In situ decoration of CoP/Ti3C2Tx composite as efficient electrocatalyst for Li-oxygen battery
Xingzi Zheng,Mengwei Yun,c,Xinqing Hung,Huifeng Li,*,Genn Sun
a Beijing Key Laboratory of Energy Conversion and Storage Materials,College of Chemistry,Beijing Normal University,Beijing 100875,China
b Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology,School of Chemistry & Chemical Engineering,Liaocheng University,Liaocheng 252059,China
c Department of Physics and Applied Optics Beijing Area Major Laboratory,Beijing Normal University,Beijing 100875,China
Keywords:MXene Transition metal phosphide Oxygen adsorption ability Li-oxygen battery Electrocatalyst
ABSTRACT Application of Li-oxygen (Li-O2) battery is in urgent need of bifunctional ORR/OER electrocatalyst.A surface-functionalization CoP/Ti3C2Tx composite was fabricated theoretically,with the optimized electronic structure and more active electron,which is beneficial to the electrochemical reaction.The accordion shaped Ti3C2Tx is featured with large specific surface area and outstanding electronic conductivity,which is beneficial for the adequate exposure of active sites and the deposition of Li2O2.Transition metal phosphides provide more electrocatalytic active sites and present good electrocatalytic effect.The CoP/Ti3C2Tx composite served as the electrocatalyst of Li-O2 battery reaches a high specific discharge capacity of 17,413 mAh/g at 100 mA/g and the lower overpotential of 1.25 V,superior to those of the CoP and Ti3C2Tx individually.The composite of transition metal phosphides and MXene are applied in Li-O2 battery,not only demonstrating higher cycling stability of the prepared CoP/Ti3C2Tx composite,but pointing out the direction for their electrochemical performance improvement.
Li-O2battery has received much attention for its high theoretical energy density (3500 Wh/kg) [1,2].The reaction principle of typical Li-O2battery is Li2O2-based reversible formation and decomposition [3].Li2O2,the product of discharge,is dielectric and insoluble,whose accumulation on the cathode surface will impede the transport of oxygen,electrons and lithium ions,thus limiting oxygen reduction reaction (ORR) and oxygen evolution reaction(OER) and causing slow kinetics of electrochemical reaction,high over potential,poor cycling stability,etc.[4,5].Over the past few decades,many studies have focused on cathodic catalysts to effectively promote Li2O2decomposition and enhance the performance of Li-O2battery [6–10].
MXene is an emerging 2D layered compound material made from metal carbides or nitrides.It is an ideal substrate material for development of high-performance electrocatalysts because of its high electrical conductivity,large surface area and extensive chemical species [11–13].Domestic and foreign researchers have done much work on it with respect to energy storage and conversion[14–24].To meet actual demand,we are also in urgent need of electrocatalysts that can catalyze OER and ORR at the same time so as to enhance its performance in Li-O2battery.
Transition metal phosphides (TMPs) have been receiving more and more attention for their excellent electrocatalytic activity [25–27].Systematic research has been done on the use of nickel phosphide,ferrous phosphide,molybdenum phosphide and cobalt phosphide as catalysts.Nonetheless,taking CoP for example,the binding energy of Co-P compound are negative,indicating that it is thermodynamically stable.Density functional theory calculations show that CoP exhibits metallic characteristics,and the bonding behavior between Co and P atoms in Co-P compounds is a combination of covalent and ionic properties [28].Therefore,CoP can be used as electrocatalyst because of its excellent redox performance.However,its performance is still unsatisfactory mainly for its poor electrical conductivity [29,30].Therefore,we may combine it with a high conductive material to enhance its electrical conductivity,thus improving its reactivity and stability.
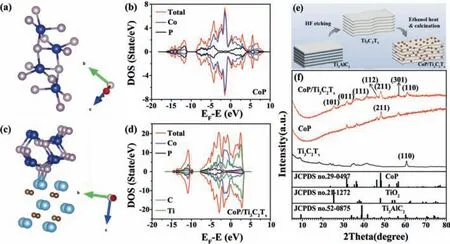
Fig.1.The optimized structures of (a) CoP and (c) CoP/Ti3C2Tx,total density of states and partial density of states of (b) CoP and (d) CoP/Ti3C2Tx structures.(e) Schematic diagram of preparation process of CoP/Ti3C2Tx.(f) XRD pattern of CoP/Ti3C2Tx,CoP and Ti3C2Tx.
Here,we develop a simple method to grow CoP nanoparticles (CoP NPs) on the surface of MXene and between the layers,thereby forming the CoP/MXene composite catalyst.It not only prevents CoP-NPs clustering and provides enough active sites,but also significantly improves the accessibility of electrolytes and promotes charge/mass transfer and quick release of gasses during electrocatalysis.The total density of states (TDOS) was used to simulate and evaluate the electrochemical activity.Figs.1a and c were schematic diagrams of the optimized electronic structure.The TDOS for CoP and CoP/Ti3C2Txcomposite were investigated as shown in Figs.1b and d.The TDOS of the CoP/Ti3C2Txremained the metallic properties of Ti3C2Txwith the DOS passing the Fermi energy.And CoP/Ti3C2Txdemonstrated the high DOS at Fermi energy than that of CoP,indicating that CoP/Ti3C2Txwas endowed with more active electron,which were easy to be accepted and lost and facilitated the electrochemical reaction [31].
The preparation process of CoP/Ti3C2Txcomposite was shown in Fig.1e.With the influence of HF etching,the Al atom layer of Ti3AlC2was successfully removed.Ti3C2Txwas mixed with Co(AC)2·4H2O and heated at 120 °C for 10 h,and then the obtained CoP/Ti3C2Txcomposite was prepared successfully by calcinated at 500 °C in sodium hypophosphite atmosphere,which was also confirmed by the X-ray diffraction (XRD) results in Fig.1f.With the effect of HF etching,the characteristic peak at 39.0° disappeared,which proved that Al atom layered in Ti3AlC2was removed and the formation of Ti3C2Tx.The accordion-like lamella of Ti3C2Txprovides growth sites for the synthesis of CoP/Ti3C2Txcomposite.The peaks at 31.6°,36.3°,46.2°,48.1° and 56.8° were corresponding to(011),(111),(112),(211) and (301) lattice planes of CoP (JCPDS No.29–0497),indicating that CoP was grown on the composite.The peak at 60.7°,as the laminate peak of Ti3C2Tx,remained in the CoP/Ti3C2Txcomposite,which proved that the laminate structure of Ti3C2Txwas not destroyed with the high-temperature calcination [32,33].The decrease of exposure intensity of Ti3C2Txin composite is due to better crystallinity and higher diffraction intensity of CoP sample.The successful preparation of the composite was also verified by Raman spectrum.Fig.S1 (Supporting information) presented a Raman spectrum of CoP.Several detectable signals were shown on small Raman shifts [34,35],and there were two distinguishable Raman activity patterns in CoP/Ti3C2Txcomposite,including characteristic peaks of CoP and Ti3C2Tx,which proved the correct synthesis of CoP/Ti3C2Txcomposite.
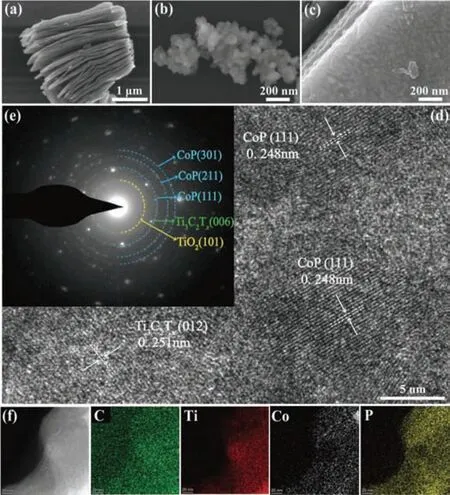
Fig.2.The SEM images of (a) Ti3C2Tx,(b) CoP and (c) CoP/Ti3C2Tx composite.The(d) HRTEM,(e) SAED and (f) EDS of CoP/Ti3C2Tx composite.
The microstructure and morphology of CoP/Ti3C2Txwere observed through scanning electron microscopy (SEM) and highresolution transmission electron microscopy (HRTEM).Compared with block Ti3AlC2(Fig.S2 in Supporting information),the layer spacing of Ti3C2Tx(Fig.2a) increased by the influence of HF etching.The lattice spacing of 0.251 nm were attributed to the (012)plane of the accordion-like Ti3C2Tx(Fig.S3 in Supporting information).The prepared CoP NPs showed obvious agglomeration phenomenon without adding the substrate of Ti3C2Tx(Fig.2b).It was obvious that the size distribution of CoP NPs was about 100 nm(Fig.S4 in Supporting information).In contrast,with the addition of Ti3C2Txsubstrate,the structure of MXene layer remained original after hydrothermal and calcination treatment,which provided enough space for loading of CoP NPs uniformly (Fig.2c).The HRTEM of CoP/Ti3C2Tx(Fig.2d) showed that the lattice spacing of Ti3C2Tx(012) was 0.251 nm.Lattice stripes at a spacing of 0.248 nm were attributed to the (111) plane of CoP NPs (JCPDS No.29–0497).The selected area electron diffraction (SAED) proved that the prepared CoP/Ti3C2Txhad good crystallinity and the single crystal electron diffraction of Ti3C2Tx(Fig.2e).The diffraction rings of CoP were consistent with (111),(211) and (301) planes.The uniform distribution of CoP NPs on Ti3C2Txwas further demonstrated by energy dispersive X-ray spectrometer (EDS) (Fig.2f).The above characterization confirmed the successful preparation of CoP/Ti3C2Txcomposite.
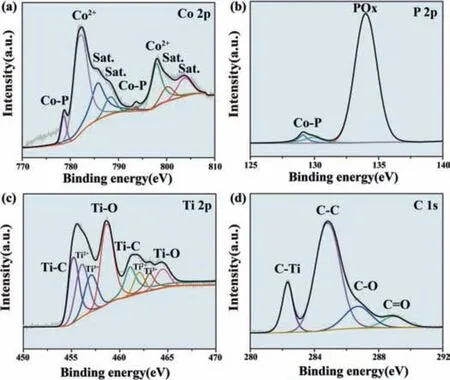
Fig.3.The XPS spectra at high resolution of (a) Co 2p,(b) P 2p,(c) Ti 2p,(d) C 1s of CoP/Ti3C2Tx composite.
In order to clarify the composition and valence distribution of elements in CoP/Ti3C2Txcomposite,we analyze the electrocatalyst by XPS.Fig.S5 (Supporting information) showed the presence of Ti,C,O,Co and P elements in the measured spectra.In Co 2p spectra (Fig.3a),the two peaks located at 778.7 and 793.7 eV were identified with Co-P bond in CoP [36],while 781.3 and 798.0 eV correspond to the oxidized Co (Co2+) species,which caused by the partial oxidation process on CoP species [37].Fig.3b presented the XPS spectra of P 2p.The two peaks which were located at 129.1 eV and 129.9 eV were subject to the Co-P bond and the binding energy at 133.8 eV corresponded to the POxspecies,respectively [38].Compared with Ti3C2Tx,metal-oxygen bonds were not identified,indicating the surface-functionalization process derived product covered the surface of Ti3C2Txfurther constructing the composite.In the spectrum of Ti 2p (Fig.3c),the peaks at 455.1,456.0,457.2 and 458.7 eV of Ti 2p3/2correspond to Ti-C,Ti2+,Ti3+and Ti-O,respectively[39].Compared with Ti 2p in Ti3C2Tx(Fig.S6 in Supporting information),the intensity of Ti-O peak in CoP/Ti3C2Txincreased notably,which caused by partial oxidation of Ti atoms in Ti3C2Tx.In Fig.3d,the spectrum of C 1s were matched to four peaks located at 282.2,284.8,286.7 and 288.9 eV which belonged to C–Ti,C–C,C–O and C=O bonds,respectively [40].
Moreover,it is well known that the specific surface area and pore size are significant to electrocatalyst performance.They were studied by N2adsorption-desorption isotherm.The specific surface area of CoP/Ti3C2Txand Ti3C2Txwere 77.31 m2/g and 24.04 m2/g,respectively (Figs.S7a and b in Supoprting information).The N2adsorption-desorption isotherm of CoP/Ti3C2Txshowed a typical hysteresis loop which caused by the presence of mesoporous.The specific surface area of CoP/Ti3C2Txincreased obviously,which was mainly due to the adhesion of CoP NPs between the surface and interlayers of Ti3C2Tx.The increased specific surface area was beneficial to fully expose the active site,which can improve the performance of Li-O2battery.Figs.S7c and d (Supoprting information) exhibited the pore size of CoP/Ti3C2Txand Ti3C2Txobtained by the BJH method.The pore size of Ti3C2Txwas about 2.440 nm,and CoP/Ti3C2Txcomposite was mostly 2.441 nm.The corresponding pore size distribution of Ti3C2Txwas located in the mesoporous range which was due to the aggregation of accordionlike nanosheets [6].These results suggested that the composition of the CoP and Ti3C2Txcould prevent the aggregation of the CoP particles and provide the more and more active sites.
As a new 2D material,Ti3C2Txfeatures a high specific surface area,superior electronic conductivity and adjustable components,etc.,which can facilitate oxygen adsorption in a reaction process of Li-O2battery and provide a proper space for discharge products storage.With good ORR/OER catalytic activity,CoP has the potential to reduce overpotential on charge process of Li-O2battery.Therefore,CoP/Ti3C2Txis expected to become an outstanding cathode material suitable for Li-O2battery.To explore electrochemical mechanism of Li-O2battery with CoP/Ti3C2Txcathode,electrochemical performance testing was carried out.As can be observed from Fig.4a,Li-O2battery with CoP/Ti3C2Txcathode provided a high discharge capacity of 17,413 mAh/g in the 1stcycle at 100 mA/g,and in the 2ndand 3rdcycles,their discharge capacity reached 11,060 mAh/g and 8536 mAh/g,respectively,which was superior to CoP and Ti3C2Tx(Fig.S8 in Supoprting information).The reason why specific capacity declines in such three cycles may be that it is difficult for discharge products to be completely decomposed during charge and thus no novel cathodes can be provided in next cycle.In comparison with CoP/Ti3C2Tx,specific capacity performance of pure CoP or Ti3C2Txwas poor;in the first cycle,their specific discharge capacity were proved to be 10,560 mAh/g and 9362 mAh/g,respectively.According to Fig.4b and Fig.S9 (Supporting information),discharge capacity of Ti3C2Tx,CoP and CoP/Ti3C2Txin the initial cycle were compared in diverse electric current densities.In the case where CoP/Ti3C2Txwas used as a cathode catalyst,the corresponding specific discharge capacity were high up to 17,413 mAh/g,9970 mAh/g and 8209 mAh/g at 100,200 and 500 mA/g,which were above those of pure CoP (10,560 mAh/g,9115 mAh/g and 6462 mAh/g) or pure Ti3C2Tx(9362 mAh/g,7215 mAh/g and 5529 mAh/g).This manifests that CoP/Ti3C2Txcomposite can effectively improve electrochemical performance of Li-O2battery.By contrast to CoP/Ti3C2Tx,Ti3C2Txwith good conductivity was short of highly active sites and thus showed insufficient electrochemical catalytic activity,enabling Li-O2battery with Ti3C2Txcathode to fail to run stably and efficiently.Even when the electric current density reached up to 500 mA/g,CoP/Ti3C2Txelectrocatalyst that combined high conductivity of Ti3C2Txand catalytic activity of CoP NPs still remained its discharge capacity at 8209 mAh/g.As presented in Fig.4c,the overpotential of CoP/Ti3C2Txcathode was 1.25 V,lower than pure CoP (1.36 V) or pure Ti3C2Tx(1.56 V),which provided that the electric current density was 500 mA/g and the specific capacity limited to 500 mAh/g.In line with such a phenomenon,it is clear that CoP/Ti3C2Txcomposite possess higher activity of ORR and OER.The cycle performance was tested as shown in Fig.4d.The CoP/Ti3C2Txcathode was able to stably run for 40 cycles at 500 mA/g.Moreover,the cyclic stability of CoP/Ti3C2Txwas significantly better than CoP and Ti3C2Tx(Fig.S10 in Supoprting information).
To learn about electrochemical catalysis of CoP/Ti3C2Txin Li-O2battery,cyclic voltammetry (CV) based tests were performed,as given in Fig.4e.The initial redox potential and the peak current intensity of CoP/Ti3C2Txwere respectively above those of pure CoP or pure Ti3C2Tx,signifying that its ORR/OER catalytic activity was superior to Ti3C2Tx.Electrochemical impedance spectroscopy(EIS) is aimed at profoundly investigating electrochemical performance of CoP/Ti3C2Txcomposite.Nyquist plots of CoP,Ti3C2Txand CoP/Ti3C2Txbefore the cycle have been depicted in Fig.4f.All EIS images were semi-circles at high frequency,but become linear at low frequency.Relevant fitting data were listed in Table S1(Supporting information).In this table,the electrolyte resistance of Ti3C2Tx(Rs=11.80Ω) was significantly lower than CoP (Rs=12.26Ω) and CoP/Ti3C2Tx(Rs=12.03Ω).With regard to charge transfer impedance,Ti3C2Tx(Rct=29.74Ω) was apparently lower than CoP (Rct=44.93Ω) and CoP/Ti3C2Tx(Rct=30.79Ω).However,the impedance of CoP/Ti3C2Txwas significantly lower than that of CoP,showing that the introduction of Ti3C2Txprovided a conductive substrate for CoP NPs.Results described above indicate that Ti3C2Txcan effectively improve electronic conductivity of cathodes.Better performance of CoP/Ti3C2Txcomposite is derived from outstanding electrical conductivity of Ti3C2Txand active catalytic sites of CoP NPs which prevent termination of electrochemical reactions.
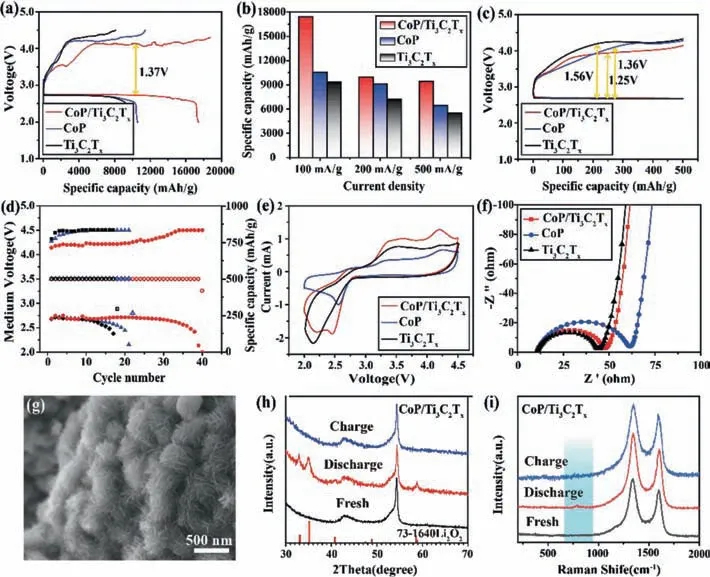
Fig.4.(a) The first cycle discharge-charge profiles of CoP/Ti3C2Tx,CoP and Ti3C2Tx cathodes at 100 mA/g.(b) The comparison of the rate performance of the CoP/Ti3C2Tx,CoP and Ti3C2Tx cathodes.(c) The first discharge-charge curves and (d) cyclic performance diagram of the medium voltage and discharge-charge capacity with 500 mAh/g capacity at 500 mA/g.(e) The CV curves and (f) the EIS of CoP/Ti3C2Tx,CoP and Ti3C2Tx cathodes.(g) The SEM of discharge products.(h) The XRD and (i) Raman of CoP/Ti3C2Tx at different states.
To explore the discharge-charge products of Li-O2battery for deeply understanding of electrochemical mechanism,the morphology changes of the CoP/Ti3C2Tx,CoP and Ti3C2Txelectrodes in the first cycle were observed in Fig.4g and Fig.S11 (Supporting information).The fresh electrode showed that the clear accordion-like surface.After the first complete discharge process (Fig.4g),a large number of flower-like discharge products deposited by nanosheets were generated on the surface of CoP/Ti3C2Tx.The reason for such flower-like discharge product is that the strong interaction between CoP/Ti3C2Txand Li2O2makes it difficult for external Li2O2monomers to aggregate into large Li2O2,leading to the isolation of Li2O2on the surface of CoP/Ti3C2Txand easier decomposition during charging process [41].However,the contact interface between Ti3C2Txand discharge products is unsatisfied,resulting in poor electron and oxygen transport in the subsequent charging process.In addition,the discharge products with small size and discrete distribution lead limited discharge capacity [42].Therefore,CoP/Ti3C2Txcomposite exhibited low overpotential and high discharge capacity.According to the XRD analysis (Fig.4h and Fig.S12 in Supporting information),there were several characteristic peaks after the first discharging.The peaks at 32.8°,34.8° and 58.5°were attributed to the crystal planes of (200),(201) and (220) in Li2O2(JCPDS No.73–1640),proving that the characteristic diffraction peaks of Li2O2were very obvious after discharging and peaks of Li2O2had disappeared on the electrode surface after charging.This result was also demonstrated by Raman spectroscopy (Fig.4i),the characteristic peak of Li2O2at 793 cm-1is obviously [7].After the charging process,the flower-shaped discharge product disappeared and the accordion-like CoP/Ti3C2Txstructure with nanoparticles grown on the surface recovered,indicating that Li2O2had completely decommissioned during the charging process,and the electrode catalyzed by CoP/Ti3C2Txhad good catalytic ability of ORR and OER with the proposed mechanism.Fig.S11 showed the surface topography of the Ti3C2Txelectrodes products.It was visibly that the number of toroid-shaped materials on the accordion Ti3C2Txwas very small after the first discharge,which meant the Ti3C2Txcathodes didn’t have enough capability to catalytic conversion more discharge product.High conductive Ti3C2Txstructure was used as conductive network,which promoted the transfer rate of electrons and Li+.CoP NPs with high catalytic activity spread evenly between the surface of CoP/Ti3C2Txlayers,which increased specific surface area of the material to accommodate more discharge product,thus the capacity of the electrode reaction was improved to achieve long cycle.
To sum up,CoP/Ti3C2Txcomposite with more active electron was designed through theoretical simulation,and the CoP NPs were uniformly distributed on the surface and interlayer of Ti3C2Tx.When using the prepared CoP/Ti3C2Txas an electrocatalyst of Li-O2battery,it not only provided high specific discharge capacity (17,413 mAh/g at 100 mA/g) but also exhibited cyclic stability and low overpotential (1.25 V).As CoP/Ti3C2Txcomposite possess certain advantages,such as the superior electronic conductivity of MXene together with excellent electrocatalytic activity of CoP,it is appropriate material that can be adopted as electrocatalyst in Li-O2battery.In summary,this study provides a prospective strategy for designing MXene-based nanocomposites applied in cathode catalysts of Li-O2battery.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Science Foundations of China (Nos.21871028,21771024) and China Postdoctoral Science Foundation (No.2020M680430).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.01.045.
 Chinese Chemical Letters2023年1期
Chinese Chemical Letters2023年1期
- Chinese Chemical Letters的其它文章
- Diabetic wound healing activated by supramolecular cascade reaction
- MBenes: Two-dimensional transition-metal borides with ordered metal vacancies
- Wet-adhesive materials of oral and maxillofacial region: From design to application
- Diverse catalytic systems for nitrogen-heterocycle formation from O-acyl ketoximes
- Fluorine-containing drugs approved by the FDA in 2021
- The development and application of dual-comb spectroscopy in analytical chemistry
