Sono-ReCORMs for synergetic sonodynamic-gas therapy of hypoxic tumor
Yue Li,Nong Lu,Qitin Lin,Hoing Wng,Zhuoming Ling,Yujun Lu,*,Pingyu Zhng,*
a College of Chemistry and Environmental Engineering,Shenzhen University,Shenzhen 518060,China
b Whittle School and Studios Shenzhen Campus,Shenzhen 518066,China
Keywords:Synergetic sonodynamic-gas therapy Carbon monoxide (CO)Rhenium(I) tricarbonyl complex Hypoxic tumor
ABSTRACT Carbon monoxide (CO) gas therapy,a novel anti-tumor technique based on the cytotoxicity from the CO released in situ,has become one of the hot topics in cancer treatment.Since the technique is oxygenindependent,it displays promising therapeutic effect for hypoxic tumor where traditional photodynamic therapy shows limited efficacy and insufficient penetration depth.To fully address these limitations of PDT,we propose a synergetic sonodynamic-CO gas releasing strategy for the therapy of hypoxic tumor.In this work,two rhenium(I) tricarbonyl complexes with different substituted ligands are investigated for US-triggered ROS generation and CO release.Our results indicated that the electron-donating NMe2-substituted complex (Re-NMe2) exhibits stronger luminescence intensity and generates more singlet oxygen (1O2) than the electron-withdrawing NO2-substituted complex (Re-NO2).In addition,Re-NMe2 displays release of CO triggered by US,thus showing high sono-cytotoxicity to tumor cells in-vitro and in-vivo.The strong ROS-generating capability combined with rapid CO-releasing feature from Re-NMe2 has made it a powerful tool for the efficient treatment of hypoxic tumor.
In recent years,gas therapy has attracted much attention due to its high biological safety and treatment efficiency [1,2].The construction of a stimulus response carrier with controlled release properties is very popular for precise gas therapy [3–6].CO is attracting increasing attention because of its role as a gasotransmitter with cytoprotective and homeostatic properties.CO releasing molecules (CORMs) are spatially and temporally controlled CO releasers that exhibit excellent pharmaceutical traits due to their unique chemical structure [7,8].Furthermore,the release of CO from CORMs does not rely on oxygen,thus showing superior therapeutic effect to hypoxic tumor where other oxygendependent methods lack efficacy (such as photodynamic therapy (PDT),chemotherapy and sonodynamic therapy (SDT)) [9–11].However,how to effectively design CORMs while achieving the precise target release of CO gas molecules with sufficient penetration depth has become a major challenge.
Transition metal carbonyls complexes,including Mn-CORMs[12,13],Ru-CORMs [14,15],and Re-CORMs [16,17],have been investigated about their light-induced decarbonylation,release of the CO molecule.In recent years,several rhenium(I) polypyridine complexes have proven to be effective photosensitizers for the controlled release of CO under visible light irradiation.The synergistic strategy of gas therapy and PDT significantly enhance the effectiveness of the cancer treatment [18–21].However,the inherent disadvantages from PDT including insufficient penetration depth and high phototoxicity have limited its applications[22,23].Compared with traditional PDT,ultrasound (US)-triggered SDT has broad application prospects in tumor treatment due to its deeper penetration depthin-vitroandin-vivo[24–26].SDT,including low-intensity US and chemical sonosensitizers,has emerged as a promising minimally invasive and selective approach for the treatment of deep tumors.US not only enhances cell membrane permeability,thereby enhancing the cellular absorption rate of chemical sonosensitizers,but also stimulates chemical sonosensitizers in deep tumor tissue to generate reactive oxygen species(ROS),thereby killing cancer cells [27–29].For instance,Linet al.successfully constructed a multifunctional nano-platform MnSiO3-Pt@BSA-Ce6 (MPBC),and realized the synergistic treatment of SDT/chemodynamic therapy (CDT).The sonosensitizer Ce6 can produce1O2to kill cancer cells under US irradiation,meanwhile,the loaded Pt is responsible for catalyzing the overexpression of H2O2in tumor microenvironment,further decomposing to produce O2,and finally overcoming tumor hypoxia and promoting SDT-induced1O2[24].In addition,US-responsive gas molecular control release has become an unique and advantageous way of drug delivery[30].Hence,it is critical to develop metal carbonyls complexes as sonosensitizers for US-triggered CO release to achieve better treatment effect in deep tumor tissues.
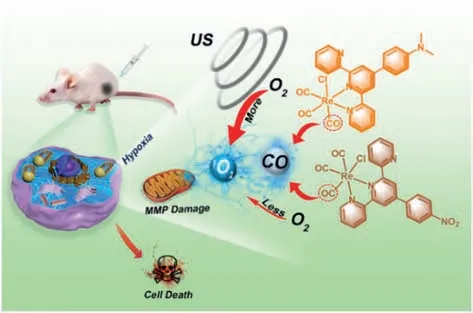
Scheme 1.The mechanism of combination of SDT and CO gas therapy based on the rhenium(I) tricarbonyl complexes.
In this work,we investigated two rhenium(I) tricarbonyl complexes sono-ReCORMs (Re-NMe2,Re-NO2) with different substituted ligands for synergistic sonodynamic-gas therapyin-vitroandin-vivo.We compared their abilities of singlet oxygen (1O2) generation and the rate of CO release in solution under US irradiation.As depicted in Scheme 1,Re-NMe2can produce more1O2than Re-NO2under same condition,but the amount of CO produced from Re-NMe2and Re-NO2is similar once stimulated by US.Our synergistic strategy combining gas therapy with SDT will provide excellent versatility in cancer treatment.The generated reactive oxygen species (ROS) and released CO from the complexes would enhance the therapeutic efficacy under normoxia while the CO gas therapy would play a critical role in treating hypoxic tumor where ROS is lacking.

Fig.1.(a) Synthetic route of the ReCORMs.(b) UV-vis absorption spectra of the ReCORMs (10 μmol/L) measured in H2O (containing 1% DMSO) at 298 K.(c) Emission spectra of ReCORMs (10 μmol/L) measured in H2O (containing 1% DMSO) at 298 K, λex=405 nm.
The synthetic scheme of two rhenium(I) complexes was depicted in Fig.1a.The detailed synthetic procedures were illustrated in the supporting information.The ReCORMs were fully characterized by ESI-MS,1H NMR and13C NMR spectroscopy (Figs.S1-S6 in Supporting information).UV-vis absorption spectra of the rhenium complexes were first studied at a molar concentration of 10 μmol/L in H2O (Fig.1b).Compared with Re-NO2,Re-NMe2displays a stronger absorption MLCT band at around 410 nm probably due to the electron-donating NMe2-substituted ligand.The complexes were relatively stable for 48 h in the PBS or Roswell Park Memorial Institute medium (RPMI-1640) solution in the dark at 298 K(Figs.S7 and S8 in Supporting information),demonstrating that the CO group cannot be released in the dark.The1H NMR spectra of Re-NMe2did not change at 298 K in the dark for 48 h (Fig.S9 in Supporting information),which further indicated that Re-NMe2was very stable in the dark.
The luminescence properties of two sono-ReCORMs were later evaluated in different solvents (Fig.1c and Fig.S10 in Supporting information).Upon excitation at 405 nm,Re-NMe2exhibited strong yellow emission (maximumca.612 nm,541 nm) in H2O and CH2Cl2at 298 K,while Re-NO2showed minimal luminescence under the same conditions.In addition,the luminescence quantum yield of Re-NMe2in CH2Cl2(Фem=0.266) or H2O (Фem=0.052)was much higher than that of Re-NO2(Фem<0.01) (Table S1 in Supporting information).The luminescence lifetime of Re-NMe2was longer in either CH2Cl2(26.27 ns) or H2O (9.72 ns) compared to that of Re-NO2(Fig.S10 and Table S1).All results suggest that these R-substituted ligands can lead to distinct photophysical properties of the rhenium(I) complexes.And the electron-donating ligands can enhance the absorption and emission compared with the electron-withdrawing ligands.
US-driven CO release from the sono-ReCORMs was further studied to investigate their compatibility for synergistic sonodynamicgas therapy,we quantitatively analysed the amount of CO release under US irradiation (1.0 MHz,0.3 W/cm2) by a hemoglobin (Hb)method [31].As shown in Figs.2a and b,the absorbance of reduced haemoglobin (Hb) was presented at 430 nm.After trapping the released CO from Re(I) complexes,Hb-CO was formed,showing a new absorbance maxima at 410 nm in the spectra.Meanwhile,the absorption at 562 nm decreased,while the other two absorption bands at 540 nm and 579 nm increased.The amount of released CO was calculated based on the absorption data at 410 nm.It is found that both Re-NMe2and Re-NO2approximately released equivalent moles of CO.

Fig.2.(a,b) UV-vis absorption spectra monitoring of the CO release process of Re-NMe2 or Re-NO2 (1 μmol/L) in the PBS by the Hb (4.2 μmol/L) method.(c) Gas chromatogram detecting of the CO release under US irradiation.(d) The diagram of US control of CO release.US irradiation: 1.0 MHz,0.3 W/cm2.
The CO fluorescent probe COP-1 [32,33] was further used to characterized CO release from the sono-ReCORMs.As shown in Fig.S11 (Supporting information),the fluorescence intensity of COP-1 in the solution containing ReCORMs increased under US irradiation.Furthermore,the fluorescence enhancement of COP-1 brought by the US-irradiated Re(I) complexes is similar to that by equivalent amount of CO.Beseides,peaks of CO released from the two complexes were detected in gas chromatography,as shown in Fig.2c.All these evidences prove that both complexes are capable to release CO under US irradiation,as shown in Fig.2d.
Generation of ROS by the Re(I) complexes was measured to evaluate their performance for SDT.The ReCORMs was mixed with 2′,7′-dichlorodihydro-fluorescein diacetate (DCFH-DA),a nonfluorescent compound which can convert into highly fluorescent 2′,7′-dichlorofluorescein (DCF) by ROS [34].In Fig.S12 (Supporting information),we observed that the fluorescence intensity of DCFHDA increased in the ReCORMs solution under US irradiation.Of note,the fluorescence enhancement of DCFH-DA in Re-NMe2solution is stronger compared with that in Re-NO2under US irradiation,indicating the electron-donating NMe2-substituted Re-NMe2can produce more ROS than electron-withdrawing NO2-substituted Re-NO2.
Furthermore,a1O2probe,9,10-diphenylanthracene (DPA) [35],was utilized to detect1O2generation by the two Re(I) complexes.As shown in Figs.3a and b,the absorbance intensity of DPA decreased with increasing US irradiation time in the presence of the ReCORMs.Additionally,the absorbance decrease resulted from the US-irradiated Re-NMe2was larger than that from Re-NO2,proving that Re-NMe2produces more1O2than Re-NO2.Their1O2quantum yieldФ(1O2) was determined by the reference of [Ru(bpy)3]2+(Ф(1O2)=0.22 in H2O) [36,37] as shown in Fig.3c and Fig.S13(Supporting information).TheФ(1O2) of Re-NMe2and Re-NO2are 0.27 and 0.16,respectively (Table S1).All the results demonstrated that Re-NMe2possesses superior1O2generation capability to Re-NO2under US irradiation (Fig.3d),further indicating that Re-NMe2could be employed as an effective sonosensitizer for US-triggered tumor treatment.

Fig.3.Absorbance changes of DPA (10 μg/mL) in the presence of (a) Re-NMe2 or(b) Re-NO2 for 1O2 measurement under US irradiation.(c) Changes in absorbance of DPA at 382 nm against US irradiation time in the presence of the compounds(10 μmol/L).(d) The diagram of 1O2 generation via US irradiation.US irradiation:1.0 MHz,0.3 W/cm2.
To evaluate the anti-cancer effect of the ReCORMsin-vitro,we first examined their cellular uptake.As shown in Fig.S14 (Supporting information),we observed strong yellow luminescence from 4T1 cells upon excitation after an incubaion time of 2 h with Re-NMe2or Re-NO2,indicating that the cellular uptake of the complexes was sufficient.A commercially available mitochondrial immobilization probe (Mito-Tracker Deep Red,MTDR) was then used to explore the intracellular location of the ReCORMs by confocal microscope (Fig.S15 in Supporting information).The result showed that the two complexes selectively accumulated in mitochondria.
We further studied the effects of different US powers on1O2and CO genenation from Re-NMe2.As shown in Fig.S16 (Supporting information),with the US power increasing,the ROS generation increased.However,the CO release almost keep the same,that is one equivalent CO released under any powers of US irradiation in Fig.S17 (Supporting information).Then we explored the suitable US irradiation time and power in order to determine the optimal conditions for cell studies (Fig.S18 in Supporting information).When the US irradiation time and power was 20 min and 0.3 W/cm2(1.0 MHz) respectively,the survival rates of control 4T1 cells reached to a satisfactory level,up to 80%.However,the control cells would be killed under 0.4 W/cm2or 30 min US irradiation.Therefore,we decided to continue our investigation of the anti-cancer activity with 4T1 cells under 20 min and 0.3 W/cm2US irradiation.
The therapeutic effect of the ReCORMs on 4T1 cells under US irradiation in normoxia and hypoxia was studied by a standard 3-(4,5-dimethylthiazol-2-yl)-2,5-tetrazolium bromide (MTT) assay[38].Without US irradiation,most of 4T1 cells were alive after incubation with the ReCORMs for 2 h.Upon US irradiation,Re-NMe2(IC50(US,normoxia)=5.34 μmol/L) caused more intended cell death than Re-NO2(Fig.4a,Fig.S19a and Table S2 in Supporting information).This is consistent with our previous results in Fig.3 that Re-NMe2is capable to generates more1O2than Re-NO2under US irradiation.In addition,Re-NMe2showed decent UStoxicity even in hypoxia (IC50(US,hypoxia)=13.24 μmol/L) (Fig.4b,Fig.S19b in Supporting information).It is evident that CO gas plays an important role in cancer treatment under hypoxia,proving the versatility of the synergistic sonodynamic-CO therapy.Given the synergistic design of our work,the US-toxicities of Re-NMe2and Re-NO2to 4T1 cells were weaker in hypoxia than in normoxia(Fig.4b,Fig.S19b and Table S2).The above results indicate that the ReCORMs are promising tools for US stimulated treatment of both normoxic and hypoxic cancer.Then,we explored the toxicity of Re-NNe2to L929 normal cells (mouse fibroblasts cells).The result showed that Re-NMe2had no obvious cytotoxicity on L929 cells without US irradiation in Fig.S20 (Supporting information),and the cytotoxicity of Re-NMe2+US (IC50>50 μmol/L) to L929 cells was weaker than that to 4T1 cells,illustrating that Re-NMe2was safe to L929 normal cells.

Fig.4.Relative viabilities of 4T1 cells after incubation with different concentrations of Re-NMe2 with/without US irradiation in normoxia (a) or hypoxia (b).(c) Fluorescence imaging of CO detection in live 4T1 cells using COP-1.4T1 cells incubated with 10 μmol/L Re-NMe2 for 2 h and then 2 μmol/L COP-1 for 20 min.COP-1:λex=460 nm; λem=520 ± 30 nm.(d) Fluorescence imaging of 4T1 cells stained with DCFH-DA (10 μmol/L) after various treatments. λex=460 nm, λem=525 ± 30 nm.Scale bar: 100 μm.
To visualize the US stimulated CO release from the ReCORMs in 4T1 cells,the COP-1 probe was again used to trap intracellular CO.As shown in Fig.4c,strong green fluorescence from COP-1 was observed after 4T1 cells treated with COP-1,Re-NMe2,and subsequent US irradiation,indicating that the presence of US induced CO release from Re-NMe2in cells.In comparison,there was no obvious green fluorescence in other control groups.Furthermore,we imaged the intracellular ROS by using DCFH-DA.As shown in Fig.4d,the 4T1 cells treated with DCFH-DA and Re-NMe2in normoxia with subsequent US exposure (1.0 MHz,0.3 W/cm2,20 min) exhibited strong green fluorescence.However,it showed weaker fluorescence due to the limitation of ROS generation in hypoxic environment.And the weak fluorescence in hypoxia probabaly due to ROS produced by released CO in cells [2].
Moreover,the killing effect of Re-NMe2on 4T1 cells in normoxia or hypoxia under US irradiation (1.0 MHz,3 W/cm2) was determined by co-staining with calcein AM (AM,live cells) and propidium iodide (PI,dead cells) (Fig.S21 in Supporting information).Re-NMe2or US treatment alone does not induce obvious cell death.However,most cells were dead in the Re-NMe2+US group in nomoxia.Additionally,stronger red fluorescence from PI was observed in nomoxia than that in hypoxia.These results indicate that the combination of CO gas therapy and SDT under normoxia is more effective than under hypoxia.In hypoxia,the killing effect on 4T1 cells relies on CO gas therapy.
Based on our studies of CO/SDT synergistic anticancer activity of the ReCORMs,we deduced that the mechanism of the antitumor effects of CO or CORMs might be linked to mitochondria activity exhaustion via the acceleration of oxygen consumption,generation of mitochondrial ROS,production of mitochondrial membrane damage,and occurrence of cell apoptosis [39–41].In order to verify the assumptions,we used the JC-1 dye as a fluorescent indicator to assess ROS-induced damage to mitochondria by measuring changes in the mitochondrial transmembrane potential(MTP) [42].As presented in Fig.S22 (Supporting information),the Re-NMe2+US group showed the strong green fluorescent signal,which indicated mitochondrial membrane depolarization.This observation indicates that Re-NMe2targets mitochondria and damages mitochondria by1O2and CO,thus inducing tumor cell death.
Based on the fact that Re-NMe2has a better US-triggered antitumor effect than Re-NO2in-vitro,Re-NMe2was chosen to further study anti-tumor activity on mice bearing 4T1 tumors.The animal care and experiments were performed in accordance with the animal research ethics committee of Shenzhen University (AEWC-201412003).The mice were randomly divided into four groups (5 mice in each group): 1) untreated; 2) treated with US irradiation(1.0 MHz,0.3 W/cm2,20 min); 3) treated with Re-NMe2(0.42 mg/kg dose injection); 4) treated with Re-NMe2and US irradiation.During 14 days of treatment,the tumor sizes and volumes were monitored and calculated with a digital caliper every other day.As shown in Fig.5a,Re-NMe2+US treatment significantly inhibited tumors growth.In comparison,tumors in other groups including control,Re-NMe2treatment without US irradiation,or US irradiation alone showed rapid growth.
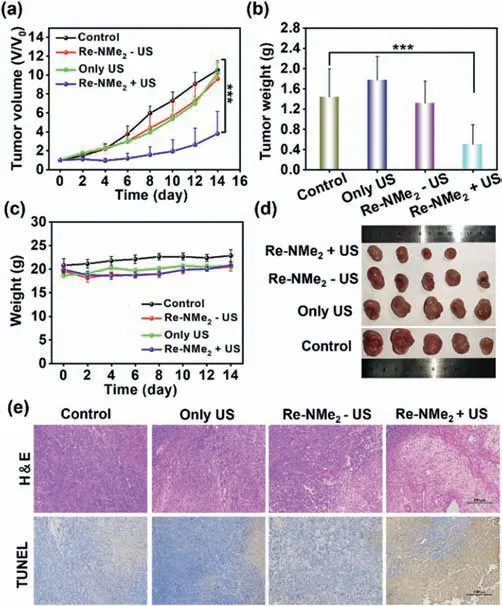
Fig.5.(a) The average tumors growth curves in mice (4T1 tumor bearing Balb/c mice) after different treatments,including Control,US alone,Re-NMe2 alone and Re-NMe2+US groups (n=5 biologically independent mice,US: 1.0 MHz,0.3 W/cm2,20 min).(b) Average tumor weights of mice at day 14 post various treatments.(c)Body weight curves of mice after different treatments.(d) Photos of tumors,which were collected from the mice at day 14 after various treatments.(e) Microscopy photos of H&E and TUNEL stained tumor slices.Tumor tissue were collected from mice at 24 h post various treatments.Scale bar: 200 μm.
The tumors of mice in the different groups were weighed and photographed (Figs.5b and d,Fig.S23 in Supporting information).Next,hematoxylin and eosin (H&E) staining and terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) were used to evaluate tumor cell apoptosis (Fig.5e).A great amount of cell death was observed in the Re-NMe2+US group,while no distinct apoptosis was observed after other treatments.These results were consistent with thein-vitroexperiments,indicating that Re-NMe2+US treatment of mice has a significant inhibitory effect on 4T1 tumors.
During 14 days treatment period,there was no obvious change in the weight of all mice (Fig.5c).The biosafety of Re-NMe2were also evaluated by H&E stained slices of main organ collected from healthy mice i.v.injected with 2 times of the therapeutic dose(0.84 mg/kg) of Re-NMe2(Fig.S24 in Supporting information).The results indicated that there was low systemic toxicity of the complex.To further illustrate the biological safety of Re-NMe2in vivo,the zebrafish (Tg(flk1:EGFP)s843) [43] were incubated with a series of concentration gradients of Re-NMe2for 4 days (Fig.S25 in Supporting information).The results showed that the blood vessels were not damaged,and the labeled green fluorescent protein fluorescence was still significant.These results indicate that Re-NMe2has good compatibility in the organism and has low side effects.
In summary,two sono-ReCORMs with electron-donating or elctron-withdrawing group (Re-NMe2and Re-NO2) were synthesized and characterized.Notably,electron-donating NMe2-substituted Re-NMe2has significant advantages in fluorescence intensity,1O2quantum yield,fluorescence lifetime,and sonocytotoxicity.Re-NMe2can generate more1O2than Re-NO2,while both Re-NMe2and Re-NO2release one equivalent CO under the same US irradiation (1.0 MHz,0.3 W/cm2).Furthermore,Re-NMe2exhibited excellent sonocytotoxicities towards both normoxic and hypoxic cancer cells.In addition,Re-NMe2can target mitochondria and the geranated1O2and CO can damaged mitochondria and finally induce tumor cell death.In-vivoexperiments,we have shown that Re-NMe2under US irradiation could effectively inhibit the growth of subcutaneous tumors.Re-NMe2has high safety and sonodynamic performance for simultaneous production of CO and1O2for anti-tumor treatment based on the synergistic strategy of sonodynamic-CO gas therapy.This research provides important insights for the development of metal complex sonosensitizers and CO releasing molecules in the future.
Declaration of competing interest
The authors declared that they do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Acknowledgments
We appreciate the financial support of the National Natural Science Foundation of China (NSFC,No.22077085),and the Science and Technology Foundation of Shenzhen (Nos.JCYJ20210324095200002,JCYJ20190808153209537).We appreciate the Instrumental Analysis Center of Shenzhen University.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.06.076.
 Chinese Chemical Letters2023年1期
Chinese Chemical Letters2023年1期
- Chinese Chemical Letters的其它文章
- Diabetic wound healing activated by supramolecular cascade reaction
- MBenes: Two-dimensional transition-metal borides with ordered metal vacancies
- Wet-adhesive materials of oral and maxillofacial region: From design to application
- Diverse catalytic systems for nitrogen-heterocycle formation from O-acyl ketoximes
- Fluorine-containing drugs approved by the FDA in 2021
- The development and application of dual-comb spectroscopy in analytical chemistry
