PTEN inhibitor bisperoxovanadium protects against noise-induced hearing loss
Bei Fan ,Fei Lu ,Wei-Jia Du ,Jun Chen,Xiao-Gang An,Ren-Feng Wang,Wei Li,Yong-Li Song,Ding-Jun Zha ,Fu-Quan Chen
Abstract Studies have shown that phosphatase and tensin homolog deleted on chromosome ten (PTEN) participates in the regulation of cochlear hair cell survival.Bisperoxovanadium protects against neurodegeneration by inhibiting PTEN expression.However,whether bisperoxovanadium can protect against noiseinduced hearing loss and the underlying mechanism remains unclear.In this study,we established a mouse model of noise-induced hearing loss by exposure to 105 dB sound for 2 hours.We found that PTEN expression was increased in the organ of Corti,including outer hair cells,inner hair cells,and lateral wall tissues.Intraperitoneal administration of bisperoxovanadium decreased the auditory threshold and the loss of cochlear hair cells and inner hair cell ribbons.In addition,noise exposure decreased p-PI3K and p-Akt levels.Bisperoxovanadium preconditioning or PTEN knockdown upregulated the activity of PI3K-Akt.Bisperoxovanadium also prevented H2O2-induced hair cell death by reducing mitochondrial reactive oxygen species generation in cochlear explants.These findings suggest that bisperoxovanadium reduces noise-induced hearing injury and reduces cochlear hair cell loss.
Key Words:acoustic trauma;Akt;oxidative stress;bisperoxovanadium;cochlear hair cells loss;inner hair cell ribbons loss;noise exposure;permanent threshold shift;phosphatase and tensin homologue deleted on chromosome ten;phosphatidylinositol 3 kinase;siPTEN
Introduction
Noise exposure is a worldwide concern,especially in industrialized countries,and is one of the major contributors to hearing loss.Noise-induced hearing loss (NIHL) greatly disrupts individual life and is a burden on the economy.Exposure to high-strength forms of noise produces irreversible hearing loss,also named permanent threshold shift (PTS).Cochlear hair cell loss is the typical pathological feature of noise-induced PTS trauma,and lost hair cells cannot regenerate.However,the underlying pathological mechanisms and effective pharmacological interventions to protect against NIHL are not enti rely clear.
Phosphatase and tensin homolog (PTEN) is a tumor suppressor gene that plays a role in multiple physiological processes,including cell proliferation,growth and survival (Simpson and Parsons,2001;Li et al.,2022).PTEN expression has been reported to exhibit a specific pattern in cochlear hair cells and sensory neurons,suggesting that PTEN plays a role in hearing function (Dong et al.,2010a).A previous study described a reduction of phosphatidylinositol 3 kinase (PI3K)-Akt survival signaling in aging cochlear hair cells caused by an increase in PTEN level (Sha et al.,2010).Strong evidence indicates that the PI3K-Akt signaling pathway maintains hair cell integrity and contributes to the protection mechanisms of cochlear hair cells under stress conditions,such as aminoglycoside or noise (Brand et al.,2011;Chen et al.,2015;Jadali et al.,2017;Kucharava et al.,2019).
Specific PTEN-inhibiting drugs have been shown to exert protective effects on diseases including neurodegeneration,ischemic brain injury,and metabolic disorders (Pulido,2018).Bisperoxovanadium (bpV),a compound of vanadate derivatives,is a known inhibitor of PTEN.A previous study reported that bpV attenuates cell apoptosis and accelerates neural functional recovery (Schmid et al.,2004).In addition to activating Akt prosurvival signaling to confer resistance to oxidative stress-induced cell death in neurons (Chen et al.,2012b),bpV activates PI3K-Akt signaling in cochlear explants and increases the survival of hair cells experiencing ototoxic damage (Jadali and Kwan,2016).However,the precise mechanism and function of PTEN in cochlear hair cells after noise exposure have not been clarified.
In this study,we explored the effect of PTEN inhibition against noise-induced hearing damage and hair cell loss,and investigated the underlying mechanism involving PI3K-Akt signaling and oxidative stress.
Methods
Animals and bpV pretreatment
Male CBA/J mice,weighing 25 ± 5 g and 6 weeks old,were obtained from the Bikai Laboratory Animals Co.,Ltd (Shanghai,China;license No.SCXK(Hu) 2018-0006),and raised and supervised at specific-pathogen-free level by professional staff at the Laboratory Animal Center of the Air Force Medical University.Male mice have usually been used in models to study noise damage (Chen et al.,2012a).The animal housing environment was 20–25°C,40–70% humidity,air cleanliness level 7,and five mice per cage.Every experimental operation protocol was performed in accordance with the regulations of Animal Care and Use Committee under the management of the Air Force Medical University (No.IACUC-20210159);approval was received on January 1,2021.Animals were randomly divided into control,DMSO,noise exposure,noise+DMSO,and noise+bpV groups.In vivoexperimental designs are shown inFigure 1A.
At 9 weeks of age,all mice were subjected to auditory brainstem response(ABR) testing before noise exposure to confirm the baseline auditory threshold.At 10 weeks of age,the mice were intraperitoneally injected with dimethyl sulfoxide (DMSO) or bpV once a day for 3 days before noise exposure.The PTEN inhibitor bpV (HOpic;Cat# sc-221377,Santa Cruz Biotechnology,Santa Cruz,CA,USA) was dissolved in DMSO and stored in a freezer at–2°C.The bpV stock solution was diluted with 0.9% normal saline for intraperitoneal injection.The final concentration of bpV was 400 µg/kg body weight (Walker et al.,2015).DMSO group animals were treated with the same concentration of DMSO (400 µg/kg).
Noise exposure and auditory brainstem response
Every animal was put in a circular wire cage (9 cm in diameter) separately,and given food and water as usual.Animals were subjected to a broadband noise ranging from 2 to 20 kHz at 105 dB sound pressure level for 2 hours,generating a PTS.A sound exposure chamber was installed to create noise using a loudspeaker (model 2450H,JBL,Los Angeles,CA,USA) driven by a power amplifier (AX-500U,Yamaha,Maebashi,Japan).Audio electronic documents were created and processed using audio editor soft ware.A sound level meter (TES 1350A,Taiwan,China) was used to adjust the noise exposure level at different positions in the sound chamber and to confirm sound consistency during noise exposure.The control group received no noise exposure.
ABR measurement was accomplished in a quiet space.The mice were anestheti zed by intraperitoneal injection of a mixture of ketamine (100 mg/kg)and xylazine (10 mg/kg) and placed in a sound isolated chamber.ABR measurement was used to assess hearing with or without noise exposure by applying the RZ6 system (Tucker-Davies Technologies,Gainesville,FL,USA)at multiple frequencies including click,8,16,24,and 32 kHz.ABR thresholds were assessed by the same researcher observing sti mulus level responses and were determined by analyzing ABR wave I amplitudes from saved waveforms.
Intratympanic delivery of PTEN silencing RNA
Predesigned PTEN silencing RNA (siRNA) (Cat# C705265700,Gene Pharma,Shanghai,China) was prepared and delivered into the adult mouse inner ear.Mice were anesthetized by intraperitoneal injection of a mixture of ketamine (100 mg/kg,Cat# 33643479,Macklin,Shanghai,China) and xylazine(10 mg/kg,Cat# 7361617,Sangon Biotech,Shanghai,China) (Wu et al.,2020) and placed on a heating pad to maintain body temperature during the surgery.The process of intratympanic delivery of PTEN siRNA (siPTEN)was performed as described in our previous study (Oishi et al.,2013).A retroauricular incision was made to expose the temporal bone.The otic bulla and facial nerve were identified and a small hole in the thin part of the otic bulla was created and enlarged to visualize a round window.A syringe connected to a customized sterile micro medical tube was used to slowly deliver 10 µL of siPTEN (60 µg/mL) or siScramble (siControl) into the round window,and the hole was covered with surrounding muscle.Finally,the small incision was sutured and the animal was maintained in the surgical position for 1 hour.Noise exposure began 72 hours after siPTEN or siControl delivery.The sequence of PTEN siRNA was 5′-GGA UUC GAC UUG ATT-3′.
Cochlear tissue preparation
After noise exposure for 1 hour,mice were anestheti zed by intraperitoneal injection of a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) and then dissected.Their temporal bones were removed and immersed in 4%paraformaldehyde solution at 4°C for 24 hours.Cochlear tissues were treated in a 4% sodium ethylene diamine tetraacetic acid solution for 3–4 days for decalcification.Cochlear specimens were dehydrated with 30% sucrose solution at 4°C overnight,embedded in opti mum cutting temperature glue,and cut into 10-µm-thick cochlear cryosections.
Immunohistochemistry of cochlear cryosections
Staining procedures were performed as described previously (Chen et al.,2020).Sections were incubated with 1% Triton X-100 in phosphate buffered saline (PBS) for 10 minutes,washed three times,and then blocked in 10%normal goat serum for 30 minutes at 37°C.Specimens were incubated with monoclonal rabbit anti -PTEN (1:100,Cell Signaling Technology,Danvers,MA,USA,Cat# 9188,RRID: AB_2253290) for 48 hours at 4°C,washed three times,and incubated overnight with Alexa Fluor 594 secondary anti body (1:200,Cat#A21207,Invitrogen/Life Technologies,Carlsbad,CA,USA,RRID: AB_141637)in the dark at 4°C.Then,4′,6-diamidino-2-phenylindole (DAPI;Cat# D9542,Sigma,St.Louis,MO,USA) was used to stain nuclei for 10 minutes.Samples were imaged and observed under a confocal microscope (FV 1000,Olympus,Tokyo,Japan).
Immunocytochemistry for hair cells
For immunohistochemistry of hair cells,cochlear samples decalcified with 4% ethylene diamine tetraacetic acid were dissected under a microscope to leave the sensory epithelium.Cochlear specimens were permeabilized using 1% Triton X-100 solution for 10 minutes,and washed with PBS solution threetimes for 5 minutes each.Specimens were blocked in bovine serum albumin solution for half an hour at room temperature and incubated with primary antibodies for 2 days at 4°C at the concentration of 1:100.The primary anti bodies were monoclonal rabbit anti -PTEN (Cell Signaling Technology,Cat#9188,RRID: AB_2253290),monoclonal rabbit anti -PI3K-p110α (Cell Signaling Technology,Cat# 4249,RRID: AB_2165248),polyclonal rabbit anti-PI3Kp85α (Cell Signaling Technology,Cat# 4292,RRID: AB_329869),monoclonal rabbit anti -phospho-Akt (Ser 473) (Cell Signaling Technology,Cat# 4060,RRID:AB_2315049) and polyclonal rabbit anti-4 hydroxynonenal (4HNE;Abcam,Cambridge,UK,Cat# ab46545,RRID: AB_722490).After the samples were rinsed with PBS,they were incubated with donkey anti -rabbit IgG secondary anti body Alexa Fluor 594 (1:200,Invitrogen/Life Technologies,Cat# A21207,RRID: AB_141637) for 24 hours at 4°C,stained with phalloidin at 1:200 (Cat#SLBP3636V,Sigma) for 30 minutes to label hair cells,and stained with DAPI(Cat# D9542,Sigma) for 10 minutes to label nuclei morphology.Samples were mounted on slides using anti -fade mounting media and imaged under a confocal microscope.
Quantification of immunofluorescence and hair cell counting
The relative fluorescence intensity of hair cell signals and hair cells were quantified as described previously (Wen et al.,2015) from confocal images taken under identical conditions and setting parameters using ImageJ V1.43 software (National Institutes of Health,Bethesda,MD,USA) (Schneider et al.,2012).Outer hair cells (OHCs) and inner hair cells (IHCs) were observed in each 0.24-mm2frame field under an upright microscope,beginning at the apex and moving down to the base.The percentage of missing hair cells was calculated and plotted as a function of distance from the apical turn to the basal turn of the organ of Corti .
Immunolabeling of inner hair cell synapses and quantification of synaptic ribbons
Following decalcification with 4% ethylene diamine tetraacetic acid,the cochlear samples were dissected under a microscope to leave the sensory epithelium.For immunolabeling of IHC synaptic ribbons,the stained presynaptic ribbons were incubated with primary monoclonal mouse anti body C-terminal-binding protein 2 (CtBP2;1:100,BD Biosciences,Franklin,NJ,USA,Cat# 612044,RRID: AB_399431) at 4°C in the dark overnight,and then with donkey anti -mouse IgG secondary anti body Alexa Fluor 594 (1:200,Invitrogen/Life Technologies,Cat# A21203,RRID: AB_141633) at 4°C for 24 hours.Nuclei were stained with DAPI to identify the position of hair cells.The number of presynaptic ribbons labeled with CtBP2 was quantified from original confocal images and counted in each 0.12-mm segment (containing about 16 IHCs) using ImageJ soft ware.
Western blotting and quanti tative reverse transcriptase-polymerase chain reaction
Cochlear tissues were removed and dissected in ice-cold PBS.Total protein was extracted using a protein extraction kit,and protein concentration was determined using the Micro BCA protein assay kit (Cat# CW0014S,Cwbio,Beijing,China).Electrophoretic gel was made and sodium dodecyl sulfatepolyacrylamide gel electrophoresis was carried out to separate protein samples,which were then electrically transduced onto polyvinylidene fluoride membranes.Then,the membranes were soaked in a blocking solution composed of 5% nonfat dried milk for 1 hour,incubated with primary antibodies as described previously,and incubated with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) anti body (rabbit,1:1000,Cell Signaling Technology,Cat# 8884,RRID: AB_11129865) for 24 hours at 4°C.After three washes with 1× Tris-buffered saline-Tween buffer solution,membranes were incubated with the corresponding polyclonal secondary anti body horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2000,Millipore,Boston,MA,USA,Cat# 12348,RRID: AB_390191) for 1 hour at 37°C.After three washes,bands were visualized using the chemiluminescence substrate reagent (Cat#WBKLS0500,Millipore) and analyzed using ImageJ soft ware.
Cochlear total RNA was extracted using TRIzol (Cat# 15596018,Life Technologies) and complementary DNA was acquired using RevertAid Master Mix (Cat# M1632,Thermo Fisher Scientific,Waltham,MA,USA) following the manufacturer’s instructions.qRT-PCR was performed using SYBR Premix Ex Taq (Cat# RR820A,TaKaRa Bio,Beijing,China).Real-time PCR reaction conditions were as follows: initi al denaturation at 95°C for 5 minutes followed by 45 cycles of 10-second denaturation at 95°C,20-second annealing at 60°C,and 20-second extension at 72°C.mRNA expression was normalized to that of GAPDH and the results were analyzed using the cycle threshold(2–∆∆Ct) method (Livak and Schmittgen,2001).The following primer sequences were used.PI3K forward primer: 5′-AAC CTT CAA CTC TGT GGT TGA GTT A-3′,reverse primer: 5′-CAG CTT GGG GTT GTA CTG AGC T-3′;Akt forward primer:5′-AGG AGG GCT GGC TGC ACA AA-3′,reverse primer: 5′-AGG CCG TTC CTT GTA GCC AAT AAA-3′;GAPDH forward primer: 5′-GAG AAA CCT GCC AAG TAT GAT GAC-3′,reverse primer: 5′-AGA GTG GGA GTT GCT GTT GAA G-3′.
Cochlear explant culture and treatment with H2O2 and bpV
Cochlear explants from 30 postnatal day 3 Sprague-Dawley rats (the Laboratory Animal Center of the Air Force Medical University) were sacrificed after anesthesia by 70% ethanol (Chen et al.,2009).Cochlea were obtained forin vitroculture,and the explant culture was performed as described in our previous study (Fan et al.,2020).Next,the cochlea basilar membranes were dissected and incubated in the culture medium at 37°C and 5% CO2for 12 hours.On the following day,the culture medium was refreshed (2 mL fresh serum-free Basal Medium Eagle) with or without the PTEN inhibitor bpV at the dose of 10 µM for 24 hours.H2O2treatment was performed to induce oxidative stress (Xiong et al.,2021).Explants were pretreated with bpV to test the prevention effects,and then were incubated with H2O2at the doses of 0,0.2,and 0.5 mM for 5 hours.In vitroexperimental designs of cochlear explant culture are shown inFigure 1B.
Mitochondrial reactive oxygen species detection
Mitochondrial reactive oxygen species (ROS) were detected using MitoSOX Red (Cat# M36008,Invitrogen,Carlsbad,CA,USA) following the manufacturer’s instructions.After culture,explants were fixed with 4%paraformaldehyde for 30 minutes and subjected to immunofluorescence staining as previously described.The explant specimens were incubated with anti -myosin VIIa (1:200,Cat# 256790,Proteus Biosciences,Ramona,CA,USA)and phalloidin to label hair cells.
Stati stical analysis
No statistical methods were used to predetermine sample sizes;however,our sample sizes were similar to those reported in a previous publication(He et al.,2021).No animals or data points were excluded in the analysis.Experimental data are shown as the mean ± standard deviation.Unpaired Student’st-test was used to assess stati stical differences.The experimental results were analyzed using GraphPad Prism V5.00 (GraphPad Software,La Jolla,CA,USA,www.graphpad.com),and differences with aP<0.05 were considered stati stically significant.
Results
Noise exposure increases PTEN expression in cochlear cells
First,we detected PTEN expression levels in cryosections by immunostaining 1 hour after noise exposure.PTEN signals were increased in the organ of Corti,including OHCs and IHCs,and in the nuclei of marginal cells in lateral wall tissues,but did not change in the nuclei of spiral ganglion neurons (Figure 1C).Noise exposure led to an elevation of PTEN level in OHCs and a decreased number of OHCs,which indicated that the model of permanent hearing impairment was successfully established (Figure 1D).Quantitative analysis confirmed that PTEN levels increased significantly in basal OHCs exposed to noise compared with the control cells (P=0.011;Figure 1E).We then evaluated PTEN expression in enti re cochlear homogenate by western blotting and found that PTEN expression was elevated 1 hour after noise exposure(Figure 1F).Quantification showed PTEN expression level after noise exposure was more than 6-fold the control level (Figure 1G).
bpV reduces the auditory threshold and prevents the loss of OHCs and IHC ribbons after noise exposure
To investigate the role of PTEN in NIHL,we first evaluated the impact of PTEN inhibitor bpV on noise-induced hearing threshold.We subjected CBA/J mice to 105 dB noise exposure for 2 hours,which significantly triggered acoustic trauma and hair cell loss.ABR measurements showed that bpV pretreatment attenuated the auditory threshold at 8,16,24,and 32 kHz,whereas DMSO had little effect on hearing impairment following 14 days of noise exposure(Figure 2A).Morphological analysis indicated severe injury of OHCs in the DMSO group,whereas fewer OHCs were lost in the bpV-pretreated group 14 days after noise exposure (Figure 2B).Noise trauma mainly damaged basal OHCs,which corresponds to hearing loss at higher frequencies.OHC and IHC loss began at a 50% and 65% distance from the apex,respectively.At the end of the basal region,OHC and IHC loss was 75% and 20%,respectively.Further analysis showed the ratio of OHC and IHC loss was reduced in the bpV-pretreated group compared with the DMSO group,suggesting that bpV suppressed noise-induced hair cell loss,especially for OHCs (Figure 2C).
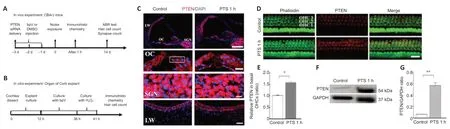
Figure 1|PTEN expression increases in cochlear cells after noise exposure.
We also measured the ABR peak I amplitudes at click and found that the bpV treatment group had higher wave I amplitudes for sound sti muli ranging from 50 to 90 dB sound pressure level compared with those of the DMSO group 14 days after the noise exposure (Figure 2D).Considering that the ABR wave I amplitude represents auditory nerve fiber activity,differences in wave I amplitudes indicate changes in synaptic function (Liu et al.,2019).We next counted the number of presynaptic ribbons immunolabeled with CtBP2.In accordance with the ABR wave I amplitude analysis,we found that bpV pretreatment reduced noise-induced loss of IHC synaptic ribbons in the apical turn region 14 days after noise exposure compared with DMSO treatment(Figure 2EandF).
PTEN inhibition alleviates noise-induced reductions in PI3K-Akt signaling in OHCs
Because PTEN regulation of PI3K-Akt signaling is related to the endogenous protection of cochlear hair cells,we hypothesized that the mechanism of bpV protection against NIHL occurs via the PI3K-Akt signaling pathway.We observed that noise exposure resulted in reductions in PI3K-p85α and PI3Kp110α levels in OHCs,and pretreatment with bpV reversed this effect (Figure 3AandB).Quanti tative analysis showed that p85α (P=0.007) and p110α (P=0.009) levels in OHCs were higher in the bpV-pretreated group than in the PTS noise exposure group (Figure 3CandD).
Phosphorylation level of Akt was decreased in OHCs after noise exposure,and p-Akt levels were recovered by pretreatment with bpV (Figure 3EandF).We also examined the mRNA levels and found that the bpV-treated group had increased expression of PI3K-Akt survival signal compared with that of the noise exposure group (Figure 3G).In addition,western blotting showed that p85α,p110α and p-Akt expression were decreased in cochlear homogenate after noise exposure compared with controls (Figure 3H).Treatment with bpV increased p85α and p110α levels in OHCs compared with those in the PTS group,but had no significant effect on p85α and p110α expression levels(Figure 3IandJ),suggesting that p85α and p110α were also localized in other types of cochlear cells such as Deiters cells.Furthermore,bpV pretreatment increased the noise-induced ratio of p-Akt/total-Akt (Figure 3K).
PTEN knockdown attenuates PTEN expression and hearing threshold
PTEN inhibitor bpV ameliorated NIHL pathology and upregulated the PI3KAkt signal.To further ascertain the function of PTEN in NIHL,intratympanic delivery of siPTEN was conducted,which showed effects on cochlear hair cells.Immunolabeling for PTEN in basal OHCs both at the cuticular plate and the nucleus were evaluated following siPTEN pretreatment (Figure 4AandB).The relative expression of PTEN was decreased in both the cuticular plate and the nucleus in the siPTEN-pretreated group compared with the siControl group (Figure 4CandD).To further demonstrate that PTEN reduction activated the PI3K-Akt survival signal,we also investigated the p85α level in OHCs and found that p85α level was increased in the siPTEN group compared with that in the siControl group (Figure 4EandF).The silencing efficiency was assessed by western blotting;analysis of the PTEN band indicated a 50% reduction after treatment with siPTEN (Figure 4GandH).Furthermore,the siPTEN group had a lower auditory threshold,especially at 32 kHz (a difference of approximately 10 dB),after noise exposure compared with that of the siControl group (Figure 4I).
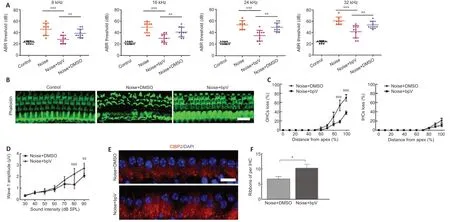
Figure 2|PTEN inhibitor bpV attenuates noise-induced changes in hearing threshold,loss of hair cells and synaptic ribbons.
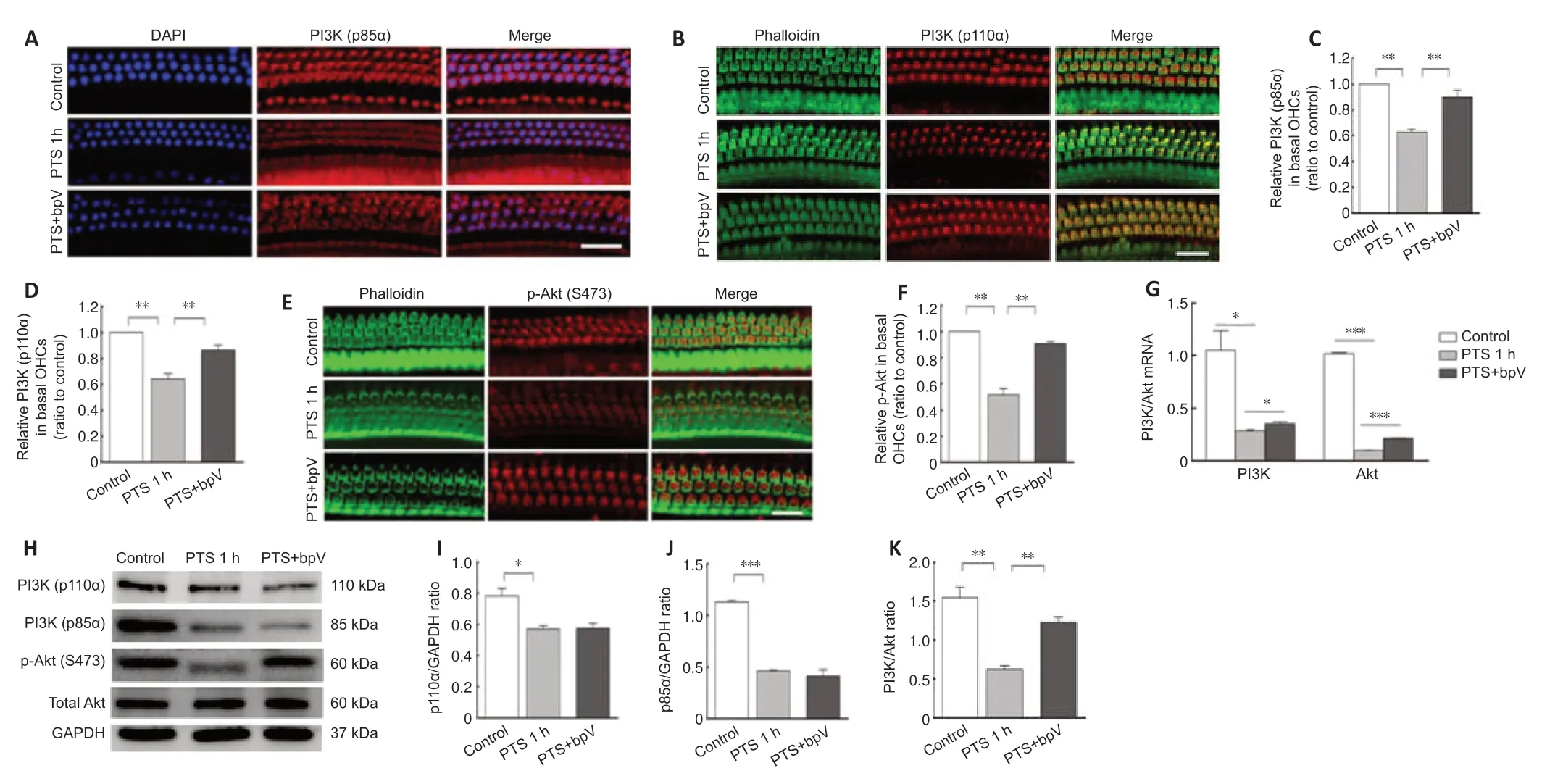
Figure 3|bpV prevents noise-induced reductions in PI3K-Akt signaling in OHCs.
bpV prevents H2O2-induced hair cell death by reducing oxidative stress in cochlear explants
Oxidative stress has been well documented as a main pathophysiological mechanism of NIHL and results in cochlear hair cell dysfunction and eventual death (Xiong et al.,2021).To explore whether the protective effect of bpV against noise-induced hair cell damage involved a reduction in oxidative stress,we used H2O2as an oxidizing agent to induce oxidative damage in cochlear explants.Hair cells in the control and bpV groups were well preserved,suggesting that bpV has no toxicity.The explants showed OHC loss when treated with H2O2,and treatment with bpV restored a normal appearance of the H2O2-treated OHCs (Figure 5A).Hair cell morphology along the entire explants was observed,and H2O2mainly induced damage in the basal region of OHCs,with no apparent change in IHCs.Quanti tative assessment of hair cells showed 50% and 80% OHC survival with 0.5 mM and 0.2 mM H2O2treatment,respectively,and bpV increased the survival of hair cells (Figure 5BandC).
Next,we evaluated mitochondrial ROS generation in cochlear explants by MitoSOX Red to determine whether bpV reduced H2O2-induced oxidative stress.We observed a low level of MitoSox Red fluorescence in hair cells treated with 0.2 mM H2O2,and the level was unchanged by pretreatment with bpV.Stronger fluorescence was observed with the higher H2O2concentration of 0.5 mM,which suggests that a high ROS level leads to serious hair cell injury.In addition,MitoSOX Red fluorescence intensity was significantly reduced by bpV pretreatment when compared with that of the 0.5 mM H2O2group (Figure 5D).To confirm that PTEN inhibitor bpV protects against NIHL by reducing oxidative stress,we further assessed the expression of 4HNE,a marker of oxidative stress (He et al.,2021),by performing immunolabeling on cochlear surface preparations.Notably,noise exposure increased 4HNE levels in OHCs,and bpV pretreatment decreased 4HNE expression (Additional Figure 1).
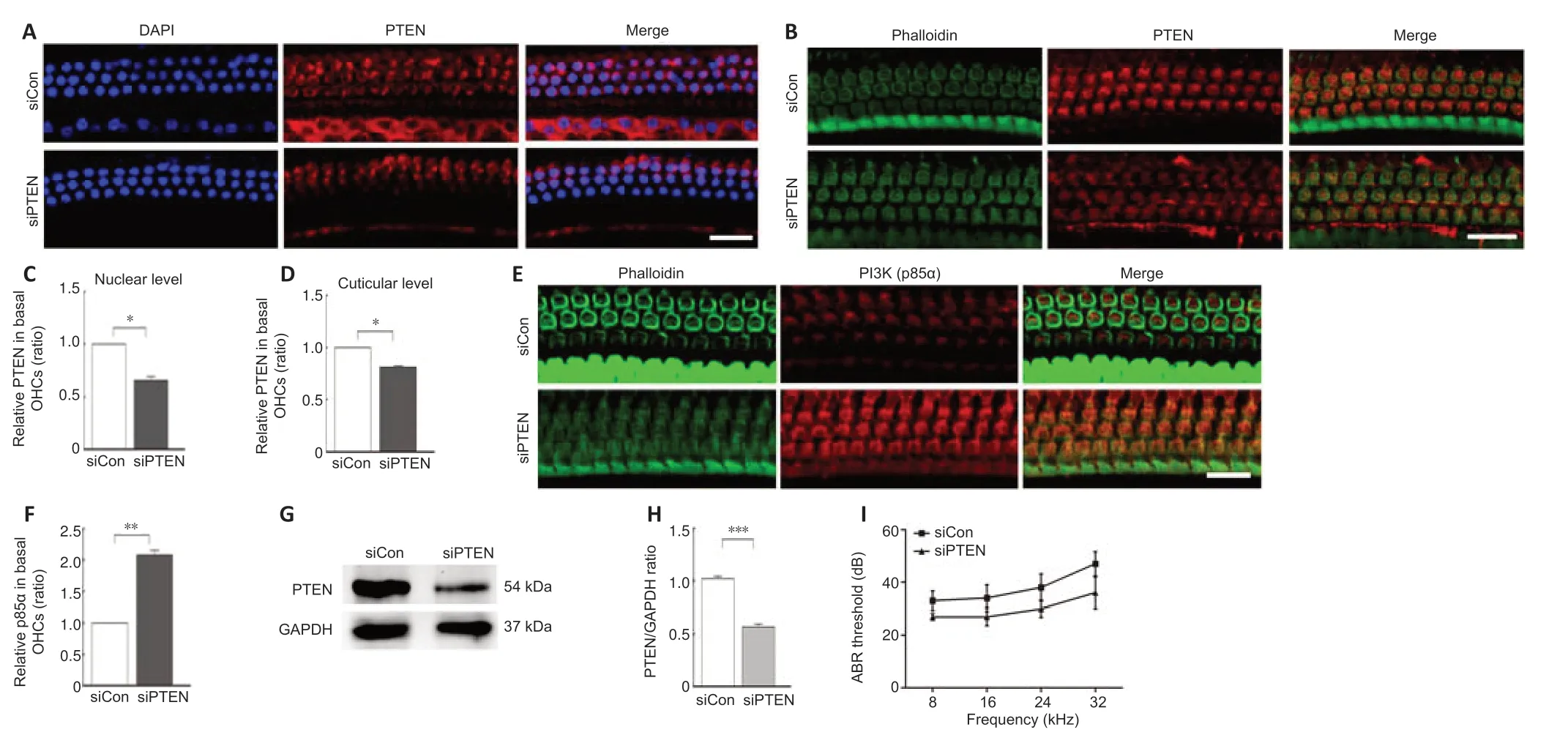
Figure 4|Intratympanic delivery of PTEN siRNA attenuates noise-induced PTEN expression and hearing threshold changes and upregulates p85α level.
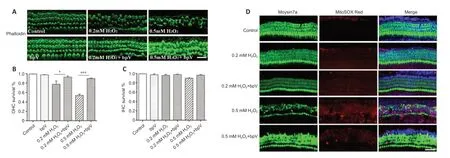
Figure 5|bpV prevents H2O2-induced hair cell loss by reducing oxidative stress in cochlear explants.
Discussion
In the present study,we demonstrated that PTEN expression was increased in inner ear tissue using an NIHL model and showed that PTEN inhibitor bpV protected against NIHL.Several studies have shown that PTEN is required for auditory system maintenance and could be a novel target for regenerating auditory hair cells (Dong et al.,2010b,2014;Sun et al.,2014;Sai et al.,2022).In addition,PTEN is an important apoptosis signal and it has been suggested that interfering with its acti vity or expression could promote axon regeneration and exert neuroprotective effects (Christi e et al.,2010;Walker and Xu,2014).bpV is a vanadate derivative that interferes with PTEN function and has protective effects in neurodegenerative disorders and cardiac dysfunction (Walker et al.,2019).Our results showed that pretreatment with bpV attenuated the auditory threshold and prevented the loss of OHCs and IHC ribbons after noise exposure,whereas the role of bpV on spiral ganglion cells needs to be further explored.Consistent with a previous report that showed gene silencing of PTEN reduces apoptosis in hippocampal cells (Zhu et al.,2006),our results showed that intratympanic siPTEN delivery decreased the auditory threshold,thus confirming the potential protective effects of PTEN inhibition.Previous studies have established that the PI3K-Akt pathway drives IHC survival and is involved in physiological processes within the inner ear (Brand et al.,2015;Xia et al.,2019).Indeed,Akt activation has been specifically detected in hair cells and spiral ganglion cells,which indicates that the protective mechanism is localized (Heinrich et al.,2015).Akt activation has also been observed in gentamicin-treated explants from STAT1–/–mice (Levano and Bodmer,2015).Furthermore,it has been reported that PI3K and p-Akt levels are reduced in OHCs after noise exposure and that downregulation of PI3K-Akt signaling enhanced vulnerability to noise (Chen et al.,2015).We found that noise exposure downregulated PI3K-Akt signaling and that PTEN inhibition preserved PI3K-Akt survival signaling in NIHL,which is consistent with the previous studies.
Oxidative stress is the main causative factor in noise-induced sensory hair cell death (Wu et al.,2020).A previous study has shown that PTEN expression increases with ROS production,whereas the PI3K-AKT signaling pathway is inhibited (Ma et al.,2020).bpV has been reported to protect against amyloid β accumulation-associated neurotoxicity and alleviate oxidative lesions in neuronal cells (Liu et al.,2017),as well as parti ally alleviate hair cell loss and activate the PI3K pathway in Fgf20–/–cochlea explants (Su et al.,2021).Taken together,our findings demonstrate that bpV protects against hair cell death by reducing oxidative stress in cultured cochlear explants,whether elevated PTEN level after noise exposure induce oxidative stress needs to be further explored.
In summary,our results demonstrated that elevated PTEN levels contributed to the pathogenesis of NIHL and that the PTEN inhibitor bpV protected against NIHL by activating the PI3K-Akt pathway and reducing oxidative stress.Further studies are required to determine the molecular mechanism of PTEN regulation on oxidative stress using PTEN conditional gene knockout mice,which will further confirm PTEN as a potenti al target for NIHL intervention.
Author contributions:FQC designed the experiments and edited themanuscript.BF performed the experiments and wrote the manuscript.FL,WJD and XGA participated in the implementation of the experiments.JC provided technical guidance for experiment implementation.RFW performed the ABR measurement.WL,YLS and DJZ helped conduct data analysis and edit the manuscript.All authors approved the final version of this paper.
Conflicts of interest:The authors declare no conflicts of interest.
Availability of data and materials:All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:This is an open access journal,andarticles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:
Additional Figure 1:bpV reduces noise-induced 4HNE expression in OHCs.
- 中国神经再生研究(英文版)的其它文章
- High-dose biotin neither fosters remyelination nor stimulates malonyl coenzyme A synthesis in the regenerating nerve
- Oncogenic BRAFV600E induces microglial proliferation through extracellular signal-regulated kinase and neuronal death through c-Jun N-terminal kinase
- Valproate reduces retinal ganglion cell apoptosis in rats after optic nerve crush
- The circ_0002538/miR-138-5p/plasmolipin axis regulates Schwann cell migration and myelination in diabetic peripheral neuropathy
- Potenti al application of let-7a antagomir in injured peripheral nerve regeneration
- Inhibiting phosphatase and actin regulator 1 expression is neuroprotective in the context of traumatic brain injury