Pharmacodynamic study of cannabidiol on bleomycin-induced pulmonary fibrosis in rats
SUN Meng-di, ZHANG Fei-yu, GAO Xin, WANG Yu, CHEN Ping-ping, LIU Shu-min✉
1. Heilongjiang University of Traditional Chinese Medicine, Harbin 150040, China
2. Institute of Traditional Chinese Medicine, Heilongjiang University of Traditional Chinese Medicine, Harbin 150040, China
Keywords:
ABSTRACT Objective: To study the protective effect of cannabidiol (CBD) on rats with pulmonary fibrosis and explore the possible mechanism of the use of CBD in the treatment of pulmonary fibrosis.Methods: Sixty SD rats were randomly divided into the normal control group,model group, prednisone group, CBD low, medium and high dose groups (12, 36, 108 mg/kg, ig),10 rats in each group.Except for the normal control group, the other 5 groups were all induced by tracheal injection of bleomycin to rat models of pulmonary fibrosis.After modeling, the rats were given intragastric administration once a day for 28 consecutive days and samples were taken.The degree of pulmonary edema was detected; the pathological changes of lung tissue were observed by HE and Masson staining; tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6) and lung tissue superoxide dismutase (SOD), malondialdehyde (MDA), hydroxyproline (HYP) contents were measured by ELISA, transforming growth factor-β1 (TGF-β1) and -smooth muscle protein( -SMA) concentration were detected by immunocytochemical method, real-time fluorescent quantitative PCR (qRT-PCR) method was used to detect the mRNA expression levels of TGF-β1,α-SMA, Nrf2 and nuclear transcription factor-κB p65 (NF-κB p65).Results: The lung organ coefficient and W/D value were significantly decreased in the CBD administration group (P<0.05); medium and high doses of CBD could reduce the number of collagen fibers and fibroblasts; the pulmonary fibrosis in the low, medium, and high dose groups of CBD was significantly lower.The levels of TNF-α, IL-1β, and IL-6 in rat serum, as well as MDA and HYP in lung tissue, were significantly lower compared to the model group.Additionally, the level of SOD was significantly increased (P<0.05); The expression of α-SMA was decreased compared with the model group (P<0.05); the contents of TGF-β1, α-SMA and NF-κB p65 mRNA in lung tissue decreased, and the expression level of Nrf2 mRNA increased(P<0.05).Especially, the high-dose group had the most significant effect.Conclusion: CBD can significantly reduce the degree of pulmonary fibrosis in rats, and its potential mechanism may be related to inhibiting inflammatory response, enhancing antioxidant capacity and inhibiting the protein expression of TGF -β1 and α-SMA.
1.Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive and irreversible lung disease[1].The interaction of various signaling pathways and cytokines causes pulmonary fibrosis.Although the pathogenesis of pulmonary fibrosis is still unclear, studies have shown that oxidative stress and excessive inflammation play an important role in it.By understanding the detailed mechanism of its pathophysiological process, we can provide a perfect plan for the clinical treatment of IPF.Currently, several drugs for pulmonary fibrosis, such as pirfenidone and Nidanib[2], have been explored, but have not significantly improved patient survival.Lung transplantation is currently an effective treatment for PF, but rejection, complications,and high treatment costs limit its clinical application.Therefore,there is an urgent need to develop new effective anti-pulmonary fibrosis drugs.
Cannabidiol (CBD), a non-psychoactive derivative of the cannabis plant, has been valued for its anti-anxiety, anti-emetic,anti-inflammatory, and anti-cancer properties[3].CBD acts as a powerful antioxidant, acting on a variety of receptor sites, including PPARγ[4], 5-HT1[5], adenosine A2A[6] and transient potential (TRP)channel receptors[7], directly or indirectly causing a wide range of anti-inflammatory and immunomodulatory effects[8].Previous studies have shown that CBD can reduce the production of related cytokines in animal models of chronic asthma[9].Recently, it has been established that CBD is involved in regulating inflammatory responses, including inflammatory lung diseases, and has a positive effect on acute and chronic inflammation[10-12].In addition, CBD is well tolerated with no significant side effects, and it may also be an effective candidate for the treatment of pulmonary fibrosis.However, there is little research on the pharmacological activity and mechanism of CBD in the treatment of IPF.Therefore, this study aimed to investigate the effect of CBD on bleomycin (BLM)-induced pulmonary fibrosis and explore its mechanism.
2.Data and methods
2.1 Experimental animals
Sixty SPF grade SD male rats with body weight (190±20) g were purchased from the Experimental Animal Center of Heilongjiang University of Traditional Chinese Medicine (lot number: SCXK(Black) 2018-003).After 7 days of adaptive feeding, no significant abnormalities in activity or feces were observed before entering the experimental stage.All relevant operations of this experiment were conducted in accordance with SPF laboratory regulations and requirements, and were approved by the Experimental Animal Ethics Committee of Heilongjiang University of Chinese Medicine.
2.2 Drugs and main reagents
CBD was purchased from Xi ‘an Lvruquan Biotechnology Co.,LTD.(Lot number: LRQ221103-1); ELISA kits for TNF-α(ml002859), IL-1β (ml037361) and IL-6 (ml064292) were purchased from Shanghai Enzyme-Linked Biotechnology Co.,LTD.Bleomycin (lot number: Z8020) was purchased from Solebol Biotechnology Co., LTD.Prednisone acetate tablets (lot number:LA22255) were purchased from Zhejiang Sienju Pharmaceutical Co., LTD.
2.3 Main Instruments
M200pro (Tecan, Switzerland); Myi QTM Optics Module Monochrome Realtime PCR detection system (Bio-Rad); Nikon Eclipse Ci-L Upright White Light Photomicrograph (Japan);KQ250DE type ultrasonic oscillator (Kunshan Ultrasonic Instrument Co., LTD.).
2.4 Establishment and grouping of animal models
Sixty rats were randomly divided into normal control group, model group, prednesone group (3.15 mg/kg), and CBD low, medium and high dose group (12 mg/kg, 36 mg/kg, 108 mg/kg)[13], and the body weight and diet of the rats were monitored weekly.The rat model of pulmonary fibrosis was established by intratracheal injection of bleomycin.Intraperitoneally injected 3% sodium pentobarbital (40 mg/kg) for anesthesia, cut the skin of the neck, exposed the trachea,gently inserted a 1mL syringe into the trachea of the rat, and placed cotton wool on the outside of the syringe tube to check whether the insertion was successful, quickly injected bleomycin (5 mg/kg), and continued to inject 0.2 mL of air to ensure that the liquid reached the lung evenly.The rat skin was sutured.Starting from the second day after modeling, the drug was given once a day by gavage for 28 d.Samples were collected, lung tissue was taken, weighed and stored in a 4% paraformaldehyde solution or in liquid nitrogen for rapid freezing and then stored at 80 ℃.
2.5 Determination of pulmonary organ coefficient and wet/dry mass (W/D) ratio of lung tissue
The rats were dissected, the complete lung tissue was removed, the surface blood was drained, the lung mass was weighed, and the lung coefficient of the rats in each group was calculated: organ coefficient= organ mass/body mass × 100%.In addition, the wet mass of the upper lobe of the right lung of rats was weighed, baked in a 60℃ oven for 72 h, and its dry mass was weighed, and the moisture content of the lung was evaluated by calculating the wet /dry weight ratio.
2.6 Histopathological observation
Lung tissue sections were stained with hematoxylin, eosin(HE) and Masson, and histological images were obtained using orthoscopic white light microscopy.Histological signs of lung tissue inflammation and fibrosis were evaluated.
2.7 ELISA was used to detect the levels of TNF-α, IL-6 and IL-1β in serum and SOD, MDA and HYP in lung tissue
The contents of TNF-α, IL-1β and IL-6 in serum and SOD, MDA and HYP in lung tissue of rats were detected by enzyme-linked immunosorbent assay (ELISA).
2.8 The contents of TGF-β1 and α-SMA were detected by immunohistochemistry
After dewaxing the 4μm thick lung tissue sections, they were incubated with 0.3% hydrogen peroxide at room temperature for 10min, then washed in 0.01 mol/L PBS for 5 min, then sealed with 5% BSA at 37 ℃ for 30 min, and then dropped with TGFβ1 (1:200) and α-SMA (1:200) at 4 ℃ overnight, respectively.Finally, goat anti-rabbit IgG antibody (1:500) labeled with HRP was incubated for 30 min, and diaminobenidine was observed under microscope after color development.
2.9 The expression of TGF-β1, α-SMA, Nrf2 and NFB p65m RNA was detected by qRT-PCR
Total m RNA was extracted by Trizol method and reverse transcribed into cDNA.PCR amplification of TGF-β1, α-SMA,Nrf2 and NF-κB p65m RNA in lung tissues of rats was performed,and Ct values were recorded.Primers were synthesized and provided by Beijing Baori Biotechnology Co., LTD., as shown in Table 1.
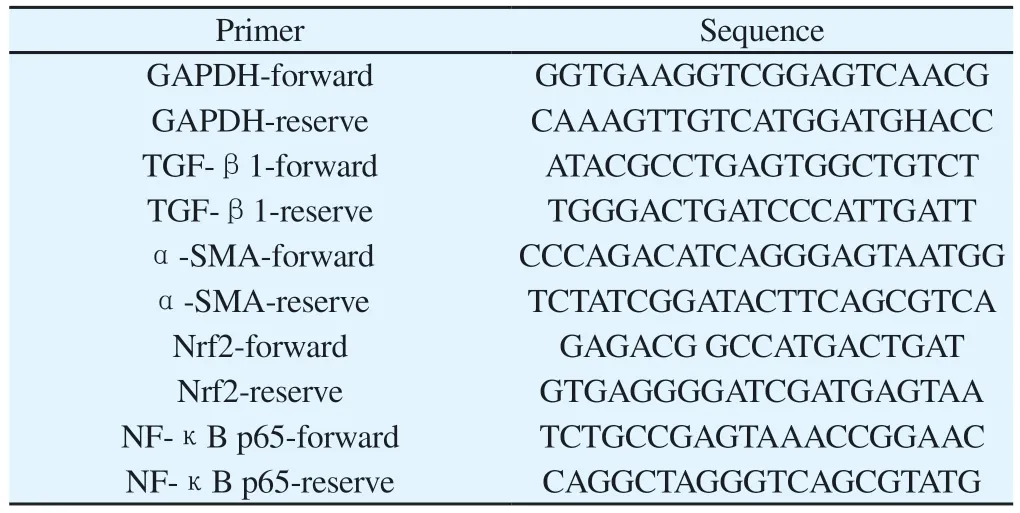
Tab 1 Primer name and related sequence
2.10 Statistical Analysis
All statistical calculations were performed using GraphPahPadPrism8.Multiple groups were compared by one-way analysis of variance.P<0.05 indicates a significant difference.
3.Results
3.1 General status observation
The rats in each group were observed and found that the rats in the normal control group were in good mental state, active and glossy, drinking, eating and defecation were normal, and body mass continued to increase normally.In the model group, the spirit was weak, the hair was dull, the breath was short, the food intake,the water intake and the body weight were decreased.After the intervention of prednisone and CBD, the general condition of the rats was improved, and the body weight of the rats in the prednisone group and the low, medium and high dose of CBD groups was significantly higher than that in the model group at 7, 14, 21 and 28 d (P<0.05).The results are shown in Figure 1.
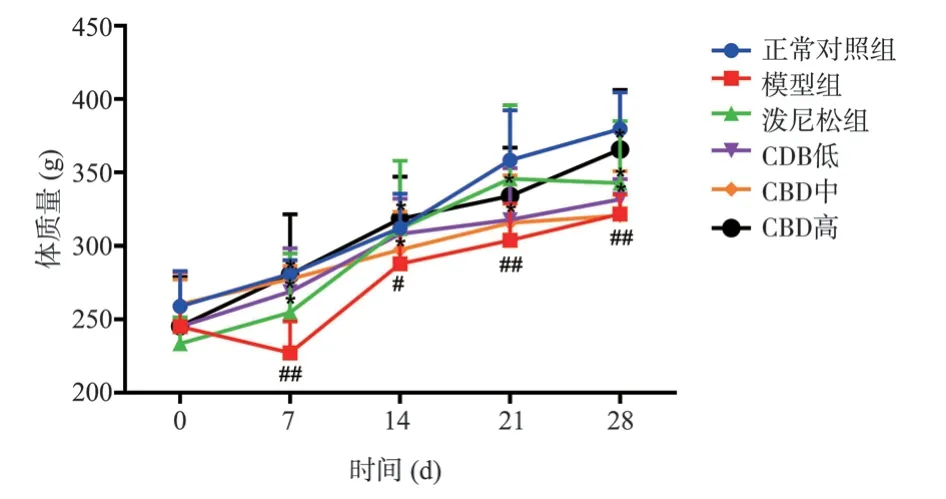
Fig 1 Effect of CBD on body weight of rats with pulmonary fibrosis
3.2 Comparison of pulmonary organ coefficients and lung tissue W/D in each group
The pulmonary organ coefficient and W/D in the model group were significantly higher than those in the control group (P < 0.01).The pulmonary organ coefficients and W/D values in CBD medium and high dose groups were significantly decreased compared with those in model group (P < 0.05) in a dose-dependent manner (Table 2).
Tab 2 Rat organ coefficient and W/D (n=6, ±s)
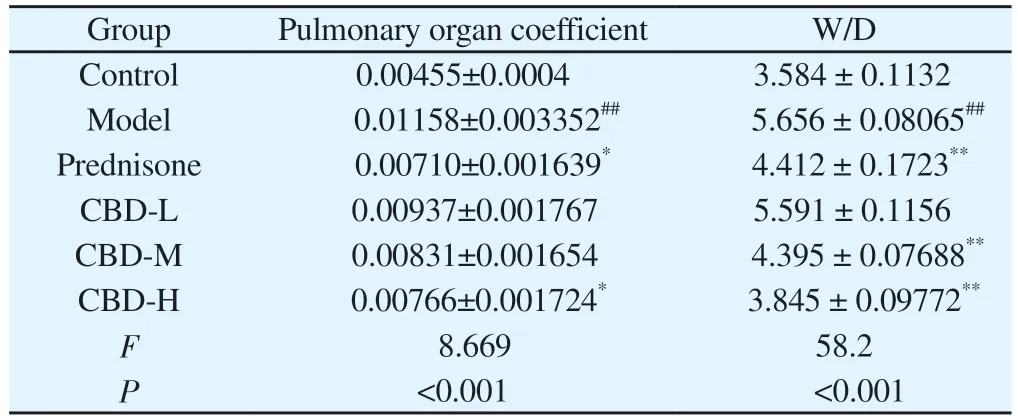
Tab 2 Rat organ coefficient and W/D (n=6, ±s)
Note: Compared with normal control group, #P<0.05, ##P<0.01; Compared with model group *P<0.05, **P<0.01.
Group Pulmonary organ coefficient W/D Control 0.00455±0.0004 3.584 ± 0.1132 Model 0.01158±0.003352## 5.656 ± 0.08065##Prednisone 0.00710±0.001639* 4.412 ± 0.1723**CBD-L 0.00937±0.001767 5.591 ± 0.1156 CBD-M 0.00831±0.001654 4.395 ± 0.07688**CBD-H 0.00766±0.001724* 3.845 ± 0.09772**F 8.669 58.2 P<0.001 <0.001
3.3 Comparison of lung histopathology in each group
HE staining and Masson staining were used to analyze the degree of lung injury and fibrosis.Compared with the normal control group,28 d after BLM modeling, the lung tissue of rats showed alveolar structure disorder, abnormal thickening of alveolar wall, large matrix deposition, and increased number of blue collagen fibers and fibroblasts.Clearly, the CBD and prednisone treatment groups significantly reduced BLM-induced lung injury and fibrosis at day 28, with no significant difference between the CBD and prednisone groups.In addition, both pulmonary fiber and alveolar inflammation scores showed that the degree of pulmonary fibrosis and alveolar inflammation in the CBD treatment group was significantly lower than that in the model group (P < 0.05), and the degree of improvement in the high-dose group was higher than that in the lowdose and medium-dose groups, indicating that CBD could improve the degree of pulmonary fibrosis and alveolar inflammation in the rats with pulmonary fibrosis.See Figure 2-4 for the results.
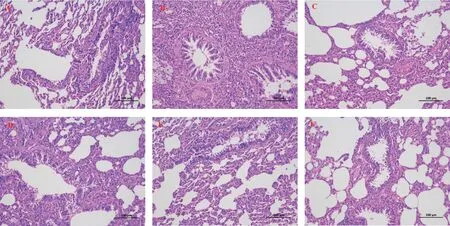
Fig 2 Comparison of pathological morphology of rat lung tissue (HE × 200)
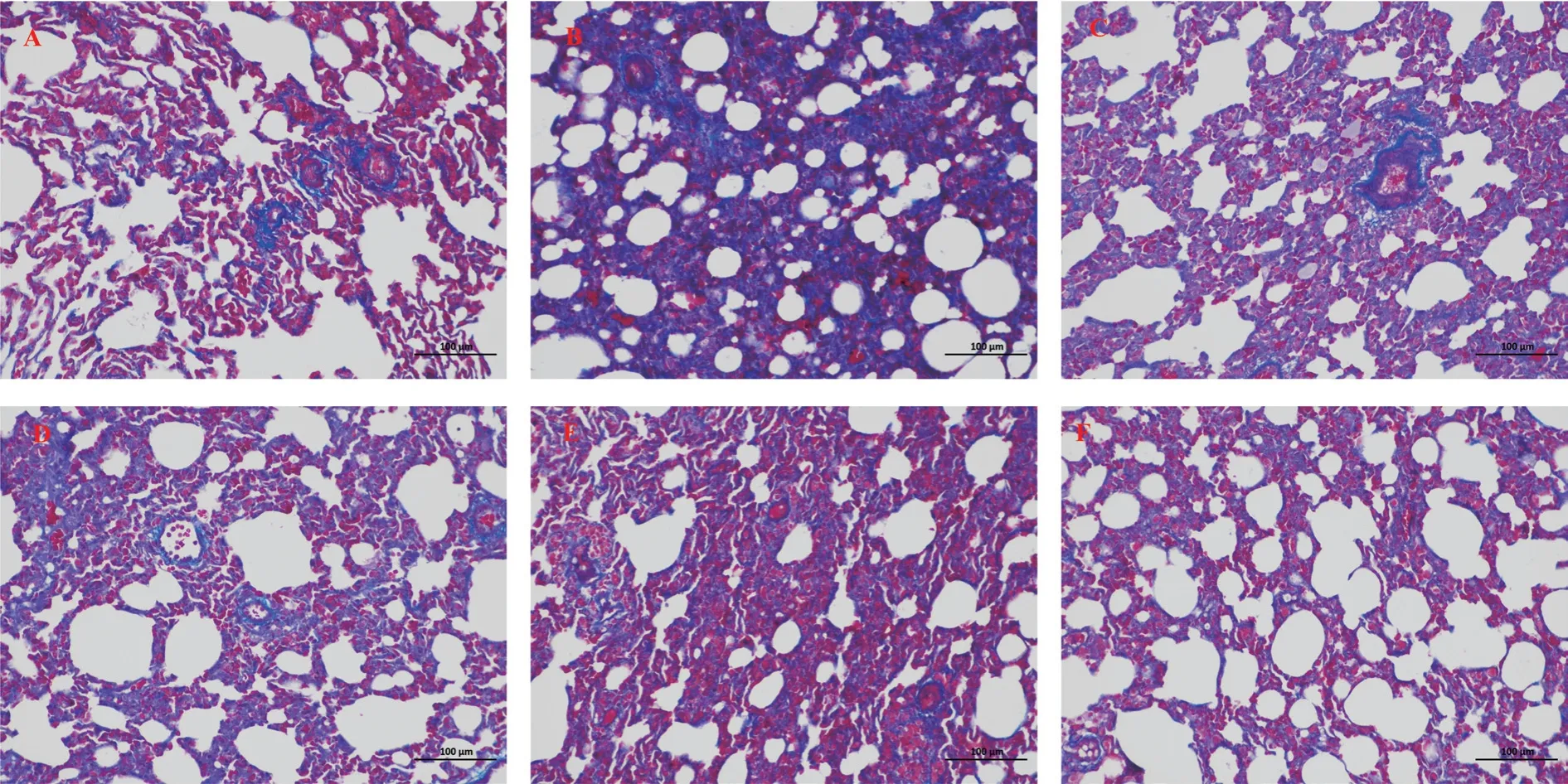
Fig 3 Comparison of pathological morphology of rat lung tissue (masson × 200)
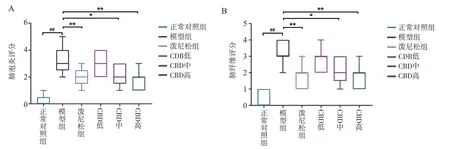
Fig 4 Rat lung tissue fibrosis and alveolitis score
3.4 Comparison of contents of TNF-α, IL-6, IL-1β in serum and SOD, MDA and HYP in lung tissue of rats in each group
Compared with normal control group, serum contents of TNF-α,IL-6 and IL-1β in model group were significantly increased, with statistical significance (P<0.01).Compared with model group, the contents of TNF-α, IL-6 and IL-1β in serum of rats in all CBD groups were decreased, the difference was statistically significant(P<0.05), and the reduction of TNF-α and IL-1β in serum of rats in high dose group was significantly higher than that in low and medium dose groups, the difference was statistically significant(P<0.01).In addition, compared with the normal control group,the contents of MDA and HYP in lung tissue of model group were significantly increased, while the activity of SOD was significantly decreased (P<0.01).Compared with model group, the contents of MDA and HYP in lung tissue of rats in CBD administration group were decreased (P<0.05), and the activities of SOD and MDA in CBD high-dose group (108mg/kg) and prednisone acetate group were significantly recovered (P<0.01).The contents of TNF-α, IL-6 and IL-1β in serum and SOD, MDA and HYP in lung tissue of rats in each group are shown in Table 3.
Tab 3 TNF-α, IL-6, IL-1β in rat serum and SOD, MDA, HYP content in lung tissue (n=6, ±s)
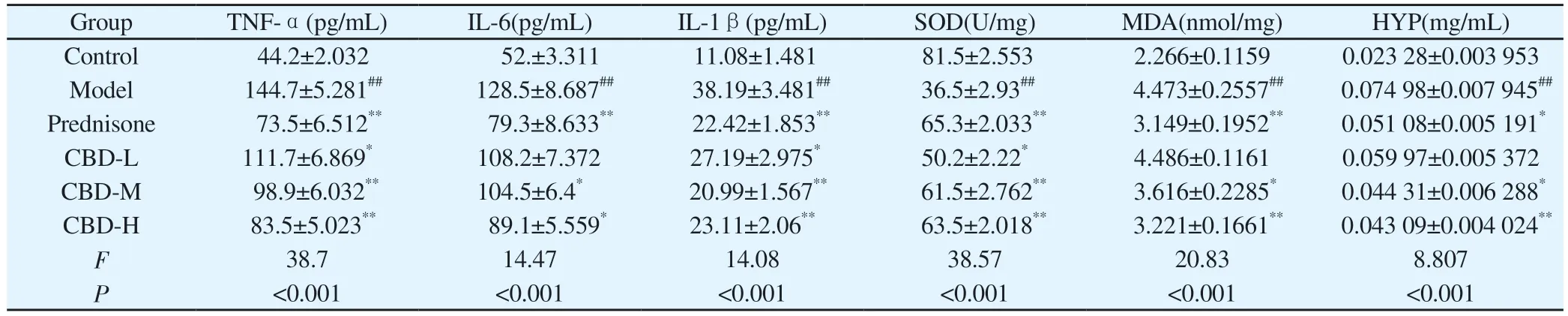
Tab 3 TNF-α, IL-6, IL-1β in rat serum and SOD, MDA, HYP content in lung tissue (n=6, ±s)
Note: Compared with normal control group, #P<0.05, ##P<0.01; Compared with model group *P<0.05, **P<0.01.
Group TNF-α(pg/mL) IL-6(pg/mL) IL-1β(pg/mL) SOD(U/mg) MDA(nmol/mg) HYP(mg/mL)Control 44.2±2.032 52.±3.311 11.08±1.481 81.5±2.553 2.266±0.1159 0.023 28±0.003 953 Model 144.7±5.281## 128.5±8.687## 38.19±3.481## 36.5±2.93## 4.473±0.2557## 0.074 98±0.007 945##Prednisone 73.5±6.512** 79.3±8.633** 22.42±1.853** 65.3±2.033** 3.149±0.1952** 0.051 08±0.005 191*CBD-L 111.7±6.869* 108.2±7.372 27.19±2.975* 50.2±2.22* 4.486±0.1161 0.059 97±0.005 372 CBD-M 98.9±6.032** 104.5±6.4* 20.99±1.567** 61.5±2.762** 3.616±0.2285* 0.044 31±0.006 288*CBD-H 83.5±5.023** 89.1±5.559* 23.11±2.06** 63.5±2.018** 3.221±0.1661** 0.043 09±0.004 024**F 38.7 14.47 14.08 38.57 20.83 8.807 P<0.001 <0.001 <0.001 <0.001 <0.001 <0.001
3.5 Effect of CBD on expression of TGF-β1 and α-SMA in lung tissue of PF rats
Compared with the normal control group, the expression of TGFβ1 and α-SMA in lung tissue of model group was significantly increased, and the difference was statistically significant (P<0.01).Compared with model group, the expression of TGF-β1 and a-SMA in lung tissues of all CBD groups was decreased, and the difference was statistically significant (P<0.05).The reduction of TGF-β1 and a-SMA in lung tissue of rats in the high-dose CBD group was significantly higher than that in the low-dose and medium-dose groups, showing similar effects to those in the prednisone group,with statistical significance (P<0.01), as shown in Figure 5-7.
3.6 Effect of CBD on expression of TGF-β1, α-SMA, Nrf2 and NF-κB p65m RNA in PF rats
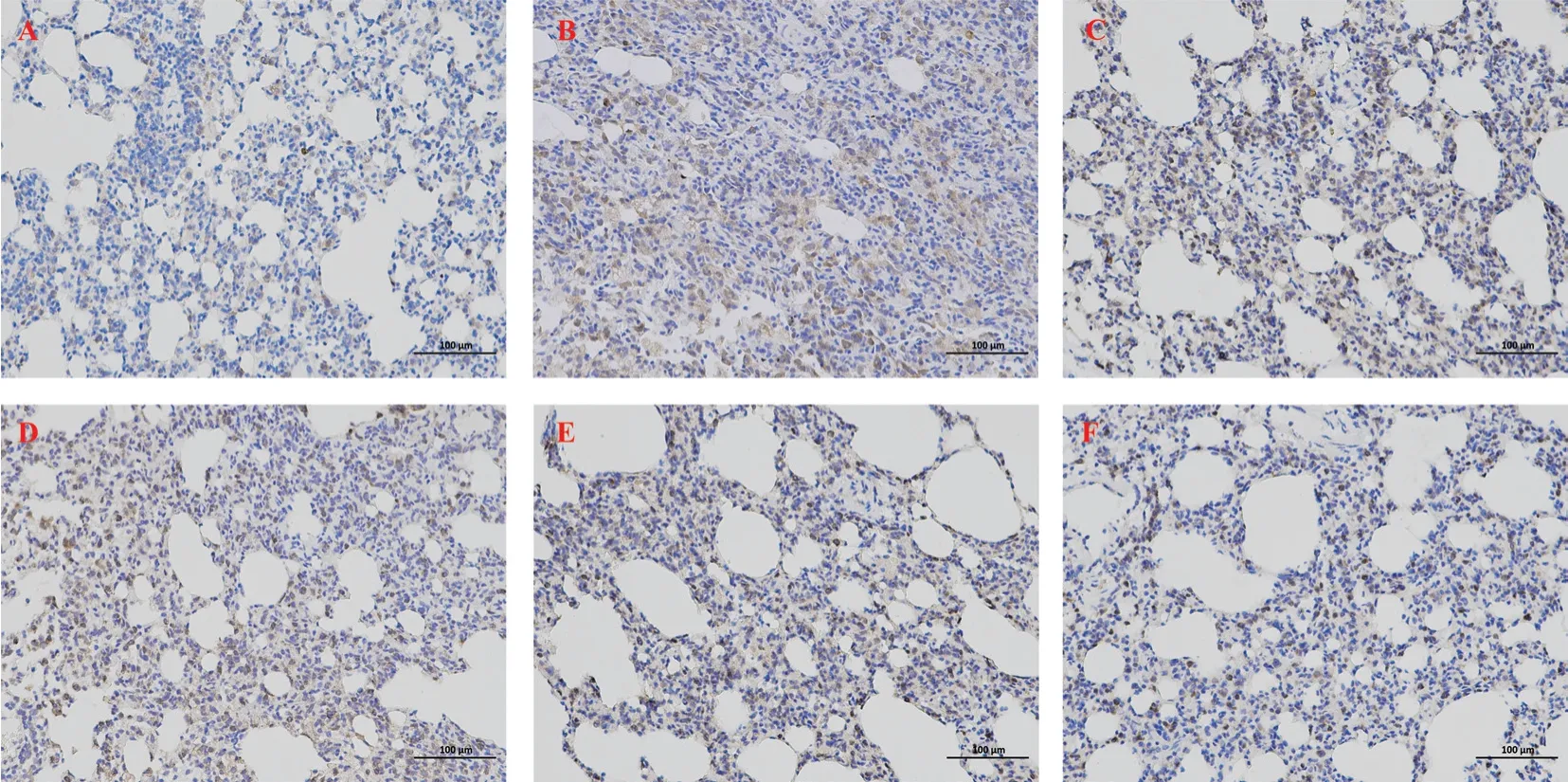
Fig 5 Expression of TGF-β1 in lung tissue
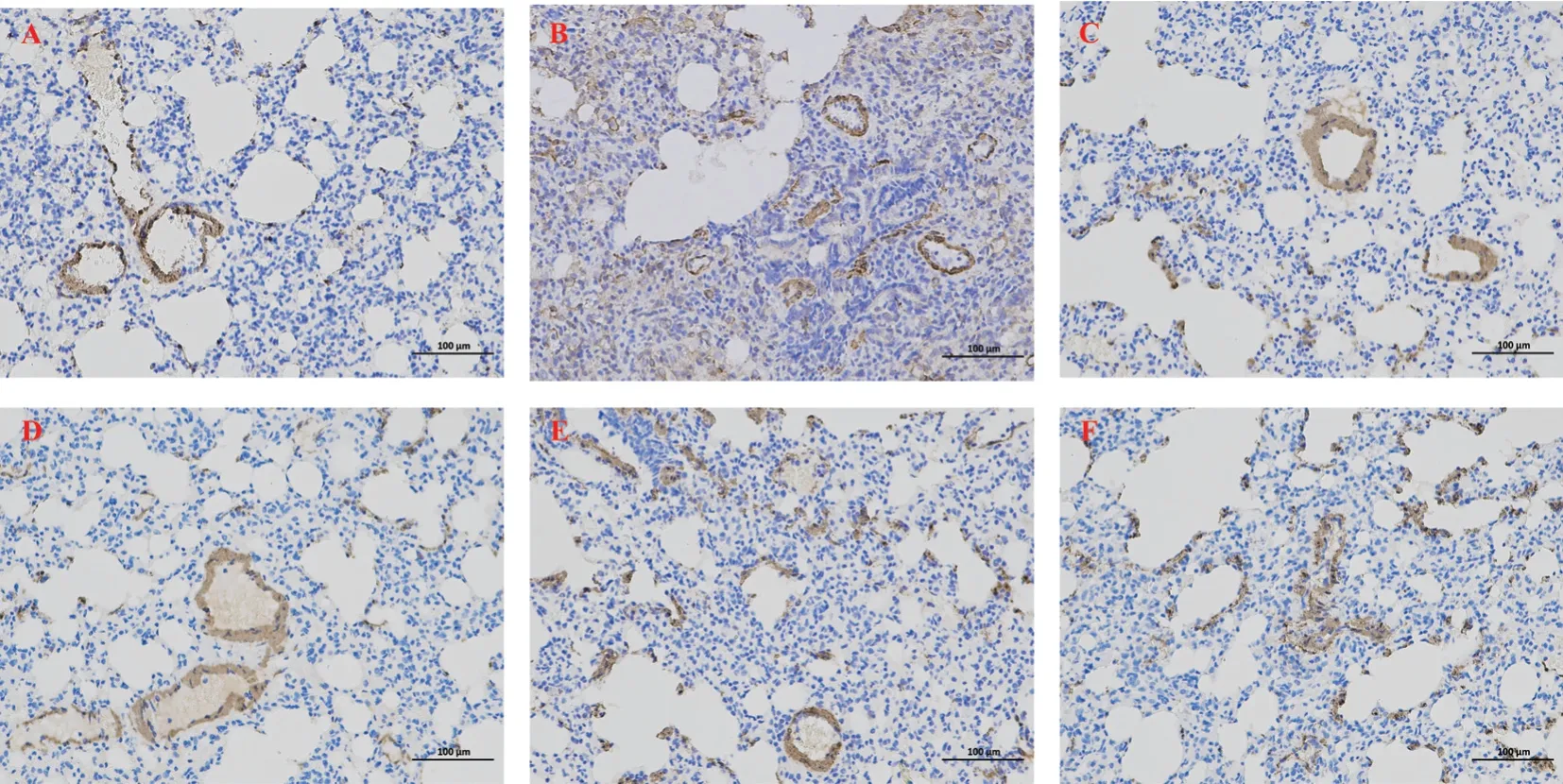
Fig 6 Expression of α-SMA in lung tissue
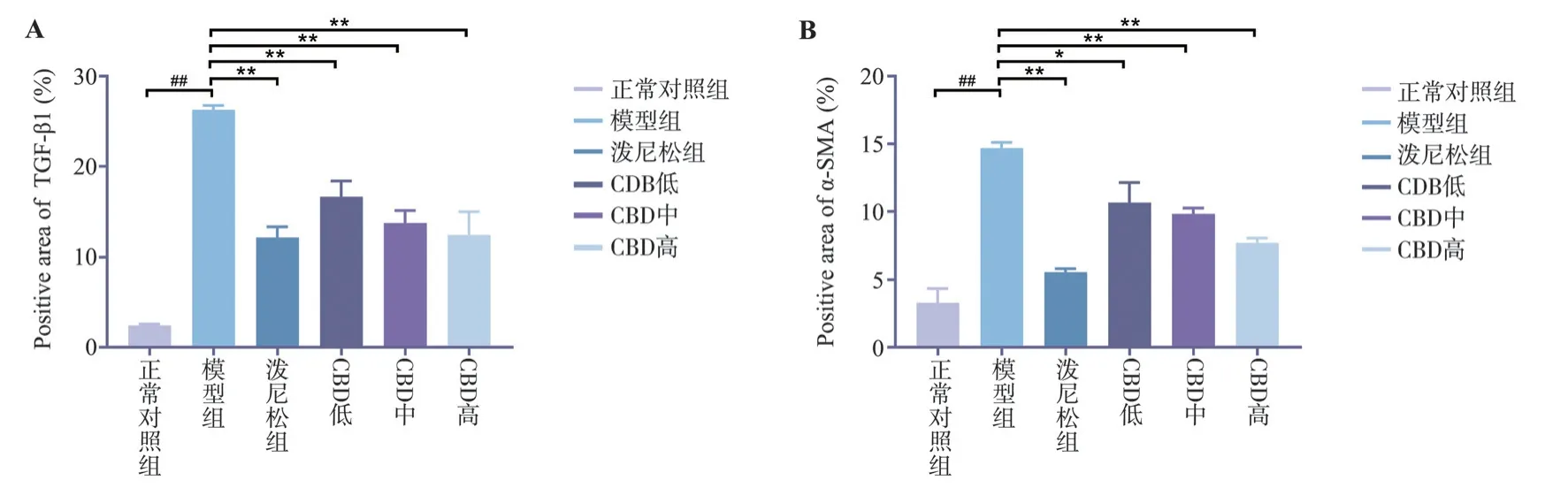
Fig 7 The expression levels of TGF-β1 and α-SMA in rat lung tissue
Compared with the normal control group, the expression of TGFβ1, α-SMA and NF-κB p65m RNA in lung tissue of model group was significantly increased, while the expression of Nrf2 m RNA was significantly decreased, with statistical significance(P<0.01).Compared with model group, the expression of TGFβ1, α-SMA and NF-κB p65m RNA in lung tissue of rats in CBD group was decreased, and the expression of Nrf2 was increased, with statistical significance (P<0.05).The reduction of α-SMA m RNA was significantly higher than that in low and medium dose groups,and the difference was statistically significant (P<0.01), as shown in Figure 8.
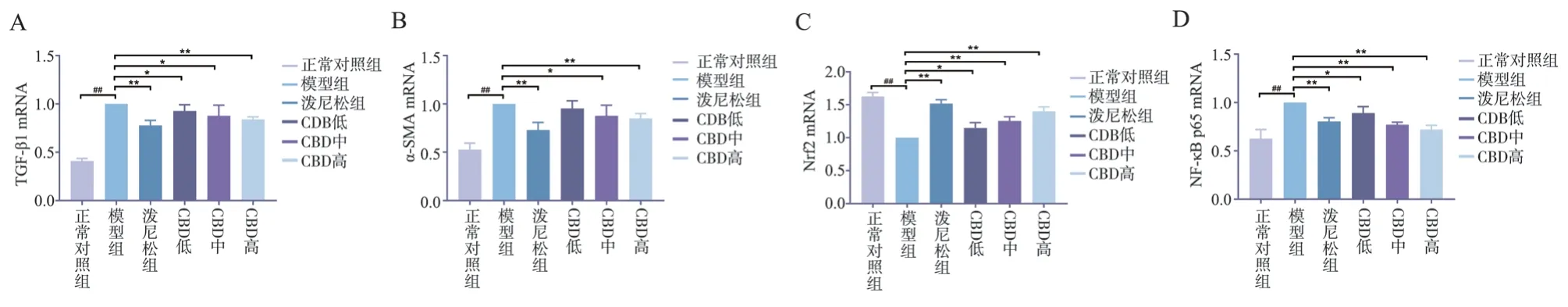
Fig 8 Expression of TGF-β1, -SMA, Nrf2 and NF-κB p65 m RNA in lung tissue
4.Discussion
IPF is a progressive lung disease caused by genetic and environmental factors or other lung diseases[14,15].Lung index is one of the indicators reflecting the degree of IPF.During the formation of IPF, the lung weight of model rats increased significantly due to inflammatory cell infiltration, cell swelling, capillary congestion and other factors.At the same time, the rats were in a disease state after modeling, resulting in weight loss.Therefore, the lung index of the model group was significantly increased.The main feature of IPF is the deposition of extracellular matrix[16].Collagen fiber is the main component of extracellular matrix, and hydroxyproline (HYP)is a unique component of collagen fiber[17], so the content of HYP can reflect the content of extracellular matrix.The experimental results showed that a single intratracheal infusion of BLM resulted in the increase of lung index and HYP content in rats, and the lung index and HYP content in rats’ lung tissue decreased after CBD intervention, suggesting that CBD may have a protective effect on BLM-induced IPF in rats.
The histopathological changes of lung tissue are the most intuitive and important indicators for determining IPF.The results under optical microscopy showed that the alveolar structure of the model group rats was severely damaged, the alveolar septum was thickened,and the collagen fibers in the alveolar septum were heavily deposited, indicating that we successfully replicated the IPF model in rats.After CBD treatment, the degree of alveolar inflammation and IPF in rats were significantly reduced, suggesting that CBD has a protective effect on BLM-induced IPF in rats.
Oxidative stress is one of the important pathogenesis of IPF[18,19].Nrf2 is the most important antioxidant factor in living organisms[20].It interacts with antioxidant reactive elements and can up-regulate various antioxidant proteins and II in the body.The expression of phase detoxification enzymes can eliminate free radicals produced by oxidative stress in the body[21].Under normal physiological conditions, Nrf2 binds to Keap1 in the cytoplasm and is inactive.When the cell is attacked by electrophilic substances or reactive oxygen species, Nrf2 dissociates from Keap1, and Nrf2 is activated in the nucleus and binds to the antioxidant reaction elements, activating the expression of downstream antioxidant enzyme proteins and playing an antioxidant role[22].Recent studies have shown that fenugreek seed extract glycosides[23],salidroside[24], and pirfenidone[25] can activate the Nrf2 pathway to inhibit the occurrence of oxidative stress, thereby inhibiting the development of BLM-induced IPF.This study found that CBD can enhance the antioxidant capacity of IPF rats by activating the Nrf2 pathway, thereby reducing the lung tissue damage caused by lipid peroxidation.
NF-κB, a dimeric compound composed of p50 and p65 subunits,is a multifunctional nuclear transcription factor with a wide range of biological activities and can be activated by TNF-α, IL-6, IL-1β and other cytokines to participate in the body’s inflammation and immune response[26].When NF-κB is activated, it moves from the cytoplasm to the nucleus to induce expression of inflammatory mediators[27].The experimental results showed that the serum inflammatory cells in the model group increased significantly after intratracheal administration of BLM.CBD can significantly reduce the number of inflammatory cells and the M-RNA expression of NFκB p65 in lung tissue, suggesting that CBD can reduce the secretion of inflammatory factors by inhibiting the NF-κB pathway.
Activation of lung fibroblasts is a key step in IPF.The continuous stimulation of pathogenic factors leads to excessive proliferation and activation of fibroblasts, and the activated fibroblasts become myofibroblasts, thereby secreting a large amount of extracellular matrix, resulting in extracellular matrix deposition, and accelerating the development of IPF[28,29].Myofibroblasts play a key role in the development of PF[30], and α-SMA is a characteristic marker of myofibroblasts.TGF-β1 is a major cytokine that causes IPF and participates in the development of PF by stimulating fibroblast activation, inducing extracellular matrix generation and inhibiting matrix degradation.The results showed that CBD could inhibit the expression of TGF-β1 and -SMA in the lung tissue of model rats.
We investigated the effect of CBD on BLM-induced pulmonary fibrosis and elucidated its underlying mechanism.In this experiment,it was observed that the expressions of TGF-β1 and α-SMA proteins and m RNA in the lung tissue of rats in the model group were significantly increased, and the expression of NF-κB in the inflammatory pathway was significantly up-regulated, while the expression of Nrf2 was significantly down-regulated.After the intervention of CBD, the expression of m RNA and protein of TGF-β1 and α-SMA in the lung tissue of rats was significantly inhibited.At the same time, the expression of NF-κB was downregulated, and the expression of Nrf2 was up-regulated, and the expressions of TNF-α, IL-6 and IL-1β of downstream inflammatory cytokines were consistent with the NF-κB pathway,especially high dose CBD had the best improvement effect, which confirmed that CBD could act on different pathways to reduce the degree of alveolar inflammation and fibrosis, and was an effective therapeutic drug.
In summary, CBD has a protective effect on BLM-induced IPF rats, and its potential mechanism may be related to inhibiting inflammatory response, enhancing antioxidant capacity and inhibiting TGF-β1 and -SMA protein expression.This study can provide some new insights into the mechanism of CBD in the treatment of pulmonary fibrosis.
 Journal of Hainan Medical College2023年22期
Journal of Hainan Medical College2023年22期
- Journal of Hainan Medical College的其它文章
- Research progress in genomics associated with prognosis of upper tract urothelial carcinoma
- A review of clinical research on using FIT screening for colorectal cancer
- Clinical efficacy of external treatment with Zi Cao Yang He Tang combined with hormone therapy for granulomatous mastitis and its effect on cellular pyroptosis proteins
- Sociopsychological impact factors of depression patients during the COVID - 19 epidemic period
- Exploring the mechanism of moist exposed burn ointment for the treatment of diabetic ulcer based on network pharmacology and molecular docking
- Clinical efficacy of leprerelin acetate with different dosage forms in central precocious puberty girls
