A dual-responsive hyaluronic acid nanocomposite hydrogel drug delivery system for overcoming multiple drug resistance
Yi Liu,Mn Zhu,Minsi Meng,Qio Wng,Yun Wng,Yu Lei,Ynmin Zhng,Lin Weng,Xin Chen
a Key Laboratories of Fine Chemicals and Surfactants in Sichuan Provincial Universities,College of Chemical Engineering,Sichuan University of Science and Engineering,Zigong 643000,China
b Institute of Precision Medicine,Zigong Fourth People’s Hospital,Zigong 643000,China
c Department of Chemical Engineering,Shaanxi Key Laboratory of Energy Chemical Process Intensification,Institution of Polymer Science in Chemical Engineering,School of Chemical Engineering and Technology,Xi’an Jiao Tong University,Xi’an 710049,China
d School of Pharmacy,Health Science Center,Xi’an Jiaotong University,Xi’an 710061,China
Keywords:Dual-responsive HA hydrogel P-gp inhibition MDR Enhance chemotherapy
ABSTRACT Chemotherapy is restricted by efficient drug outflow due to the multiple drug resistance (MDR) in heterogenous nature of tumor.Herein,we present a dual-responsive hyaluronic acid (HA) nanocomposite hydrogel that can not only response to the tumor microenvironment but also enhance chemotherapy.This HA hydrogel consists of a core-shell SiO2 (GOD@SiO2-Arg) and mesoporous silica nanoparticles (MSNs)with doxorubicin (DOX) as the cargo (DOX@MSN).It could rapidly release the GOD@SiO2-Arg nanoparticles at the low pH tumor-specific environment due to the cleavage of imine bond.GOD@SiO2-Arg activated by over-expressed glutathione (GSH) in tumor cells releases GOD due to the cleavage of disulfide bonds,which could oxidize glucose to produce hydrogen peroxide (H2O2) for in situ NO generation via reaction between Arg and H2O2.The validity of this study might provide a method to modulate the tumor microenvironment for enhancing chemotherapy.
Cancer is one of the most deadly diseases in world wide.Chemotherapy drugs such as doxorubicin (DOX) used in tumor chemotherapy can effectively treat multiple cancers,such as breast cancer,liver cancer,hepatocellular carcinoma cancer [1–6].The organic and inorganic materials-based nanomaterials have been achieved for the treatment of tumors [7,8].However,cancer cell resistance to therapeutic drugs plays a significant role in its survival [9].The multiple drug resistance (MDR) and premature drug release have become major obstacles in chemotherapeutic treatment [3].Although the smart drug delivery system with zero premature release,specific cancer cell targeting,and controlled release had been widely used to enhance the chemotherapy,it is still unable to completely eradicate tumor bulk due to MDR of cancers[10,11].Among various pathways of resistance,the P-gp,a critical protein for drug efflux which is upregulated expression in cancer chemotherapy has been considered as one key factor of MDR in different cancer cells [12–15].This efflux transporter is in charge of a variety of substrates efflux from cells and their up-regulated expression in cancer cells results in the insufficient concentration of drug [12].
As to inhibit P-gp induced MDR,nitric oxide (NO) therapy with negligible side effects have received significant attention on cancer research [16,17].NO is a gaseous molecule that plays a significant role in cells [18,19].Plenty of research results indicate that NO as a chemosensitizer,can effectively reverse MDRviareducing the Pgp expression levels and restoring the cytotoxicity of chemotherapeutics against tumor cells [20–22].In the field of cancer therapy,high concentrations of NO can kill tumor cells through nitrosation of mitochondria and DNA,and low concentrations of NO also had a direct toxic effect on tumors because it can sensitize cancer cells and contribute to the reversal of MDR [19,23].NO assisted adjuvant chemotherapy to enhance the chemotherapy efficacy had attracted wide attention [24–26].Unfortunately,NO is highly reactive,and makes tumor sensitive to physiological substances,greatly hindering its application for tumor therapy [27].Although many NO composites have been developed,delayed action of NO also has a negative influence on chemotherapy [15,20].Arginine (Arg),a natural NO donor,is able to continuously release NO in the presence of H2O2[28].Recently,NO cooperated with cancer chemotherapy treatment have been tested,but have hysteresis in restraining delayed drug excretion which will result in drug leakage [24].Thus,a significant releasing time interval is needed and will be beneficial for NO and DOX sequential release in MDR cells,where the early release of NO can reduce the P-gp expression and promote the accumulation of following release of DOX for killing cancer cells.
Hyaluronic acid (HA) is a well-studied biomaterial for its biocompatibility,biodegradability,and non-immunogenicity [29–32].Similar to the natural extracellular matrix (ECM),hydrogels including HA based on polysaccharides and peptides are more suitable for clinical use [33].MSN have been demonstrated as one of the most promising drug carriers due to its uniform pore size,high surface area,high drug loading capability,remarkable biocompatibility,and easy surface functionalization [34–36].Silica nanoparticles are biocompatible nanocarriers that has been generally recognized safe by the US Food and Drug Administration [37].Enzyme can be immobilized in silicas and become highly resistant to leaking [38].Specific tumor micro-environment includes low interstitial pH,over-expressed enzymes,and a high GSH level [39,40].GSH abundance in the tumor cells can break the disulfide bond [41].
Herein,we fabricated a pH and enzyme dual-responsive hydrogel to successfully achieve NO generation and subsequent DOX delivery in tumor cells for promoted chemotherapy.The MCM-41 type mesoporous silica nanoparticles (MSNs) were introduced to load DOX.In consideration of the essential role of glucose in providing energy in the tumor,we strategically imported GOD which can exhaust the within-tumor glucose generate gluconic acid and H2O2encapsulated to the SiO2.Simultaneously,the concentration gradient elevated concentration of H2O2can help to accelerate the oxidization of Arg into NO [25,42].The major part of this hydrogel was formed by cross-linking between MA/EDA (provided the functional hyaluronic acid (HA) with carbon double bond and amino group and crosslinked by diglycidyl ether (EO-PEG-EO))co-grafted hyaluronic acid (HA-MA-NH2) and thiol modified drug carrier (MSNs containing DOX as cargo and polyester as the cap,named as DOX@MSN-SH)viaclick reaction,which was further conjugated with NO generator (glucose oxidase loaded silica nanoparticle with disulfide bonds in the shell and arginine on the surface,named GOD@SiO2-Arg) using pH cleavable imine bond.After implantation around tumor tissue,the tumor-specific acidic environment was able to immediately break the amido bond to release GOD@SiO2-Arg,which would be taken by tumor cellsviaendocytosis [43–45].This intracellular GOD@SiO2-Arg was further activated by over-expressed GSH in tumor cells to expose GOD due to the cleavage of disulfide bonds [45–47],which could oxidize glucose to produce H2O2forin situNO generationviareaction between Arg and H2O2[25,48].Subsequently,the over-expressed hyaluronidase would gradually catalyze the HA frame of the hydrogel to degrade slowly and release DOX@MSN-SH,following tumor-targeted DOX delivery,esterase triggered degradation and DOX release,as well as the final NO promoted chemotherapy (Scheme 1) [49,50].
First,HA-MA was synthesized by the reaction between HA and MA,then HA-MA-NH2nanocomposites were obtained from the modification of HA-MAviaEDA.These nanocomposites were verified by1H nuclear magnetic resonance spectroscopy (1H NMR).As shown in Figs.1a and b,characteristic proton signalsδ= 3.195,δ= 3.155,indicating the successful HA modification.The Fourier transform infrared (FTIR) was used to verify the modification of HA as well (Fig.1c).
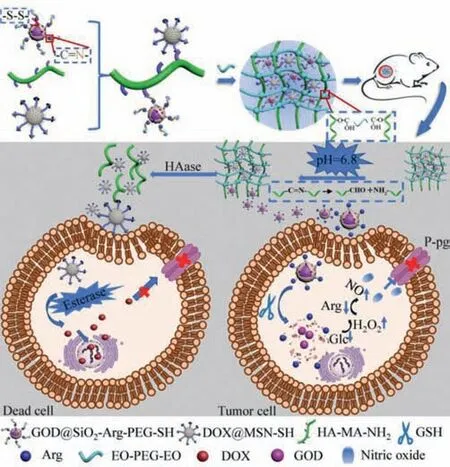
Scheme 1.Schematic illustration of acid-responsive and esterase-responsive hyaluronic hydrogel.HAase would gradually degrade the HA frame of the hydrogel to slowly release DOX@MSN-SH and GOD@SiO2-Arg.The NO was triggered by the reaction between GSH released Arg and H2O2 in tumor cells to inhibit Pglycoprotein (P-gp) function.DOX loaded MSN particles were released by the esterase from the tumor microenvironment.The strong synergistic anti-cancer activities were triggered by both P-gp inhibition and DOX chemotherapy.
FTIR present characteristic vibrations of C=O (1720 cm-1) from the HA,C=C (1302 cm-1) from the MA confirmed that MA was successfully modified on HA,CO-NH (1640 cm-1) confirmed that EDA was successfully modified on HA-MA.The peaks of1H NMR spectra and clear C=C and CO-NH signals of FTIR spectra indicated that the successful synthesis of HA-MA-NH2.To fabricate a responsive drug delivery system,the core-shell GOD@SiO2-Arg-PEG-SH nanoparticle and the ester terminated DOX@MSN-SH nanoparticles were synthesized respectively.The images showing approximate 50 nm diameter spherical GOD@SiO2were captured by transmission electron microscopy (TEM) (Fig.1d).GOD@SiO2was subsequently modified to GOD@SiO2-Arg-PEG-CHO.FTIR spectra showed the specific signals of N-H (1560 cm-1) and indicated the successfully GOD@SiO2-NH2modified.The CO-NH bond (1630 cm-1)indicated GOD@SiO2-NH-Arg successfully being modified,and the CHO bond (1685 cm-1) indicated GOD@SiO2-NH-Arg-peg-CHO being successfully modified (Fig.1e).The zeta potential of pure GOD@SiO2nanoparticles was -13.0 mV.The potential increased to+32.5 mV after the amino group bonding to GOD@SiO2.The potential decreased to +13.9 mV when Arg bound to the amino group of GOD@SiO2-NH2.Finally,the potential was slightly changed to+15.03 mV after GOD@SiO2-NH2-Arg reacted to dialdehyde-based PEG (Fig.1f).TEM showed an approximate 200 nm diameter for the spherical and porous MSN (Fig.1g).FTIR showed a clear -SH signals at 2900 cm-1and the signal of Si-OH at 2550 cm-1disappeared in MSN-SH nanoparticles (Fig.1h).The potential changed from -12.0 mV to -21.3 mV also supporting the correct modification to yield MSN-SH particles (Fig.1i).These results proofed that those dual nanoparticles and HA-MA-NH2were successfully synthesized.
The hydrogel was fabricated by HA-MA-NH2and EO-PEG-EO.HA-MA-NH2(1 wt%) was used to dissolve nanoparticles,then MSN or GOD@SiO2-NH2-Arg loaded hydrogel were formed and the sol state hydrogel transformed into fluidless hydrogel (Fig.2a).To test chemical composition of the hydrogel,the thermogravimetric analysis was carried out at first.As shown in Fig.2b,hydrogel was completely decomposed before reaching 1000 °C,and the mass ratio was 0%,while hydrogel with GOD@SiO2-Arg had a 6.00% mass remained at 1000 °C.Meanwhile,hydrogel with GOD@SiO2-Arg and MSN had a 10.34% mass left at 1000 °C.It indicated that MSN mass ratio was 4.34% (Fig.2b).The structure of the hydrogel was further measured by scanning electron microscopy (SEM).As can be seen from the figures,the hydrogel had a uniform pore structure with pore size between 10 μm and 20 μm (Fig.2c).The GOD@SiO2-Arg nanoparticles showed a 50 nm diameter on the surface of hydrogel (Fig.2d) and the 200 nm diameter of MSN nanoparticles appeared on the hydrogel surface as well (Fig.2e).The characteristic peaks of C,N,O and Si were investigated by energy dispersive spectrometry (EDS).It can be seen from the figure that hydrogel contained C,N and O.However,Si element was detected besides C,N,and O elements in the composite hydrogel,which verified the GOD@SiO2-Arg and MSN loading to the hydrogel fact (Fig.2f).Furthermore,FITC labeled GOD@SiO2-Arg and RhB labeled MSN were loaded to the hydrogel and both nanoparticles were able to be observed by fluoresce microscopy (Figs.2g–i).
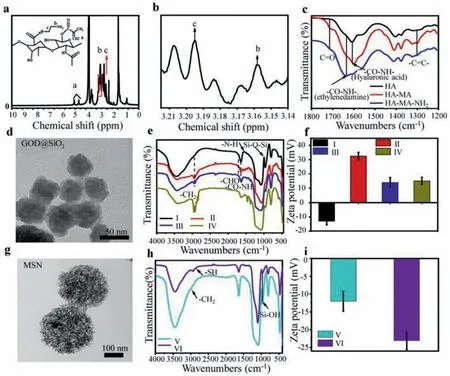
Fig.1.Synthesis of GOD@SiO2 and MSN-SH nanoparticles.(a) 1H NMR spectra of HA-MA-NH2.(b) Peaks of the methylene group.(c) FTIR spectra of HA,HA-MA and HA-MA-NH2.(d) Transmission electron microscopy images of GOD@SiO2.(e) FTIR spectra of I: GOD@SiO2,II: GOD@SiO2-NH2,III: GOD@SiO2-NH-Arg,and IV: GOD@SiO2-NHArg-PEG-CHO.(f) Zeta potential I: GOD@SiO2,II: GOD@SiO2-NH2,III: GOD@SiO2-NH-Arg,and IV: GOD@SiO2-NH-Arg-PEG-CHO.(g) Transmission electron microscopy images of mesoporous silica (MSN).(h) FTIR spectra of V: MSN and VI: MSN-SH nanoparticles.(i) Zeta potential of the V: MSN and VI: MSN-SH nanoparticles.
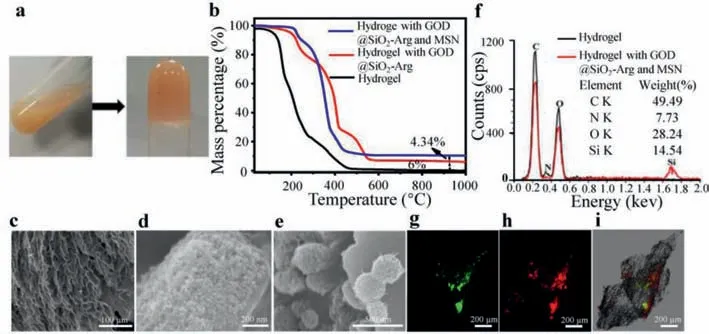
Fig.2.Hydrogel properties.(a) The hydrogel formed from the sol state.(b) Thermogravimetric (TG) analysis of different hydrogels.(c–e) Scanning electron microscopy (SEM)image of HA-MA-NH2+GOD@SiO2+MSN.(f) EDS analysis of the elements in the hydrogel.(g–i) Fluorescence microscopy images of the hydrogel.FITC and RhB fluorescence presented FITC labeled GOD@SiO2-Arg and RhB labeled MSN respectively.
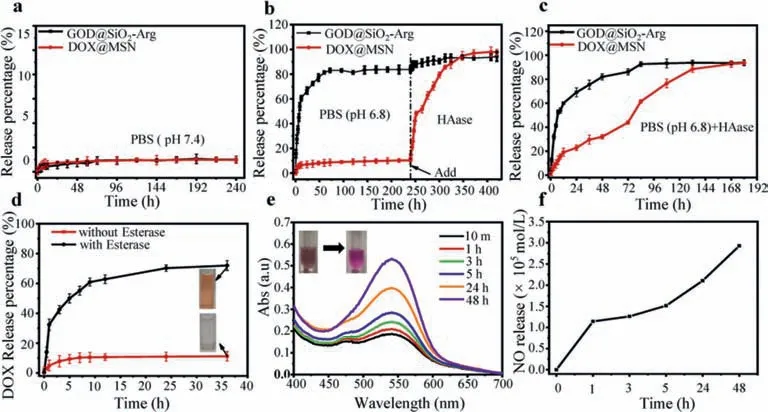
Fig.3.Hydrogel release profiles of the (a) release kinetics of hydrogel in PBS (pH 7.4).(b) Release kinetics of hydrogel in PBS (pH 6.8) for 240 h then HAase was added.(c)Release kinetics of hydrogel in HAase with PBS (pH 6.8).(d) DOX@MSN particles release profiles in the presence of esterase and the absence of esterase.(e) NO production in vitro.GOD@SiO2-Arg (FITC labeled GOD) particles were reacted with GSH and glucose.Sulfanilamide solution and N-1-naphthylethylenediamine solution were added to test NO followed spectrophotometer analysis.(f) NO was determined by the standard curve.
The release kinetics of responsive hydrogel was investigated under different pH values to mimic physiological conditions (pH 7.4)and tumor microenvironment (pH 6.8).The hydrogel had barely released any of the nanoparticles under such condition (Fig.3a).However,it showed expected low pH and HAase response.The Schiff base bond was acid-responsive and GOD@SiO2-Arg (FITC labeled GOD) nanoparticles were released from hydrogel after approximate 24 h incubation in the pH 6.8 environment.The release rapidly reached the maximal and about 80% GOD@SiO2-Arg had been released in 240 h.In contrast,DOX@MSN nanoparticles were barely released until HAase was added after 240 h (Fig.3b).When hydrogel was exposed to both low pH and HAase conditions,the pH response was still faster than HAase response (Fig.3c).For individual treatment of pH 6.8 or HAase,GOD@SiO2-Arg and DOX@MSN were both released from the HAase environment(Fig.S1a in Supporting information).GOD@SiO2-Arg was rapidly released from the pH 6.8 environment,but DOX@MSN was only responsive to the HAase environment (Fig.S1b in Supporting information).The esterase response release profile of ester terminated DOX@MSN nanoparticles was also determined.In the presence of esterase,DOX was released completely in 60 min.Fig.3d showed that the color of the centrifuged solution was changed when DOX was released by esterase.Otherwise,the transparent supernatant was presented.GOD@SiO2-Arg-PEG-SH and DOX@MSN-SH showed excellent stability (Figs.S2 and S3 in Supporting information).GOD@SiO2-Arg was able to generate NO in the simulated tumor microenvironment.GOD was released when actived by GSH faster than the Arg release at low pH value.GOD participated in the glucose consumption,functioning as a starving therapy of tumor cells,and H2O2was generated by the catalysis of GOD.Arg was a NO pre-donor to react with H2O2,and the resulting NO functioned as P-gp inhibitor to accumulate the chemotherapy drug in targeted tumor cells.Also,endogenous H2O2in the tumor microenvironment could offer more reactions with Arg to create NO.As can be seen from Figs.3e and f,NO concentration was determined by the spectrophotometer.The amount of NO was calculated according to the standard curve and it had reached 3.0×10-5mol/L after 48 h.
To estimate cellular uptake of the nanoparticles released from the hydrogel,hydrogel leachouts of FITC labeled MSN and RhB labeled GOD@SiO2-Arg were incubated with tumor cells.Laser confocal microscopy and flow cytometry were used to determine cell uptake (Figs.4a and b).Cells showed weak signals when incubated with MSN-FITC or GOD@SiO2-Arg-RhB nanoparticles from hydrogel leachouts (pH 7.4).As expected,the strong red fluorescence signals of GOD@SiO2-Arg-RhB were captured with pH 6.8 hydrogel leachouts.However,when cells were incubated with the HAase hydrogel leachouts which had previously adjusted its pH to 6.8,both strong red and green fluorescence signals were captured.As Fig.4b shows,only 10.5%–11.0% of cells were positive to the nanoparticle hydrogel leachouts.As a comparison,86.0% positive uptake of the GOD@SiO2-Arg-RhB and 32.5% positive uptake of the MSN-FITC were presented.For the HAase and pH 6.8 hydrogel leachouts,GOD@SiO2-Arg-RhB and MSN-FITC uptake ratio have been increased to 87.2% and 77.3%.Meanwhile,the cellular uptake ratio was extremely low when cells were treated with nanoparticle from a pH 7.4 hydrogel leachouts (Figs.S4a and b in Supporting information).These results not only demonstrated that cells could uptake MSN and GOD@SiO2–Arg nanoparticles when hydrogel released these nanoparticles in specific tumor environments but also indicated that only whenin situNO was triggered in advance,chemotherapy has a better effect.h-BMSC and MCF-7/ADR tumor cells were used to evaluate the cell cytotoxicity of the responsive hydrogel.The GOD@SiO2-Arg and DOX@MSN loaded hydrogel was co-cultured with h-BMSC and tumor cells respectively(Fig.4c).CCK-8 reagent was used,and its absorbance at 450 nm wavelength was measured to calculate the cell viability The results showed low cytotoxicity of this hydrogel to normal cells (h-BMSC)compared to tumor cells.Normal cells had more than 90% viability after 12 h treatment.The cell viability of tumor cells decreased continuously with prolonged time.However,the normal cells had barely changed the cell viability during 96 h treatment.the tumor cells had a 10% viability at 96 h.These results proved that the drug-loaded hydrogel has acid and enzyme responses in the tumor environment.The cytotoxicity of 96 h co-culture with different hydrogel or hydrogel leachouts was estimated as well (Fig.4d).The cell viability was still as high as 90% for the HA hydrogel group,indicating that the hydrogel had good biocompatibility to normal cells.GOD@SiO2-Arg loaded hydrogel showed the acid response and the low cell viability caused by the more NO generated inside tumor cells.Naked DOX or hydrogel leachouts (pH 6.8 and HAase pre-treated GOD@SiO2-Arg and DOX@MSN loaded hydrogel for 108 h) of hydrogel contained DOX showed no selective cell inhibition.Thus,GOD@SiO2-Arg and DOX@MSN loaded hydrogel enabled the nanoparticles not only acid response but also enzyme response.These results demonstrated that dual nanoparticles loaded hydrogel has the tumor-selective function and it has the best tumor cell inhibition after 96 h treatment.
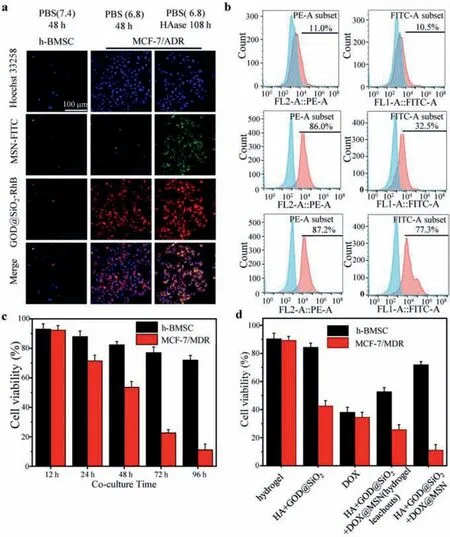
Fig.4.Cell uptake of nanoparticles and cytotoxicity of different nanoparticles loaded hydrogel in vitro.(a) Laser confocal images of cellular uptake efficiency of MSN-FITC and GOD@SiO2-Arg-RhB with different hydrogel leachouts which was dealed with different pH values and HAase.The nucleus was stained by Hoechst 33258.(b) Cellular uptake efficiency of nanoparticles by flow cytometric analysis.(c) Cell viability of tumor cells and h-MBSC cells after co-cultured with drug loaded hydrogel.(d) Cell viability after 96 h co-cultured with drug free hydrogel,GOD@SiO2-Arg loaded hydrogel,GOD@SiO2-Arg and DOX@MSN loaded hydrogel leachouts (pH 6.8 and HAase pre-treated GOD@SiO2-Arg and DOX@MSN loaded hydrogel for 108 h) and GOD@SiO2-Arg and DOX@MSN loaded hydrogel.
To investigate thein vivoanti-cancer effect of the responsive hydrogel,a subcutaneous xenograft tumor model in nude mice was used.The animal experiments were conducted according to regional authority guidelines (SYXK shaan 2015-002)based on China animal-care regulations.As shown in Figs.5a and b,nanoparticles loaded hydrogel treated groups showed significant inhibition on tumor growth compared to the control group of HA-MA-NH2.The hydrogel in the group of HA-MANH2+GOD@SiO2-Arg+DOX@MSN demonstrated the best inhibitory effect among the three groups,and the inhibitory effect of the HA-MA-NH2+DOX@MSN loaded hydrogel was better than that in group of HA-MA-NH2+GOD@SiO2-Arg.The spleen was obtained and weighed at the end of the study and the results in Fig.5c demonstrated no difference.No significant difference was observed among all the groups,demonstrating no visible damage was caused by the gels.For histological observation of tumors from nude mice in different groups,H&E assay was conducted,and the results were shown in Fig.5d.In HA-MA-NH2hydrogel group,a relatively complete cellular structure could be observed,but the cellular structure was destroyed by the hydrogel.The result of the immunohistochemical assay on ki-67 was also shown in Fig.5d.Ki-67 expression in the other three groups was significantly decreased compared to HA-MA-NH2hydrogel group,indicating the decrease of the proliferative cells.The survival assay in Fig.5e demonstrated that the survival time of the animals in the responsive hydrogel groups was longer than that in the pure hydrogel.Most animals survived in the group of HA-MA-NH2+GOD@SiO2-Arg+DOX@MSN loaded hydrogel at the end of the study.Throughout the study,the body weight was also registered and the result was shown in Fig.5f.All these results revealed that the responsive hydrogel for drug delivery is a promising strategy for anti-cancer-therapy.
In summary,we have developed a dual-responsive hyaluronic acid-based nanocomposite hydrogel drug delivery system for overcoming MDR.This hydrogel rapidly was able to release the coreshell SiO2nanoparticles contain Arg and GOD at the low pH tumor-specific environment rapidly,to accumulate the glucose consumption and generate the NO against the drug efflux assisted by P-gp on the MDR tumor cell membrane.MSNs loaded DOX was released from the HA hydrogel by esterase to induce the apoptosis of tumor cells.This HA hydrogel has provided a great synergistic tool that benefits MDR tumors treatment.Thein vitroandin vivoresults confirmed that the sequential release hydrogel could be used to overcome chemoresistance in cancer therapy.These performances make this HA hydrogel promising to achieve enhanced effects in MDR tumor therapy.
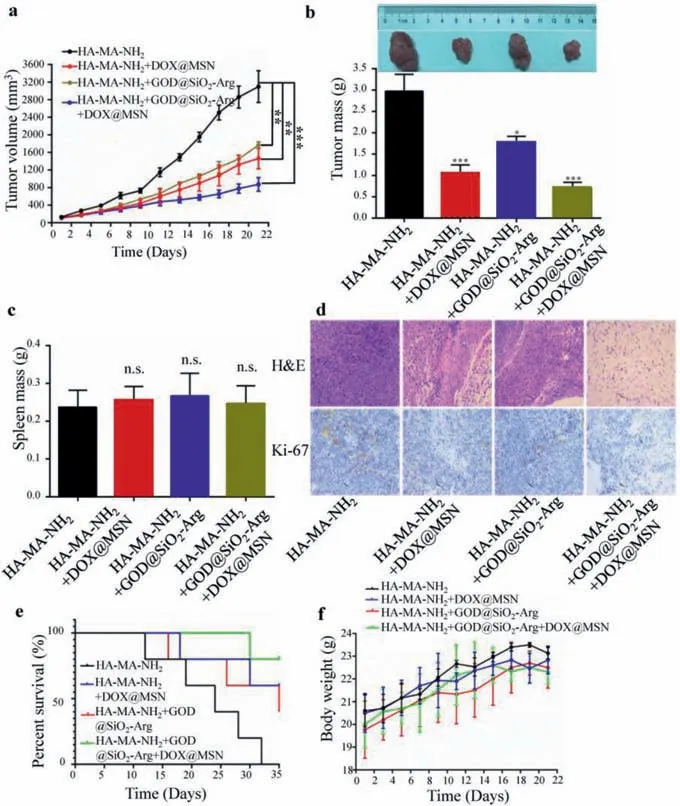
Fig.5.Inhibition of the tumor growth by hydrogel with no visible damage in vivo.(a) Tumor volume changes of different groups.(b) Tumor weight of different groups.(c)Spleen weight of tumors from different groups.(d) Histology of H&E,and immunohistochemical assay (ki-67) of tumors tissue.(e) Survival assay of nude mice in different groups.(f) Body weight of nude mice from different groups.Values are presented as mean ± SD. n=5.*P<0.05,**P<0.01 and ***P<0.001 vs. the control group.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by Sichuan Science and Technology Program (No.2022NSFSC0363),the Introduction Program of Scientific Researcher of Sichuan University of Science & Engineering (No.2020RC40) and Key Laboratories of Fine Chemicals and Surfactants in Sichuan Provincial Universities (No.2020JXY02).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.06.006.
 Chinese Chemical Letters2023年1期
Chinese Chemical Letters2023年1期
- Chinese Chemical Letters的其它文章
- Diabetic wound healing activated by supramolecular cascade reaction
- MBenes: Two-dimensional transition-metal borides with ordered metal vacancies
- Wet-adhesive materials of oral and maxillofacial region: From design to application
- Diverse catalytic systems for nitrogen-heterocycle formation from O-acyl ketoximes
- Fluorine-containing drugs approved by the FDA in 2021
- The development and application of dual-comb spectroscopy in analytical chemistry
