fenton oxidation,have been used to treat TC pollution [3–8].However,the adsorption method cannot degrade the TC,and the adsorbent often requires expensive and toxic solvents for regeneration,the regenerated liquid waste also requires additional treatment and disposal.Fenton technique requires oxidation reagent,and the deposition of large amounts of ferric ion sludge increase cost dramatically [9].UV radiations and ultrasonic treatment have limited penetration power in the aqueous solution,and they are harmful to eyes and skin[10].Among many technologies,photocatalysis has attracted much attention to purify industrial and culture wastewater because of its low cost and high efficiency [11,12].Metal oxides such as ZnO and ZnFe2O4have been widely used in photocatalysis due to their fast hole transport,underwater stability and high light absorption coefficient [13–16].Therefore,we constructed ZnO/ZnFe2O4nanocomposite with yolk-shell structure,which can not only provide large surface area,but also enhance the light absorbance as much as possibleviamultiple reflections inner the microsphere [17,18].
Surface reactive oxygen species (ROS) exhibit strong activity in the photodegradation of TC [19,20].However,due to the high recombination of charge carrier,the existence of charge and energy transfer process,and the insufficient of dissolved oxygen in water after reaction,the ROS production is usually inefficient.If additional energy is supplemented,the reaction process will be continued and accelerate.Controlling the activation of oxygen is of great significance to achieve efficient and stable ROS generation.Direct conversion of solar energy into heat to drive oxygen activation is a new and effective way to utilize solar energy.However,most semiconductors only absorb ultraviolet light or part of visible light,how to effectively extend the light absorption range to convert long wavelength light into heat energy and improve the photocatalytic performance of the system is a challenging task.
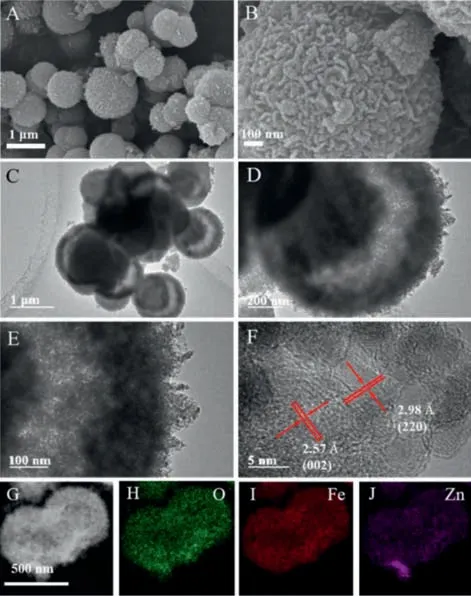
Fig.1.(A,B) SEM images of ZOv/ZFOv 500.(C–E) TEM images of ZOv/ZFOv 500.(F)HRTEM image of an individual microsphere and (G–J) the corresponding elemental mapping images.
Construction of oxygen vacancies is an effective way to enhance light adsorption and improve the separation efficiency of photogenerated carriers [21–24].Currently,the wide usage of oxygen vacancy in photocatalysis is about the photoelectrons capture and light absorption extension.In fact,the oxygen vacancy induced photothermal effect may also be an important factor influencing photocatalytic process.In photocatalytic process,semiconductors with oxygen vacancies continuously generate heat energy,which affects the transport of photogenerated carriers and significantly improves the photocatalytic performance [25–27].Additionally,evaporation of water by the photothermal effect can concentrate pollutant and achieve high removal efficiency [28].But rare attention was devoted to the photothermal effect of oxygen vacancy during photodegradation process.
In this paper,ZnO/ZnFe2O4with oxygen vacancy (ZOv/ZFOv)was synthesized by a solvothermal-calcination method.Detailed characterizations confirmed the yolk-shell ZOv/ZFOvnanoparticles possess superior multi-reflection absorption capability.By optimizing the oxygen vacancy concentration to tune the optical absorption,ZOv/ZFOvfeatures outstanding TC degradation performance through photocatalysis coupled with photothermal effect.The influence of photothermal effect,possible mechanism and pathway of TC degradation on ZOv/ZFOvwere fully explored.
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images of the as-prepared ZOv/ZFOv500 were recorded to characterize its morphologies.As shown in Figs.1A and B,the sample exhibits sphere-like morphology with diameter of about 1 μm,consisting of small nanoparticles.Figs.1C–E show that ZOv/ZFOv500 possesses a special yolk shell structure,and the average shell thickness as estimated from the histograms is 0.191±0.020 μm (Fig.S3 in Supporting information).This special structure makes the microspheres inside have enough light scattering and multiple reflections to improve the light absorption and photothermal conversion.Fig.1F displays the typical high resolution TEM image,and the spacing of lattice fringes isca.0.298 nm,corresponding to the (220) crystal plane of ZnFe2O4.Elemental mappings (Figs.1G–J) exhibit the distribution of Zn,Fe,and O elements in ZOv/ZFOv500,demonstrating the nanocomposite with yolk-shell structure has been successfully prepared.
X-ray photoelectron spectroscopy (XPS) spectra was performed on ZOv/ZFOv500 to explore the surface chemical composition and chemical states.Survey spectrum in Fig.S6 (Supporting information) illustrates the prepared material contains O,Fe and Zn elements,further confirming the formation of ZOv/ZFOv500.The C 1s XPS spectrum could be deconvoluted into three peaks centered at 282.6,284.0 and 286.4 eV,corresponding to C-C,C-O and C=O,respectively (Fig.S7A in Supporting information).In Fig.S7B (Supporting information),there are two binding energy peaks centered at 735.0 and 708.5 eV,corresponding to Fe 2p1/2and Fe 2p3/2,respectively.XPS spectrum of Fe 2p3/2could be fitted into 708.2 and 710.2 eV,belonging to tetrahedral and octahedral Fe3+.Besides,the peak at 724 eV is consistent with the Fe 2p1/2photoelectron response of Fe3+.As shown in Fig.S7C (Supporting information),the high-resolution XPS spectra of Zn exhibits the binding energies of 1019.3 eV (Zn 2p3/2) and 1042.4 eV (Zn 2p1/2),indicating the presence of Zn2+in ZOv/ZFOv.According to the O 1s XPS spectrum (Fig.S7D in Supporting information),the three fitted binding energy peaks located at 531.0,527.7 and 529.6 eV correspond to the adsorbed water,surface lattice oxygen of ZFOvand ZOv,respectively[29].
Photothermal catalytic TC degradation was performed by using the as-prepared catalysts under visible light and near infrared (NIR) light.As shown in Fig.2A,under visible light irradiation,ZOv/ZFOvexhibited superior degradation efficiency,and the best degradation performance occurred in ZOv/ZFOv500.However,excessive defects will become new electron-hole recombination centers thus reducing the photocatalytic performance.As a result,ZOv/ZFOv600 exhibits worse activity than that of ZOv/ZFOv500.Fig.2B shows the degradation rate constant of all the samples.The photodegradation rate constant of TC by ZOv/ZFOv500(0.01650 min-1) is 1.5 times and 14.1 times higher than that of ZOv(0.01118 min-1) and ZFOv(0.001170 min-1),indicating the superior performance of the nanocomposites.Temperature can increase the production rate of ROS and affect the catalytic activity.For comparison,as shown in Fig.S9 (Supporting information),TC cannot be degraded by visible light irradiation alone,and temperature has negligible impact on TC degradation.The performance of some photocatalysts for pharmaceutical personal care products’degradation is listed in Table S2 (Supporting information) [30–37].To explore the temperature changes of the solution under light irradiation,temperature monitoring was carried out in TC degradation process with all the as-prepared samples.As shown in Fig.2C,compared with ZO/ZFO,ZOv/ZFOvexhibits better photo-thermal conversion capacity,and the best sample reaches equilibrium within 60 min,the light-to-heat conversion efficiency achieved 61.3%,suggesting that oxygen vacancy can more efficiently convert light energy into heat energy [38].As shown in Figs.2D–F,the catalysts under NIR irradiation exhibits a considerably worse photocatalytic performance andΔTthan the visible light irradiation,indicating the photocatalysis and photothermal conversion mainly rely on visible light.
The photothermal effect plays an important role in enhancing the photocatalytic degradation performance,and the diffuse reflection spectra (DRS) results indicate that ZOv/ZFOv500 has excellent absorption capability from visible light to NIR.Therefore,we explored the temperature changes of ZO/ZFO 500 and ZOv/ZFOv500 in visible and NIR by infrared thermal imager.As shown in Fig.S10A (Supporting information),at the initial time,the temperature of ZO/ZFO 500 is consistent with the room temperature.After turning on the lamp,the infrared image color of ZO/ZFO 500 became bright with the increase of time.After 16 min of illumination,the temperature rose to 73 °C,and then remained basically unchanged.Under the same irradiation condition,it can be seen from Fig.S10B (Supporting information) that the temperature of the ZOv/ZFOv500 nanocomposite increased to 72.1 °C within two minutes,and then continued to rise to 90.1 °C.As shown in Fig.S11 (Supporting information),ZO/ZFO 500 heats up slowly under NIR and the temperature is not high.The heating rate of ZOv/ZFOv500 under NIR is slightly faster than that of ZO/ZFO 500,and the result is consistent with those measured in solution.These experiments demonstrate that oxygen vacancy induced photothermal effect is mainly dependent on visible light rather than NIR.
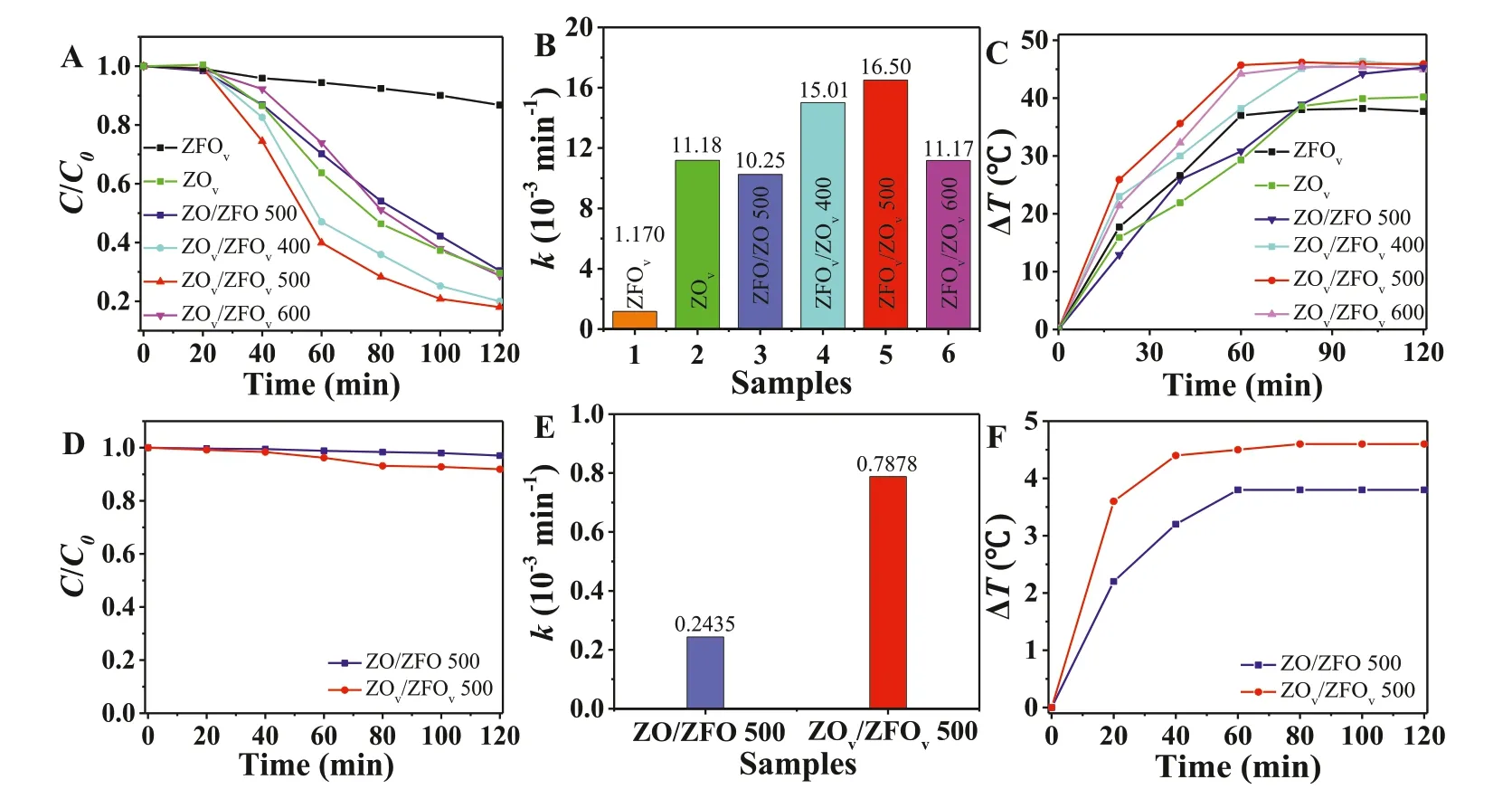
Fig.2.Degradation performance (A),photodegradation rate constants (B) and ΔT (C) of ZOv/ZFOv nanocomposite under visible light irradiation (λ >420 nm,0.29 W/cm2)in water bath.Degradation performance (D),photodegradation rate constants (E) and ΔT (F) of ZOv/ZFOv nanocomposite under NIR irradiation (λ >765 nm,0.07 W/cm2).
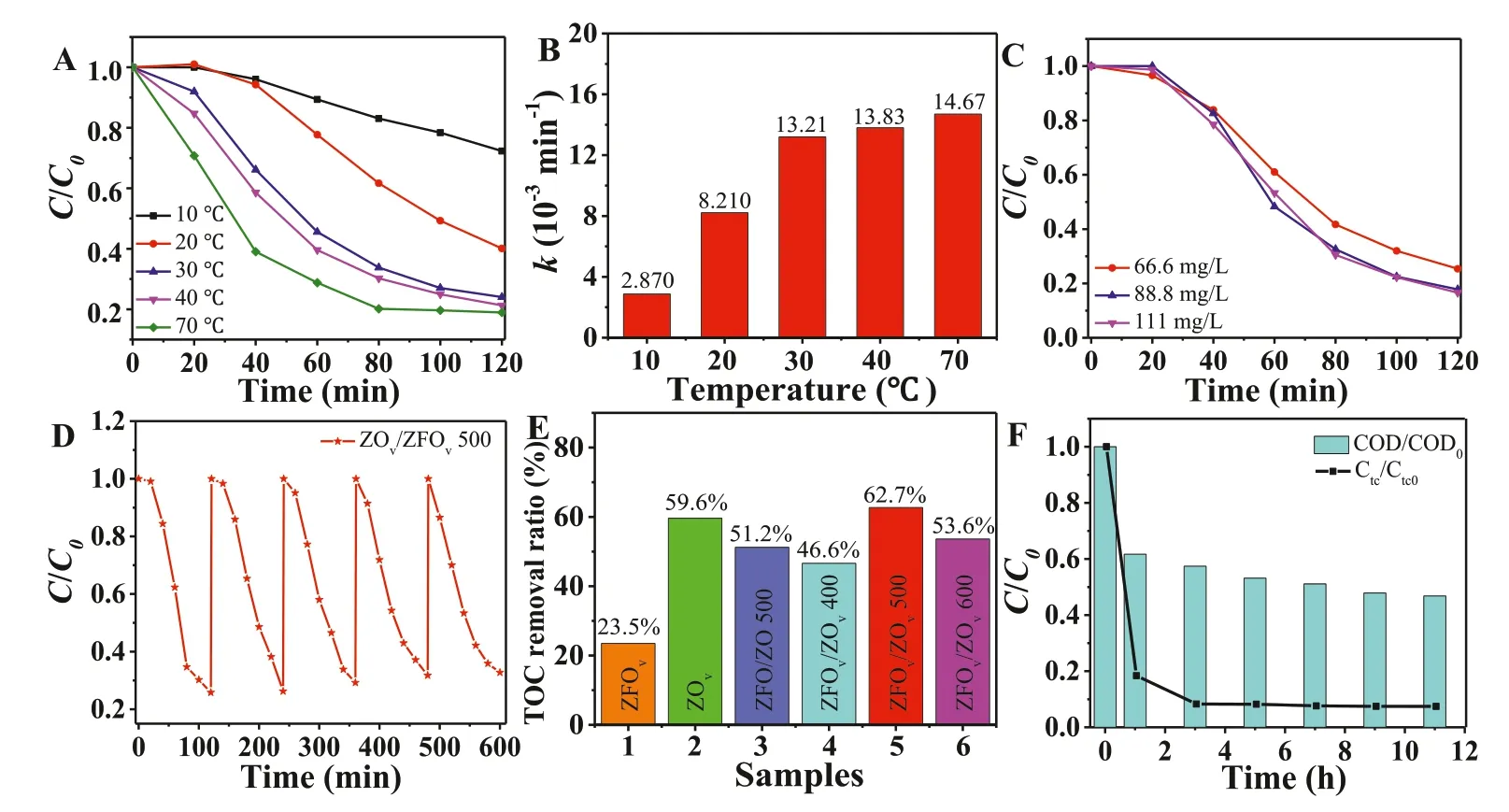
Fig.3.Photodegradation performance (A) and degradation rate constants (B) of ZOv/ZFOv 500 at different temperature.(C) Impact of TC concentration.(D) The cycling experiment for the degradation of TC by ZOv/ZFOv 500.(E) TOC removal by all the as-prepared samples.(F) TC degradation and COD removal in real wastewater.
As shown in Fig.3A,temperature changes have significant impact on TC degradation.The TC degradation efficiency of ZOv/ZFOv500 at 10 °C,20 °C,30 °C,40 °C and 70 °C are 27.7%,59.9%,76.0%,78.7% and 81.5%,respectively,demonstrating that the higher temperature,the higher degradation efficiency.Combined with Fig.2C,it can be analyzed that oxygen vacancy can improve the photothermal conversion efficiency,which is conducive to the photocatalytic degradation of TC.Fig.3B shows the degradation rate constants of ZOv/ZFOv500 at different temperature,The rate of TC degradation reactions follows the Arrhenius law (Fig.S12 in Supporting information).As shown in Fig.3C,photodegradation effi-ciency increases with the increase of TC concentration,and the degradation efficiency exhibits little change when the concentration reaches 111 mg/L.This result indicates that the catalyst also has a good removal effect on high concentration TC.As shown in Fig.3D,the photocatalytic activity of ZOv/ZFOv500 remains 86.2% after 5 cycles,and XRD patterns demonstrate that the catalyst structure did not change after the test (Fig.S13 in Supporting information),suggesting ZOv/ZFOv500 has good stability.To further explore the mineralization ability of all samples towards TC,the total organic carbon (TOC) concentration was evaluated after photodegradation experiments.Fig.3E shows that the TOC removal efficiency of ZOv/ZFOv500 is 62.7%.In order to explore the degradation performance of ZOv/ZFOv500 in the real waste water environment,the water sample from sewage treatment plant of Shaanxi University of Science and Technology was selected to carry out photocatalytic experiments.As shown in Fig.3F,after 11 h of visible light irradiation,the TC and chemical oxygen demand (COD)removal reachesca.92.6% and 53.2%,indicating that ZOv/ZFOv500 possesses great potential in practical application.
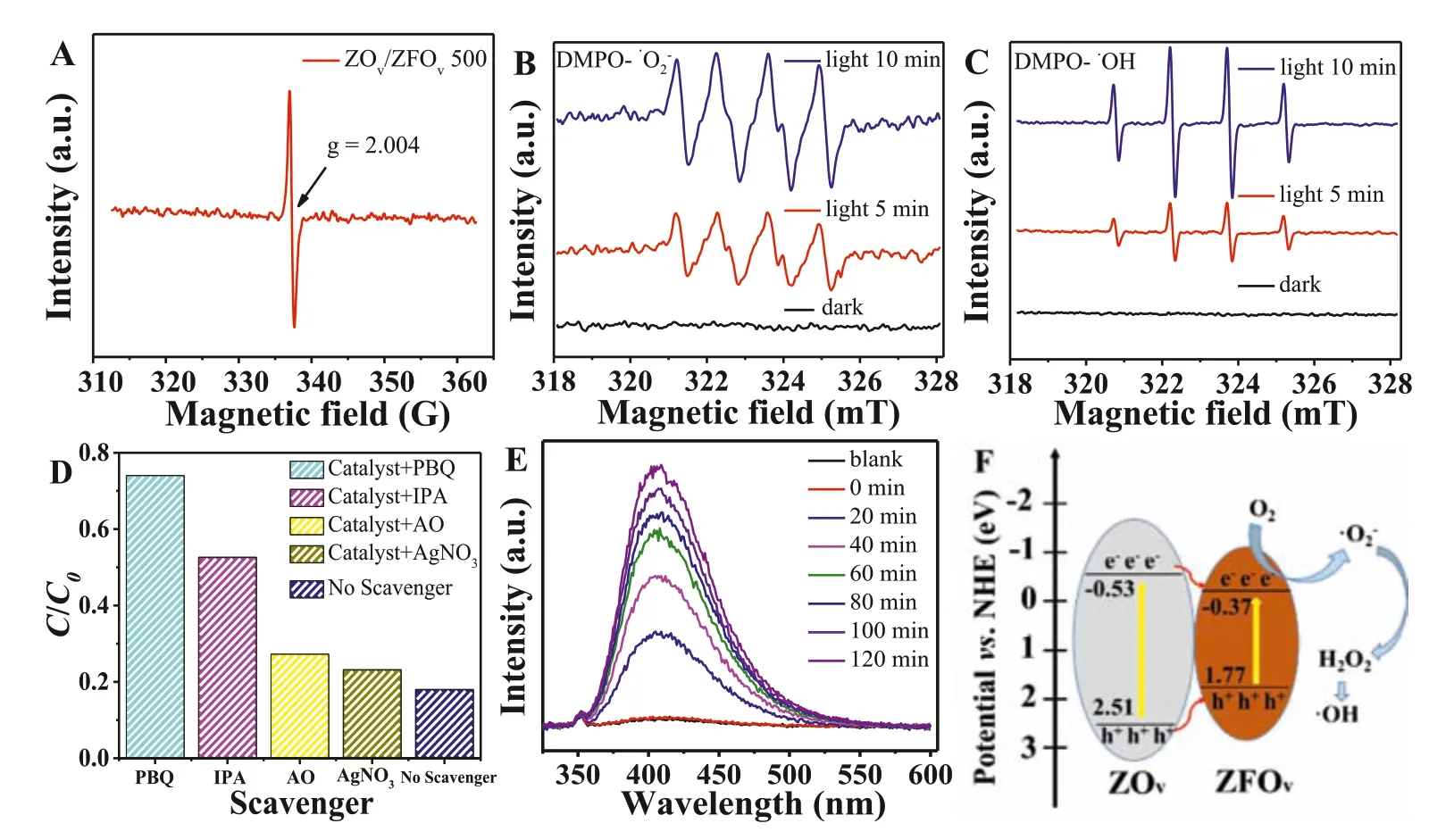
Fig.4.(A) EPR plots of ZOv/ZFOv 500.(B) DMPO spin-trapping EPR spectra for ·O2-.(C) DMPO spin-trapping EPR spectra for ·OH.(D) Active species quenching experiments over ZOv/ZFOv 500.(E) Hydrogen peroxide trapping experiments of ZOv/ZFOv 500.(F) Schematic of the possible carrier’s separation process on the interface of ZOv/ZFOv 500 under visible light irradiation.
Electron paramagnetic resonance (EPR) was performed to verify the oxygen vacancy in ZOv/ZFOv500.As shown in Fig.4A,ZOv/ZFOv500 displayed a symmetrical signal atg=2.004,which can be attributed to the unpaired electrons on the oxygen vacancy sites,illustrating that ZOv/ZFOv500 was successfully synthesized.EPR was also carried out to confirm the existence of reactive oxygen species,which are produced under visible light in the process of photoreaction.As shown in Fig.4B,no signal can be observed for ZOv/ZFOv500 in darkness.After irradiation,the signal with four peaks corresponding to·O2-were appeared,and the intensity of this signal was enhanced with the extension of irradiation time.Similarly,the signal characteristics of the DMPO-·OH were also observed after irradiation (Fig.4C).
In order to identify the main active species in degradation,active species trapping experiments were performed.Isopropyl alcohol (IPA),p-benzoquinone (PBQ),AgNO3and ammonium oxalate monohydrate (AO) were selected as the capture agent for·OH,·O2-,e-and h+,respectively (Fig.4D).The removal rate of TC reached 82.0% without any scavenger.After adding PBQ into the suspension,the TC degradation rate was dramatically decreased,indicating that·O2-plays a significant role in the degradation process.The presence of IPA modestly inhibited the degradation,and the photocatalytic activity was only slightly inhibited after addition of AO and AgNO3,respectively,demonstrating that e-and h+are inconsequential to TC degradation.
Combining with the results of Mott-Schottky (Fig.S5 in Supporting information) and DRS experiments (Fig.S4 in Supporting information),the nanocomposite cannot produce·OH radicals through hole directly.In order to study whether the catalyst can produce·OH radicalsviaH2O2in the degradation process,the fluorescence intensity of H2O2and its evolution with time were explored.As shown in Fig.4E,the yield of H2O2was gradually increased with the increase of time,proving that·OH radicals were produced from H2O2.
According to the above discussion,a probable photocatalytic degradation mechanism was proposed in Fig.4F.Because the conduction band (CB) of ZOv(-0.53 eV) is more negative than that of ZFOv(-0.37 eV),the excited electrons on ZOvcould be transferred to the CB of ZFOvby electron injection.The electrons in ZFOvcould react with surface adsorbed oxygen to form superoxide radicals,and parts of superoxide radicals further converted to hydrogen peroxide and thus generates hydroxyl radical to participate in degradation reaction.Because the valence band (VB) potential of ZFOvis more negative than the OH-/·OH potential,the positively charged holes in VB can only oxidize TC instead of oxidizing H2O or OH-to·OH.
The degradation intermediate products were analyzed by high performance liquid chromatograph-mass spectrometer (HPLC-MS)to investigate the photocatalytic pathways.Based on the HPLCMS analysis,degradation and removal process can be divided into two main pathways (Fig.S14 in Supporting information).The first pathway is by the ring-opening reaction and the deprivation of methyl from the tertiary amine to generate the intermediate withm/zof 391.16 (A1),which is further fragmented to the product A2 (m/z=332.16)viathe deprivation of amino group.Afterwards,the intermediates with them/zvalues of 290.10 and 226.06 were degradedviathe oxidation,dehydroxylation reaction,deethylation reaction and deacetylation reaction.Meanwhile,tetracycline was fragmented to B1 (m/z=360.15) by removal of the amide group,hydroxyl group and methyl.The intermediates with them/zvalues of 316.16,272.16,261.17 and 245.18 are generated by the cleavage of specific functional groups and the ring-opening reactions.In the end,the intermediate products would be finally oxidized to CO2,H2O and small inorganic molecules.
In conclusion,we successfully synthesized yolk-shell structure of ZOv/ZFOvby a solvothermal-calcination method.The ZOv/ZFOvcatalyst with suitable oxygen vacancy concentration can effectively degrade TC under visible light irradiation.The superior photocatalytic performance of the nanocomposite can be ascribed to the following advantages: (1) Photothermal effect accelerates the migration of ROS and speeds up the reaction rate; (2) Oxygen vacancy and heterostructure extend the light absorption and improve the charge carriers’separation; (3) Yolk-shell structure leads to the superior multi-reflection absorption capability.The present work not only gives insights into the TC photodegradation by using ZOv/ZFOv,but also offers a solution to the practical application of photocatalysts in wastewater treatment.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.21976116,52161145409),Shaanxi Provincial Foreign Expert Project (No.2021JZY-004),SAFEA of China (Highend Foreign Expert Project),Alexander-von-Humboldt Foundation of Germany (Group-Linkage Program),and the scientific research startup fund of Shaanxi University of Science and Technology.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.03.031.
 Chinese Chemical Letters2023年1期
Chinese Chemical Letters2023年1期
- Chinese Chemical Letters的其它文章
- Diabetic wound healing activated by supramolecular cascade reaction
- MBenes: Two-dimensional transition-metal borides with ordered metal vacancies
- Wet-adhesive materials of oral and maxillofacial region: From design to application
- Diverse catalytic systems for nitrogen-heterocycle formation from O-acyl ketoximes
- Fluorine-containing drugs approved by the FDA in 2021
- The development and application of dual-comb spectroscopy in analytical chemistry
