ASb(SO4)2 (A=Rb,Cs): Two short-wave UV antimony sulfates exhibiting large birefringence
Yng Ln ,Jinxun Ren ,Pu Zhng ,Xuehu Dong ,Ling Hung,∗ ,Liling Co ,Dojing Go,Guohong Zou
a College of Chemistry and Materials Science,Sichuan Normal University,Chengdu 610066,China
b College of Chemistry,Sichuan University,Chengdu 610065,China
Keywords:Antimony sulfates Birefringent crystals Large birefringence Short UV absorption edge Stereochemically active lone pair
ABSTRACT Herein,two antimony sulfates,named RbSb(SO4)2 (1) and CsSb(SO4)2 (2),have been successfully synthesized with the introduction of Sb3+ cation with stereochemically active lone pairs (SCALP) into sulfates by the conventional hydrothermal method.Both two compounds endow short ultraviolet (UV) absorption edges (281 nm and 278 nm,respectively) and large birefringence (0.171@546 nm and 0.174@546 nm,respectively),which means that they are promising short-wave UV optical materials.Interestingly,though both of the two compounds exhibit similar 1D chained structures,and possess the same functional moieties including SbO4 seesaws and SO4 tetrahedral groups,they exhibit significantly opposite macroscopic symmetries,i.e.,compound 1 crystallizes in a centrosymmetric (CS) manner (P21/n) and compound 2 in a noncentrosymmetric (NCS) manner (P212121),due to the size of cations [r(Rb+)=1.56 ˚A,r(Cs+)=1.67 ˚A] affects the orientation of SCALP of the adjacent Sb3+.
Short-wave ultraviolet (UV) birefringent crystals have attracted increasing interest due to their applications in the laser industry,optical communications,polarimetry,scientific instrumentations and other fields [1–10].With the continuous development of science and technology,some birefringent crystals have been synthesized and commercialized over the last few decades,e.g.,CaCO3[11],YVO4[12],MgF2[13],α-BaB2O4(α-BBO) [14].However,some birefringent crystals are limited by defects such as low transmittance,small birefringence and harsh growth conditions,which severely limit their applications in the UV area.Hence,discovering new compounds with large birefringence and wide band gaps remains of great scientific and technical importance.
Based on the structure-property relationship of birefringent crystals,materials scientists have found an effective method to enhance the birefringence of optical crystals [15–18],which is to introduce the distorted metal cations contained polyhedra with stereochemically active lone pairs (SCALP) into the system,can significantly increase the probability of obtaining compounds with large birefringence.For example,a variety of high-performance Sn2+/Bi3+-containing birefringent materials,e.g.,Sn2BO3I (0.393@1064 nm) [19],Sn2PO4I (0.468@1064 nm) [19],α-SnF2(0.163@1064 nm) [20],Bi(IO3)(SO4) (0.087@1064 nm) [21],CdBi(IO3)(SO4)2(0.1@1064 nm) [21],KBiSO4Cl2(0.098@1064 nm)[22],have been successfully designed and synthesized by researchers.Recently,our group has conducted a series of explorations on Sb3+cation,which also has an electronic configuration similar to that of the Sn2+/Bi3+cations,and has synthesized a number of birefringent materials with excellent properties [23],such as CsSbF2SO4(3.0×KDP,0.112@1064 nm) [24],K2Sb(P2O7)F(4.0×KDP,0.157@546 nm) [25],BaSb(H2PO2)3Cl2(5.0×KDP,0.09@546 nm) [26],K2Sb2(C2O4)F6(0.1×KDP,0.097@546 nm) [27].In these compounds,the Sb3+cation exhibits a variety of rich coordination patterns,e.g.,SbO4F square pyramid [25],SbO2F2and SbOF3seesaw [28,29],which all have large distortions that facilitate the enhancement of optical anisotropy of compounds.Also,in the total oxygen system,the Sb3+cation is easy to coordinate with oxygen atoms to form asymmetric structural units,e.g.,SbO3triangular pyramid,SbO4seesaw,SbO5square pyramid and SbO6octahedron.By virtue of its structural diversity and flexibility,birefringent crystals with rich structures can be better obtained,and the optical properties may be improved.Recently,some total oxygen antimony-based compounds with excellent performance have been found,such as Sb4O4(SO4)(OH)2(1.2×KDP,0.147@1064 nm) [30],Sb6O7(SO4)2(2.0×KDP,0.052@1064 nm) [31],SbB3O6(3.5×KDP,0.290@546 nm) [32].Therefore,for the synthesis of optical crystals with excellent properties,the introduction of Sb3+into the total oxygen system is also a good choice.
However,the introduction of metal cations containing SCALP will redshift the UV absorption edge of the compound.In order to ensure that the compound has a low UV absorption edge,the introduction of tetrahedral groups with non-π-conjugated groups(PO4,SO4,SeO4) into the system is effective due to their high transmittance in the UV/deep-UV range.Plenty of high-quality phosphates and sulfates with wide transmittance bands were synthesized,e.g.,LiCs2PO4(7.02 eV) [33],RbNaMgP2O7(6.07 eV) [34],Ba3P3O10X (X=Cl,Br) (>6.22 eV) [35],NH4NaLi2(SO4)2(>6.66 eV)[36],K2SrP4O12(>6.22 eV) [37],Li8NaRb3(SO4)6·2H2O (>6.52 eV)[38].
On this basis,we have carried out a systematic study on the Sb2O3-A2SO4(A stands for the alkali metal cations) system,and two antimony sulfates named RbSb(SO4)2(1) and CsSb(SO4)2(2)have been obtained by introducing Sb3+cations with SCALP into sulfates.The structure of CsSb(SO4)2has been reported by Zhaoet al.[39].Nevertheless,its optical properties have never been investigated.In this work,we investigated its nonlinear optical property and birefringence for the first time.Both the reported compounds exhibit wide transmission ranges (281 nm and 278 nm,respectively) and large birefringence (0.171@546 nm and 0174@546 nm,respectively).Interestingly,compounds1and2show exactly opposite macroscope symmetries due to the difference in size of the alkali metal cations.Herein,detailed characterizations and optical property measurements of the two stoichiometrically equivalent materials were performed.
The compound1crystallizes in the CS space group ofP21/n(No.14) and contains one independent Rb,one Sb,two S and eight O atoms in the asymmetric unit (Tables S1,S2 and S4 in Supporting information).The Sb atom forms the SbO4seesaw with Sb-O bond length of 2.038(11)-2.278(12) ˚A,and the S atom forms the SO4tetrahedron with S-O bond length of 1.412(14)-1.539(11) ˚A (Fig.1a).In thebcplane,the SbO4seesaws and the SO4tetrahedra interlink by shared O atoms to form a three-dimensional (3D) framework structure.The Rb+cations are distributed to balance the charges in the channel extending along thea-axis (Fig.1b).Each repeating unit in the 3D structure can be viewed as an infinite 1Dchain,which contains alternating four-membered rings (4-MRs) and 8-MRs,by rotating it 90° (Fig.1c).In this chain,each of the two SbO4units shares O atoms with S2O4to form a 4-MRs.Two S1O4and two S2O4form the 8-MRs basic unit by connecting with the terminal oxygen of the four SbO4groups.
The compound2crystallizes in the NCS space group ofP212121(No.19) and contains one independent Cs,one Sb,two S and eight O atoms in the asymmetric unit (Tables S1,S3,S5 in Supporting information).Similar to RbSb(SO4)2,the Sb atom adopts the SbO4seesaw coordination mode with Sb-O bond length of 2.043(3)-2.298(4) ˚A.The S atom adopts the SO4tetrahedron coordination mode with S-O bond length of 1.419(4)-1.544(3) ˚A.This compound also has a 3D frame structure similar to RbSb(SO4)2.The Cs+cations are distributed to balance the charges in the channel extending along thea-axis (Fig.1d).Similarly,rotating the repeating unit by 90°,an infinite 1Dchain containing 6-MRs can be observed (Fig.1e).In this chain,three SO4tetrahedra form the 6-MRs basic unit by connecting with the terminal oxygen of the three SbO4groups.The 6-MRs units are arranged in an up-and-down staggered pattern along thea-axis.
From the structural descriptions of the two compounds,it is clear that the differences in the radii of the alkali metal cations cause the two examples to show different macroscopic symmetries,with compound1being CS while compound2being NCS.As shown in Fig.S1 (Supporting information),the ionic radius of Cs+is larger than that of Rb+,and the greater steric hindrance causes the SbO4seesaw polyhedra to rotate,ultimately inducing compound2to crystallize in a NCS space group.
The powder X-ray diffraction (PXRD) of compounds1and2were measured.The PXRD patterns match well with the calculated data (Fig.S2 in Supporting information),indicating that the powder samples were pure phases and can be further tested.
The thermogravimetric analysis results of compounds1and2are shown in Fig.S3 (Supporting information).Compounds1and2could be stabilized up to 425 °C and 420 °C,respectively.And the decomposition products were confirmed to be Rb2SO4and SbO2,Cs2SO4and SbO2,respectively,as demonstrated by the PXRD patterns of melted samples (Fig.S4 in Supporting information).
The infrared (IR) spectra for compounds1and2are shown in Fig.S5 (Supporting information).Theυ3(SO42−) asymmetric stretching vibrations for compounds1and2are located at 1223,1186,1056,1006 cm−1and 1252,1171,1069,1007 cm−1,respectively.The absorption peaks of 850,885,and 849,887 cm−1are attributed to the symmetric stretching vibration ofυ1(SO42−),respectively.The bands at 661,582,477 cm−1of compound1belong to Sb-O asymmetric stretching and the bending.Similarly,we can observe absorption peaks for compound2of the Sb-O bond at 665,614,578,488 and 455 cm−1.The results of the characteristic absorption peaks above are consistent with previously reported compounds [24].
The optical diffuse-reflectance spectra of compounds1and2are shown in Figs.2a and b.The results show that their band gaps are 4.41 eV and 4.45 eV,respectively.The corresponding UV absorption edges are 281 nm and 278 nm,which indicate that both two titled compounds are potential short-wave UV optical materials.
The birefringence of compounds1and2were measured using a ZEISS Axio A1 polarizing microscope (Fig.2c).The reported compounds exhibit the birefringence of 0.171@546 nm,0.174@546 nm,respectively.As shown in Fig.S6 (Supporting information),we can see that the birefringence of the titled compounds are significantly larger than some other representative sulfates and phosphates,e.g.,LiCs2PO4(0.008@1064 nm) [33],NH4NaLi2(SO4)2(0.008@1064 nm) [36],K2SrP4O12(0.016@1064 nm) [37],Li8NaRb3(SO4)6·2H2O(0.021@1064 nm) [38],CsSO3F (0.031@546 nm) [40],(NH4)2PO3F(0.035@532 nm) [41],NaNH4PO3F·H2O (0.035@532 nm) [41],Sr(NH2SO3)2(0.056@589.3 nm) [42],[SO2(NH2)2] (0.07@589.3 nm)[43],(N2H6)(HPO3F)2(0.077@1064 nm) [44].And obviously,the birefringence of these two reported compounds are larger than the antimony-based sulphates/phosphates reported to date,e.g.,Rb6Sb4(SO4)3F12(0.01@1064 nm) [45],Sb6O7(SO4)2(0.052@1064 nm) [31],K4Sb(SO4)3Cl (0.066@546 nm) [46],NH4SbFPO4·H2O(0.08@1064 nm) [47],K2SO4·SbF3(0.08@1064 nm) [48],NH4SbSO4Cl2(0.09@1064 nm) [49],Rb2SO4·SbF3(0.09@1064 nm)[48],RbSbSO4F2(0.10@1064 nm) [28],Rb2SO4·(SbF3)2(0.11@1064 nm) [50],RbSbSO4Cl2(0.11@1064 nm) [51],CsSbSO4F2(0.112@1064 nm) [24],(NH4)SbSO4F2(0.138@1064 nm) [47],Sb4O4(SO4)(OH)2(0.147@1064 nm) [30],(Gu)SbFPO4·H2O (0.151@546 nm) [52],K2Sb(P2O7)F (0.157@546 nm) [25].The calculations show that the densities of both antimony-based polyhedra and tetrahedral anions of the two reported compounds are in the middle to upper range (Table S6 in Supporting information),and the cooperative effect of the two moieties helps them to exhibit large total optical anisotropy.
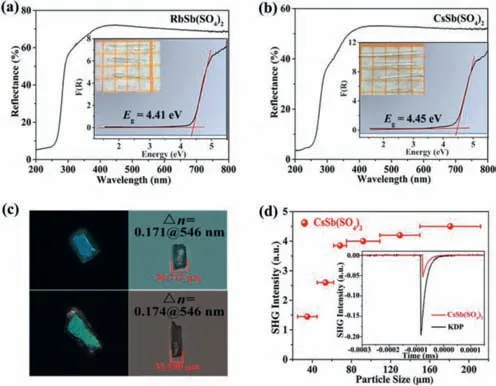
Fig.2. (a,b) The diffuse-reflectance spectra for compounds 1 and 2 (the insert is the image of 1 and 2 crystals).(c) Birefringence measurements on the 1 and 2 crystals.(d) Phase-matching curve for compound 2.The SHG intensity for the samples(150-212 μm) of compound 2 and KDP are shown in the insets.
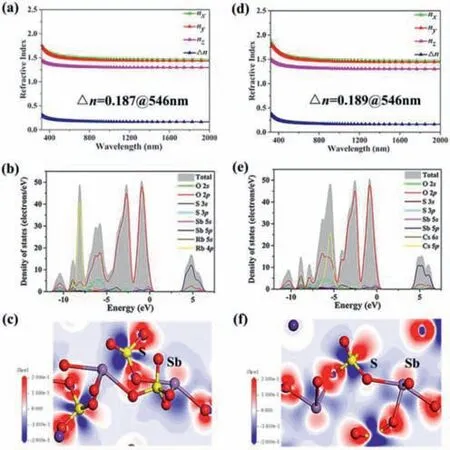
Fig.3. Calculated refractive indices of compounds (a) 1 and (d) 2.TDOS and PDOS of compounds (b) 1 and (e) 2,and the Ef is normalized to 0 eV.EDD maps of compounds (c) 1 and (f) 2.
For assessing the NLO coefficient of compound2and the powder SHG signal has been measured with 1064 nm Q-switched laser by the Kurtz-Perry method (Fig.2d) [53].The result indicates that the SHG efficiency is about 0.35 times that of KDP.As the particle size increases,the SHG intensity signal gradually increases,indicating that compound2is type-I phase matching.In order to further investigate the source of the SHG coefficient for compound2,dipole-moment calculations on crystallographically independent groups (SbO4,SO4) have been carried out (Table S7 in Supporting information).The results show that the SHG response of CsSb(SO4)2is mainly contributed by SbO4groups.
To further investigate the relationship between structures and optical properties,two compounds were calculated systematically based on DFT.The calculated band gaps of compounds1and2are 3.97 and 4.35 eV,respectively,which are smaller than the experimental values,due to the GGA-PBE underestimates the band gap(Fig.S7 in Supporting information) [54].
As shown in Figs.3a and d,the refractive index dispersion curves show that both compounds have strong anisotropy and that the birefringence values at 546 nm are 0.187 and 0.189,respectively,which are consistent with the test results.
According to the fact that compound2crystallizes in theP212121space group and Kleinman symmetry,it has three nonzero independent frequency doubling coefficient tensors (d14,d25,d36).As shown in Fig.S8 (Supporting information),the absolute value of the maximum nonlinear optical coefficientd14is 0.34×10−9esu@1.165 eV,corresponding to 0.34×KDP,which is in agreement with the experimental value.
The partial and the total densities of states (DOS) for compounds1and2have been investigated and displayed in Figs.3b and e.The upper regions of the valence band (VB;-10 eV to 0 eV) are mainly from O-2s,O-2p,S-3p,Rb-4p and O-2s,O-2p,S-3p,Cs-5p for compounds1and2,respectively.The conduction band(CB) bottom (0 eV to 10 eV) are both mainly attributed to the Sb-5p,O-2p,S-3p.Evidently,the electrical states in the vicinity of the forbidden band consists mainly of O-2s,O-2p,Sb-5p and S-3p orbitals,which shows that the optical characteristics of compounds1and2mainly derive from the cooperation of the SbO4seesaws and SO4tetrahedra,which can be illustrated by electron-density difference (EDD) map (Figs.3c and f).It is clearly described that Sb3+has SCALP electrons and there exists charge transfer from S to O atoms.
In summary,two alkali metal antimony sulfates,RbSb(SO4)2(1) and CsSb(SO4)2(2) with the same stoichiometric ratio were successfully synthesized by the conventional hydrothermal synthesis method.The optical properties have been enhanced by introducing Sb3+cations with SCALP into the sulfates,therefore the two title compounds exhibit the large birefringence (0.171 and 0.174@546 nm,respectively).Particularly,the compound2shows the largest birefringence value among the antimony-based sulphates/phosphates reported to date.The two title compounds endow short UV absorption edges (281 nm and 278 nm,respectively),which means that they are promising short-wave UV optical materials.Interestingly,the structural symmetries of the two compounds can be modulated by the discrepant size of alkali metal cations.This work will guide the tuning of crystal structures through metal cations and open a new door for further designing high-performance birefringent materials.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank Dr.Daichuan Ma at Analytical and Testing Center,Sichuan University for technical help in the Material Studio calculations.This work was supported by the National Natural Science Foundation of China (Nos.22122106,22071158,21971171),the Fundamental Research Funds from Sichuan University (No.2021SCUNL101).
Supplementary materials
Electronic Supplementary Information (ESI) available: [additional crystallographic data,XRD patterns,IR spectra,TGA,the residue of TGA,the calculation of densities and theoretical calculation of RbSb(SO4)2and CsSb(SO4)2.CCDC-2242196 for RbSb(SO4)2,2242197 for CsSb(SO4)2].Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108652.
 Chinese Chemical Letters2023年12期
Chinese Chemical Letters2023年12期
- Chinese Chemical Letters的其它文章
- Spin switching in corrole radical complex
- Benzothiadiazole-based materials for organic solar cells
- Mono-functionalized pillar[n]arenes: Syntheses,host–guest properties and applications✰
- Recent advances in two-step energy transfer light-harvesting systems driven by non-covalent self-assembly✩
- From oxygenated monomers to well-defined low-carbon polymers
- Doping-induced charge transfer in conductive polymers
