Vesicle fusion induced by zwitterionic amphiphilic channels☆
Qingyu Huan,Tao Lin,Yong-Hong Fu,Jun-Li Hou
Department of Chemistry,Fudan University,Shanghai 200433,China
Keywords:Vesicle fusion Pillar[5]arene Electrostatic interaction Lipid bilayers
ABSTRACT A new strategy to induce vesicle fusion has been developed by employing pillar[5]arene derivatives that were channel-like and were prepared by appending side chains onto pillar[5]arenes backbones.The channels feature with hydrophilic negatively and positively charged groups at both ends and hydrophobic Trp residues at the outer surface,which endows the channels with amphiphilicity.The zwitterionic amphiphilic channels could spontaneously incorporate into the bilayer membranes of lipid vesicles to induce vesicle fusion driven by the electrostatic interactions between negatively charged and positively charged groups.
In biological systems,membrane fusion is one of the central processes for cargo of biological molecules [1–3],which involves the merging of the membranes of two different organelles and the mixing of the compartments encapsulated by the membranes[4–7].The process of the fusion consists of the membrane adhesion,outer leaflets fusion,formation of the fusion pore,and merger of the membrane to achieve the final full fusion [8,9].In biological systems,this process is induced by fusion proteins [10].Inspired by the proteins,chemists have great interests in constructing artificial systems to mediate vesicle-vesicle fusions because of the importance for drug delivery,cancer cell apoptosis,and disease diagnosis [11–15].Studies of membrane fusion have extensively employed lipid vesicles as artificial analogues of cells.In the past decades,a large number of strategies have been developed to induce membrane fusion,including mediated by chemical reagents,such as lantibiotic [16],calcium salt [17],modified DNA[18],and synthetic proteins and peptides [19,20],or triggered by physical methods,such as electric field pulse [21–23],laser irradiation [24],electrostatic interaction [25,26],and so forth.In the context,electrostatic interaction-induced fusion is a promising approach due to its high efficiency in promoting the membrane adhesion.In this approach,it requires premixing of the positively or negatively charged reagents with the lipid to prepare the positively or negatively charged lipid vesicles,which would affect the stability and yield of the vesicles.Herein,we report a new strategy to induce vesicle fusion by post-addition of zwitterionic amphiphilic pillar[5]arene derivatives.
Pillar[5]arene,a new kind of macrocyclic paracyclophane,was first synthesized by Ogoshietal.and has been extensively used in supramolecular and material chemistry due to its convenient preparation,rigid backbone,and deep cavity [27–52].It has been established that conformations of pillar[5]arene derivatives are channel-like with attached sidechains pointing to two opposite directions along the pillar axle [53–56].We have demonstrated that the unimolecular tubular structures could be efficiently constructed by appending linear sidechains onto the pillar[5]arene backbones [57–60].The tubular structures could be positively and negatively charged or even zwitterionic by introducing different groups at both ends of the structures [61,62].The structures with hydrophobic surface can spontaneous incorporated into lipid bilayers because of their amphiphilic structural feature.
We envisioned that the zwitterionic tubular molecules with amphiphilic backbone would insert into the bilayers of lipid vesicles to induce the fusion of the vesicles driven by electrostatic interaction (Fig.1a).Thus,the zwitterionic tubular molecules1–3were designed (Fig.1b).The terminal amino and carboxylic acid groups and the hydrophobic peptide chains endow the molecules with amphipathy.The tryptophan (Trp) residues in the peptide chains can further enhance the bilayer-incorporation ability of the tubular molecules driven by hydrogen bonding between indole amide and lipid headgroup oxygens and dipole interaction between the hydrophobic indole ring and the lipid tails [63–65].The compounds were synthesized by attaching Trp-contained side-chains onto pillar[5]arene backbones via click-chemistry (see Supporting information).

Fig.1. (a) Schematic for the vesicle fusion induced by the zwitterionic tubular molecules.(b) The chemical structure of compounds 1–3.
The possibility of1–3to induce the fusion of the lipid vesicles was firstly investigated using Förster resonance energy transfer (FRET) experiments.For the experiments,large unilamellar vesicles (LUVs) consisting of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DOPC),andN-(7-nitro-2,1,3-benzoxadiazol-4-yl) (NBD) labeled and Rhodamine (Rho) labeled 1,2-dioleoyl-snglycero-3-phosphoethanolamine (DOPE) were prepared,where the NBD and Rho moieties act as FRET energy donor and acceptor,respectively.Under a certain DOPE/DOPC ratio,NBD and Rho moieties have chance to move to close with each other and generate FRET that was monitored by exciting NBD moiety at 450 nm and detecting emission at 530 nm.The fusion of NBD -and Rholabeled vesicles with vesicles without labeling can decrease the DOPE/DOPC ratio.As a result,the distance between NBD and Rho moieties would increase,which leads to lower FRET (Fig.2a).
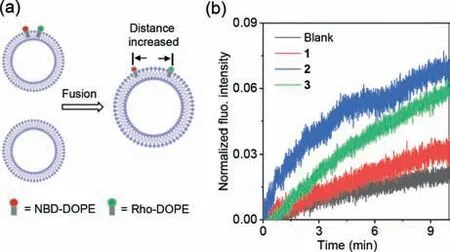
Fig.2. (a) Schematic for the experimental setup.The vesicle fusion will cause the increasement of the distance between NBD and Rho moieties,leading to the decrease of the FRET between the two moieties.(b) Changes in the normalized fluorescent intensity of NBD (λex=450 nm,λem=530 nm) with time after mixing the NBD-and Rho-labeled vesicles with the unlabeled vesicles in the presence of 1–3(mRL=7.8%).
By mixing the labeled vesicle assay with the vesicle assay without labeling in the absence of the zwitterionic pillar[5]arene derivatives,minimal NBD fluorescence intensity change was observed (Fig.2b),indicating the absence of the vesicle fusion.However,mixing both vesicle assays in the presence of1–3(molar ratio relative to lipid (mRL,%)) led to increasement of the fluorescence intensity with time,which clearly indicates the decreasing of FRET.This result provides the first evidence for the fusion of the vesicles mediated by1–3.Compound2exhibited higher ability to mediate vesicle fusion in comparison with1and3as indicated by its faster fluorescence intensity increasing speed.The different ability of these three compounds to mediate vesicle fusion was ascribed to their different lipid membrane-incorporation ability.Compound2possessed the highest membrane-incorporation ability due to its length (3.3 nm) matched well with the thickness of the lipid bilayers (3.5 nm).
The fusion of the vesicles was further verified by intervesicle salt transport experiments.For the experiments,LUVs loading MgCl2(LUVMgCl2) and Cl−sensitive fluorescent probe 3-(6–methoxy-1-quinolinio)propanesulfonate (SPQ,LUVSPQ) were prepared respectively.Mixing of both vesicle assays in the presence of compound2caused the significant decrease of SPQ fluorescence intensity (Fig.3a),indicating the fluxing of MgCl2from LUVMgCl2into LUVSPQ.Vesicle-based MgCl2transport experiments demonstrated that this compound could not mediate MgCl2transmembrane transport (Fig.S7 in Supporting information),due to the high dehydration energy of divalent Mg2+that prevents this cation from entering the cavity of the channel molecules and blocks the cotransport of Mg2+and Cl−.The blockage excluded the possibility of MgCl2transport in the pathway of LUVMgCl2-bulk-LUVSPQ.Thus,above SPQ fluorescence intensity decreasing under mixing both vesicle assays in the presence of2should be a result of the blending of the vesicle inner solution that was caused by the vesicle fusion.The extent of the fluorescence intensity decreasing was dependent on the dose of compound2,supporting that the vesicle fusion was mediate by this compound.
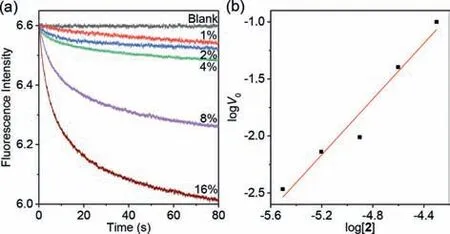
Fig.3. (a) Changes in the fluorescent intensity of SPQ (λex=344 nm,λem=443 nm)with time after mixing LUVMgCl2 with LUVSPQ in the presence of 2.(b) Plot of logV0 vs. log[2],indicating the linear relationship of logV0 with log[2].The slop of the line was determined to be 1.2±0.1.
The molecularity of compound2in the lipid bilayers was further evaluated by calculating the initial transport rate (V0) from the slope of the stop-flow curves att=0 s.The logV0values were found to have a linear relationship with log[2] (Fig.3b),and the slope were determined to be 1.2±0.1.The fact that the slope was close to unity should be the evidence for the formation of unimolecular vesicle fusion trigger.This observation also suggested that,under the experimental conditions,the zwitterionic compound did not aggregate in the lipid bilayers.
Giant unilamellar vesicles (GUVs) usually act as a model for assessing membrane fusion because of their large dimensions for direct visualizing the fusion process.Therefore,GUVs were also employed in this work to investigate the membrane fusion.For the experiments,GUVs were prepared by swelling DOPC lipid on top of dried polyvinyl alcohol (PVA) surface.The vesicle fusion was triggered after the addition of the zwitterionic compound2onto the top solution of GUVs (Supplementary Video).Three vesicles that were suspended in the solution moved to close and contact with each other (Fig.4a).Subsequently,the bilayer membranes of the contacting area fused with each other to form a hemifusion intermediate in a short term (Fig.4b).The hemifusion membrane further expanded to result in a larger vesicle with sphere shape(Fig.4c).The fusion process could be completed in around 2 min,which indicates a quick fusion process.The quick fusion should be a result of the strong electrostatic interaction between the zwitterionic pillar[5]arene derivatives locating in the adjacent bilayers.The zwitterionic compound2features with high charge density at both ends,which contributes to the strong electrostatic interaction.

Fig.4. Microscopy images of the GUVs recorded at (a) 30 s,(b) 45 s,(c) 118 s after addition of compound 2,highlighting the fusion of GUVs induced by 2.
In conclusion,we have developed a new strategy to induce vesicle fusion by employing zwitterionic pillar[5]arene derivatives.The derivatives were channel-like and were prepared by appending side chains onto pillar[5]arenes backbones.The pillar[5]arene channels contains hydrophilic negatively and positively charged groups at both ends and hydrophobic Trp residues at the outer surface.This structural feature endows the channels with amphiphilicity.The zwitterionic amphiphilic channels can efficiently incorporate into the bilayer membranes of lipid vesicles and induce vesicle fusion driven by the electrostatic interactions between negatively charged and positively charged groups.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was support by the National Natural Science Foundation of China (NSFC,Nos.21921003 and 21971046) and the Science and Technology Commission of Shanghai Municipality (STCSM,No.22JC1403700).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108566.
 Chinese Chemical Letters2023年12期
Chinese Chemical Letters2023年12期
- Chinese Chemical Letters的其它文章
- Spin switching in corrole radical complex
- Benzothiadiazole-based materials for organic solar cells
- Mono-functionalized pillar[n]arenes: Syntheses,host–guest properties and applications✰
- Recent advances in two-step energy transfer light-harvesting systems driven by non-covalent self-assembly✩
- From oxygenated monomers to well-defined low-carbon polymers
- Doping-induced charge transfer in conductive polymers
