Efficacy of suspended moxibustion stimulating Shenshu (BL23)and Guanyuan (CV4) on the amygdala-HPA axis in rats with kidney-Yang deficiency symptom pattern induced by hydrocortisone
MIN Youjiang,YAO Haihua,WANG Zhiqin,LUO Kaitao,SUN Jie,YUAN Zheng,WU Huiqi,CHENG Lihong
MIN Youjiang,School of Traditional Chinese Medicine,Nanchang Medical College,Nanchang 330053,China
MIN Youjiang,YAO Haihua,YUAN Zheng,Traditional Chinese Medicine Department,Shanghai Eighth People’s Hospital,Shanghai 200235,China
MIN Youjiang,WANG Zhiqin,SUN Jie,WU Huiqi,CHENG Lihong,Acupuncture Department,Affiliated Hospital of Jiangxi University of Traditional Chinese Medicine,Nanchang 330006,Jiangxi,China
LUO Kaitao,Acupuncture Department,Jiaxing Hospital of Traditional Chinese Medicine,Jiaxing 314001,China
Abstract OBJECTIVE: To investigated the effects of suspended moxibustion stimulating Shenshu (BL23) and Guanyuan(CV4) acupoints on the amygdala and HPA axis in our rat model and elucidated the possible molecular mechanisms of moxibustion on kidney-Yang deficiency symptom pattern (KYDS).METHODS: Sixty male Sprague Dawley rats were randomly divided into a control group (n= 12) and an experimental group (n= 48).Rats in the experimental group were given intramuscular injections of hydrocortisone to establish a KYDS model.The 48 rats successfully modeled were then randomly divided into a model group (model,n= 12),a carbenoxolone intraperitoneal injection group (CBX,n=12),a moxibustion group (moxi,n= 12),and a moxi+CBX group (n= 12).In the moxi,the Shenshu (BL23) and Guanyuan (CV 4) acupoints were treated with moxibustion for 14 d.After treatment,measures were taken of serum levels of corticosterone (CORT),adrenocorticotropic hormone (ACTH),and corticotropinreleasing hormone (CRH).The expression of mineralocorticoid receptors (MRs),glucocorticoid receptors (GRs),11beta-hydroxysteroid dehydrogenase type 1 (11β-HSD1),CRH,and ACTH in the rats'amygdala,hypothalamus,or pituitary (as appropriate)was detected.Data were analyzed using one-way analysis of variance.RESULTS: Compared with those of the control group,the serum levels of CRH,ACTH,and CORT;the mRNA and protein expressions of MR,GR,and 11β-HSD1 in the amygdala;the mRNA and protein expressions of 11β-HSD1 in the hypothalamus;the CRH mRNA expression in the amygdala and hypothalamus;and the ACTH mRNA expression in the pituitary of the rats in the model group were all significantly decreased (P < 0.05 or 0.01).After treatment with moxibustion,all the aforementioned observation indices except for 11β-HSD1 mRNA expression were ameliorated compared with those in the model group (P < 0.05 or 0.01).CONCLUSIONS: Suspended moxibustion can effectively improve the serum levels of ACTH,CRH,and CORT and can up-regulate the mRNA and protein expressions of MR,GR,11β-HSD1,CRH,and ACTH in the amygdala and hypothalamus of KYDS rats.This may be one of the molecular mechanisms with which moxibustion alleviates KYDS.
Keywords: moxibustion;kidney-Yang deficiency;amygdala;hypothalamus;receptors,mineralocorticoid;receptors,glucocorticoid;11-beta-hydroxysteroid dehydrogenase type 1
1.INTRODUCTION
Kidney-Yangdeficiency syndrome (KYDS) is one of the main syndromes in Traditional Chinese Medicine (TCM).It is a type of cold syndrome caused by a deficiency and decline in kidney-Yangdysfunctional temperature regulation,and loss of gasification power.IntroductoryMedicine,a classic TCM scholarly work,theorizes that moxibustion treats the kidney-Yang,deficiency in KYDS by restoring Yuan Yangand re-warming theQi.
Moxibustion is a traditional therapy in Chinese medicine that uses a preparation of burned dried mugwort (moxa).It is widely used in China for the treatment of various chronic diseases and deficiencies.Suspended moxibustion is an indirect form of moxibustion in which the dried mugwort is placed over an acupoint without skin contact to stimulate the circulation by warming the acupoint and improving the flow of blood andQi.This facilitates health and recovery from disease.1Research and clinical observation indicate that moxibustion therapy is an effective treatment for KYDS,2-5but the exact mechanisms remain to be fully elucidated.
The hypothalamic-pituitary-adrenocortical axis (HPA axis) is an important part of the neuroendocrine system.The HPA axis is affected both through negative feedback by hormones secreted within it and by upper regulating centers such as the hippocampus and amygdala.Stimulation of amygdala in rats induces corticosterone(CORT) secretion,and damage to the amygdala can reduce the secretion of adrenocorticotropic hormone(ACTH) and CORT.6Activation of corticotropinreleasing hormone (CRH) peptides in the central amygdala increases cortisol levels,leading to anxiety,fear,and activation of the sympathetic nervous system.7The amygdala also contains high concentrations of CRH,CRH receptors,CRH binding proteins,and corticosteroid receptors.8This suggests that the amygdala may have the ability to synthesize and secrete CRH,directly activating the HPA axis.It may also be regulated by CRH and corticosteroid feedback.The CRH systems of the hypothalamus and amygdala jointly contribute to chronic stress.8
Glucocorticoid (GC) is a terminal product of the HPA axis that plays a key role in the amygdala-HPA axis and the associated stress response.9Mineralocorticoid receptors (MRs) and Glucocorticoid receptors (GRs)participate in the regulation of the HPA axis by binding to GC.10The negative feedback regulation effects of GC on the target regions of the brain,including the HPA axis,depending on the levels of GR and MR in the upper regulating centers and the GC concentration in the target areas.In addition to being influenced by the concentration of free GC in plasma,the level of GC in the target regions is regulated by the GC metabolic enzyme,11 beta-hydroxysteroid dehydrogenase (11β-HSD).11,12This is a molecule that regulates the rate at which GC is metabolized by catalyzing the REDOX reaction between the ketone group (inactive) and the hydroxyl group (active) at position 11 of the GC.1311β-HSD type 1 (11β-HSD1) is expressed mainly in the hippocampus,amygdala,neocortex,and cerebellum of adult rats.This helps to maintain CORT levels and plays an important role in promoting the growth and maturation of tissues.14Carbenoxolone (CBX),a derivative of glycyrrhiza,is a non-selective inhibitor of 11β-HSD that can inhibit both 11β-HSD1 and 11β-HSD type 2 (11β-HSD2).15The inhibition of 11β-HSD1 by CBX has been demonstrated using 11β-HSD1 knockout mice.16
Recent studies have shown that the main material basis of KYDS is functional disorder of the HPA axis.17Our previous research found that suspended moxibustion at the Shenshu (BL23) and Guanyuan (CV4)acupoints with a moxa stick can effectively improve dysfunctions of the HPA axis.18Thus,we hypothesized that a possible mechanism by which moxibustion improves KYDS may be up-regulation of the mRNA and protein expressions of MR,GR,and 11β-HSD1 in the amygdala-HPA axis of KYDS rats.This,in turn,would improve the serum levels of ACTH,CRH and CORT.This study aimed to further elucidated the possible molecular mechanisms through which moxibustion treats KYDS.
2.METHODS
2.1.Animals and grouping
Sixty healthy,clean male Sprague-Dawley rats aged 8 weeks [weighing (200 ± 20) g] were purchased from the Experimental Animal Center of Jiangxi University of Traditional Chinese Medicine [Jiangxi,China,certificate No.JZLLSC (Jiangxi) 2018-0033].The rats were fed with standard fodder,with food and water freely available in a controlled environment at a constant temperature of 20-25 ℃ and a 12/12-h light/dark cycle.All procedures were conducted in accordance with guidelines reviewed and approved by the Institutional Animal Care and Use Committee of Jiangxi University of Traditional Chinese Medicine,China.
Sixty rats were randomly divided into a control group(control,n=12) and an experimental group (n=48).Using a procedure modified from previous research,19each rat in the experimental group was given an intramuscular injection of hydrocortisone (Huazhong Pharmaceutical Co.Ltd.,national drug approval No.H420201507) at a dose of 0.03 mg/g daily for 14 d in the left and right hind limbs of the rat alternately.The criteria for model success were decreased body weight,withered hair,hunched back,aversion to cold,tendency to cluster,slowed reactions,weakness,decreased activity,scrotal shrinkage,and significantly increased urine output.20According to the modeling success criteria,all 48 rats were successfully modeled and could be used in our study.
The 48 successfully modeled rats were randomly subdivided into four groups (12 in each group): a model group (model),a carbenoxolone intraperitoneal injection group (CBX),a moxibustion group (moxi),and a moxibustion+carbenoxolone intraperitoneal injection group (moxi+CBX).Moxibustion treatment began on the first day after completion of modeling.Before treatment,the rats were shaved at the relevant acupoints to expose the skin.In the moxi group,the Guanyuan(CV4) and Shenshu (BL23) acupoints (acupoints were located with reference toExperimental Acupuncture21)were selected and heated by suspended moxibustion using a moxibustion cigar made of mugwort (length 12 cm,diameter 0.6 cm;custom made for use with animals in the Affiliated Hospital of Jiangxi University of Traditional Chinese Medicine,Nanchang,China).The cigar was suspended at a height of approximately 3 cm over the hairless areas of skin for 20 min once a day for 14 d.The rats were fixed on a splint for the moxibustion treatment.Rats in the CBX group were given intraperitoneal injections of carbenoxolone at a dose of 10 mg/kg15once a day for 14 d.Fixation was performed as for the moxi group once a day for 14 d but without suspended moxibustion.Rats in the moxi+CBX group received moxibustion in the same manner as those in the moxi group.After moxibustion treatment,the rats were given intraperitoneal injections of carbenoxolone in the same manner as those in the CBX group.Rats in both the control and model groups were fixed as for the moxi group but without moxibustion and given intraperitoneal injections of saline 10 mg/kg once a day for 14 d.
2.2.Sampling
After 14 d of treatment,the rats were anesthetized with intraperitoneal injections of sodium pentobarbital 3%(weight/volume) at a dose of 40 mg/kg.Arterial blood was extracted and centrifuged to extract the supernatant.This was stored at -20 ℃ for later analysis.Tissues from the amygdala,hypothalamus and pituitary were collected and stored at -80 ℃ for later analysis.
2.3.Enzyme-linked immunosorbent assay (ELISA)
The levels of CORT,ACTH and CRH were determined using an ELISA kit purchased from Shanghai bogu biotechnology Co.,Ltd.(Shanghai,China).The ELISAbased method was conducted according to protocol provided by manufacturer.
2.4.Reverse transcription real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from amygdala,hypothalamus and pituitary of rats in each group using TRIZOL reagent(Invitrogen,Carlsbad,CA,USA).The mRNA expression levels of MR,GR,11β-HSD1,CRH and ACTH were measured using an RT-qPCR system with SYBR Green (Thermo Fisher Scientific,Waltham,MA,USA).cDNA was amplified by PCR using primers for each target gene.cDNA was synthesized from each RNA using a random primer and RevertAid™ M-MuLV reverse transcriptase (Fermentas,Carlsbad,CA,USA),according to the manufacturer's instructions.RT-qPCR conditions were as follows: 94 ℃ for 5 min,followed by 40 cycles of 95 ℃ for 15 s,60 ℃ for 45 s and 72 ℃ for 30 s.The fluorescence signal was detected at 60 ℃,and the samples were finally extended at 72 ℃ for 7 min.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH)was used as an internal control.The relative mRNA levels of target genes were calculated using the 2-ΔΔCtmethod.The primers employed are listed in Table 1.
2.5.Western blot assay
All amygdala and hypothalamus tissues obtained in each group were homogenized in lysis buffer (JRDUN Biotechnology,Shanghai,China).The sample was centrifuged at 12 000 r/min for 20 min at 4 ℃,and an aliquot of the supernatant was taken for protein concentration estimation using the BCA assay.Equal amounts of proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis,and then the resolved proteins were transferred to polyvinylidene fluoride (PVDF) membranes (Millipore,Bedford,MA,USA).The membranes were incubated with primary antibodies overnight at 4 ℃.Monoclonal antibodies used for Western blotting included a rat monoclonal anti-MR antibody (1:1000;Santa Cruz Biotechnology,Santa Cruz,CA,USA).The antibodies used in the present study were rat monoclonal antibodies,1:1000 and were purchased from Santa Cruz Biotechnology (Santa Cruz,CA,USA),except for those specifically indicated.The immuneoreactive bands were visualized using an enhanced chemiluminescence reagent (Beyotime Biotechnology,Shanghai,China).The relative expression of protein was calculated based on the ratio of target grayscale values to loading control grayscale values.
2.6.In situ hybridization
The paraffin blocks were cut into 4-7-μm-thick serialsections.After being dewaxed and dehydrated,each section was incubated in 50 μL hybridization buffer containing 10 µM oligonucleotide probe at 95 ℃ for 5 min and 37-40 ℃ for 12 h,then washed with saline sodium citrate and treated with blocking buffer at 37 ℃for 15 min.Each section was then incubated in 30 µL biotinylated anti-digoxin antibody (1:50) at 37 ℃ for 1 h,incubated in streptavidin-biotin-peroxidase complex at 37 ℃ for 30 min.Subsequently,the sections were developed in 3,3,diaminobenzidine,counterstained with hematoxylin,dehydrated in alcohol,permeabilized in xylene,mounted with proof quench mounting agent,and photographed with a fluorescence microscope.The upper,middle,lower,left and right visual fields were randomly selected for each section and the image-pro Plus 6.0 software was used to detect the integrated optical density(IOD) of positive cells,the average value was used for statistical analysis.The negative control was incubated in 0.01M PBS without primary antibody.The primers employed are shown in Table 2.
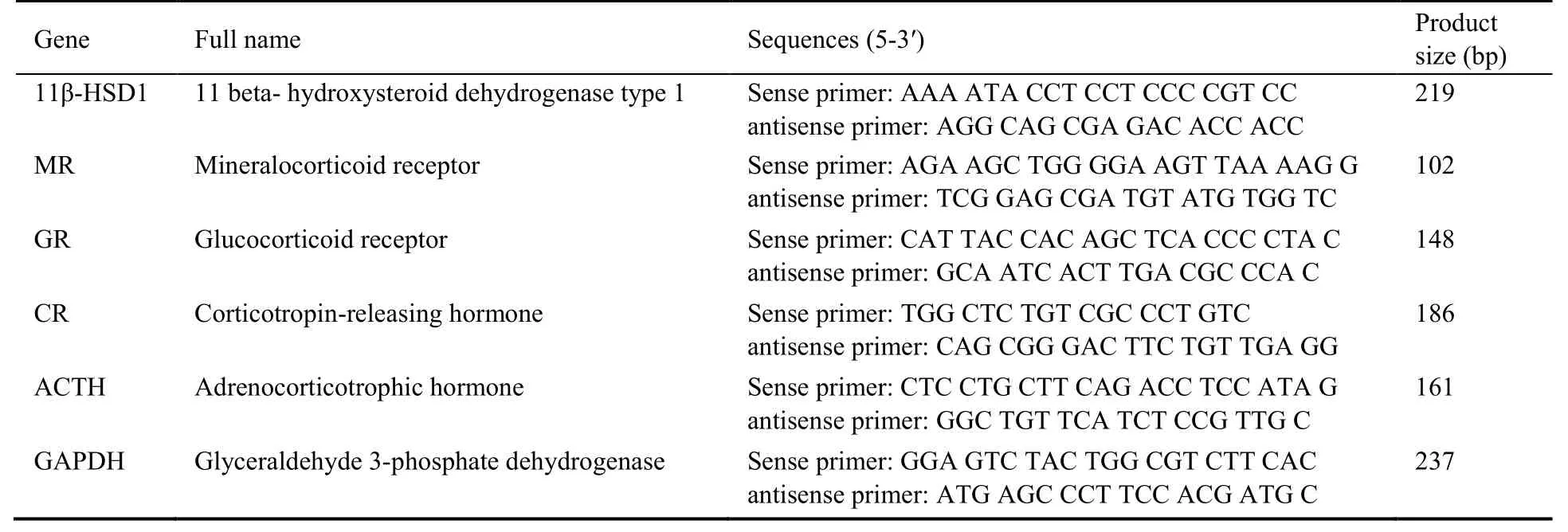
Table 1 Primer sequences for real-time quantitative polymerase chain reaction
2.7.Statistical analyses
All data were presented as mean ± standard deviation ().Data were analyzed using one-way analysis of variance followed by a posthoc Student-Newman-Keulstest using SPSS 19.0 software (IBM Corp.,Armonk,NY,USA).A value ofP <0.05 was considered statistically significant.
3.RESULTS
3.1.General condition of the rats
The rats injected with hydrocortisone lost weight,were less active,less responsive,less interested in novel things,hadarched backs,clustered together,lost hair luster,and had shrunken scrotums.After treatment with moxibustion,the weight and activity of these rats significantly increased and their hair gradually recovered its luster.The limbs and tail were less cold than before treatment and less cold than those of rats in the model group.
3.2.Serum levels of CORT,ACTH,and CRH measured using ELISA
The levels of serum CORT,ACTH,and CRH of the rats in the model group were significantly lower (P <0.01)than those in the control group.After treatment,the serum levels of CORT and ACTH in the moxi and moxi+CBX groups were significantly higher than those in the model group (P <0.05 or 0.01).The serum levels of CRH in the moxi,moxi+CBX,and CBX groups were significantly higher than those in the model group (P <0.05 or 0.01).The serum levels of CORT and CRH in the moxi group were significantly higher than those in the CBX group (P <0.01),and the serum levels of ACTH in the moxi and moxi+CBX groups were significantly higher than those in the CBX group (P <0.01 or 0.05)(Table 3).
3.3.mRNAexpressions of MR,GR,11β-HSD1,CRH,and ACTH measured using qRT-PCR
The mRNA expressions of MR and GR in the amygdala of rats in the model group were significantly lower than those in the control group (P <0.01).After treatment,the mRNA expressions of MR and GR in the amygdala of rats in the moxi group were significantly higher than those in the model group (P >0.01) but those in the CBX and the moxi+CBX groups were not.The mRNA expressions of 11β-HSD1 and CRH in the amygdala of rats were significantly lower in the model group than those in the normal control group (P <0.01).After treatment,except for11β-HSD1,the mRNA expression of CRH in the amygdala of rats in the moxi and the moxi+CBX groups were significantly higher than those in the model group (P <0.01 or 0.05).The mRNA expressions of 11β-HSD1 and CRH in the amygdala of rats were significantly higher in the moxi group than those in the CBX group (P <0.01 or 0.05) (Table 4).
The mRNA expressions of 11β-HSD1 and CRH in the hypothalamus of rats were significantly lower in the model group than those in the normal control group (P <0.01).After treatment,the mRNA expressions of CRH inthe hypothalamus of rats in the CBX,moxi,and moxi +CBX groups were all significantly higher than those in the model group (P <0.01).The 11β-HSD1 expression in the hypothalamus of rats in the moxi group was significantly higher than that in the model group (P <0.05).The mRNA expressions of 11β-HSD1 in the hypothalamus of rats in the moxi and the moxi+CBX groups and the CRH expression in the moxi group were significantly higher than those in the CBX group (P <0.01).The mRNA expression of ACTH in the pituitary of rats in the model group was significantly lower than that in the normal control group (P <0.01).After treatment,ACTH mRNA expression in the pituitary of the rats in the moxi and the moxi+CBX groups was significantly higher than that in the model group (P <0.01 or 0.05).The ACTH mRNA expression in the moxi group was significantly higher than that in the CBX group (P <0.01) (Table 5).Except for the GR mRNA expression in amygdala,all the afore mentioned indicators were no significant differences between the moxi and the moxi+CBX groups (P >0.05) (Tables 4,5).
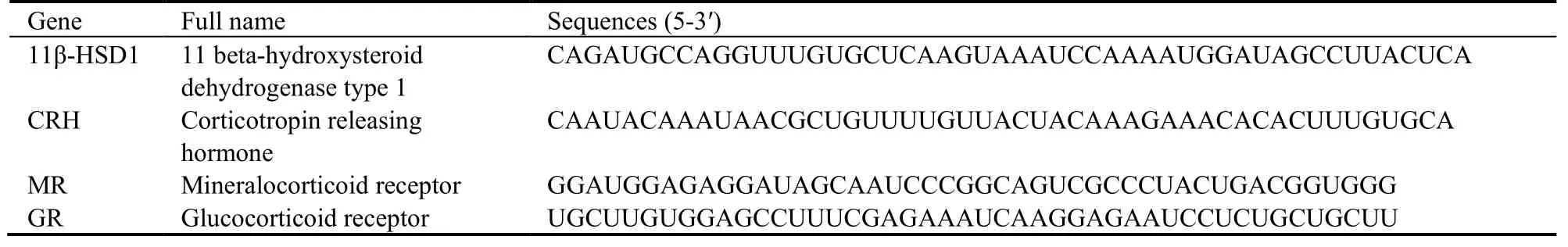
Table 2 Primer sequences for Hybridization in situ
Table 3 Comparison of the level of CORT,ACTH and CRH in serum (ng/L,)
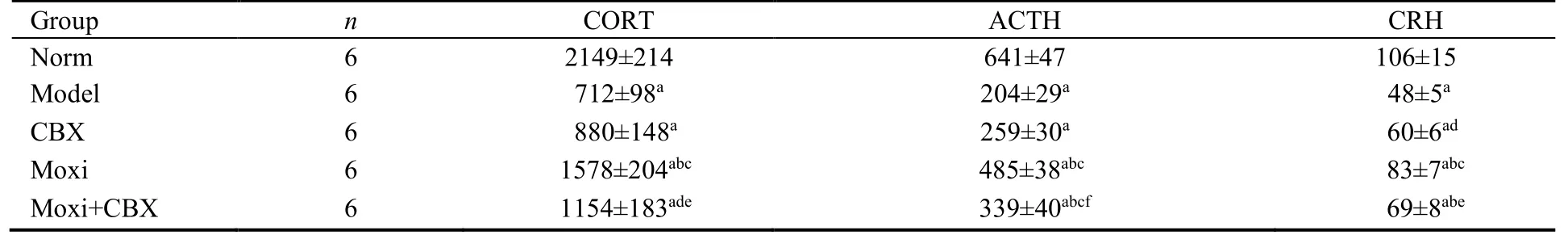
Table 3 Comparison of the level of CORT,ACTH and CRH in serum (ng/L,)
Notes: norm: normal control group;model: model group;CBX: carbenoxolone intraperitoneal injection group;moxi: moxibustion group;moxi+CBX: moxibustion plus carbenoxolone intraperitoneal injection group.CORT: corticosterone;ACTH: adrenocorticotrophic hormone;CRH:corticotropin-releasing hormone.aP < 0.01 versus norm;bP < 0.01,dP < 0.05 versus model;cP < 0.01,fP < 0.05 versus CBX;eP < 0.05 versus moxi.
3.4.Protein expressions of MR,GR,and 11β-HSD1 measured using Western blotting
The protein expressions of MR,GR,and 11β-HSD1 in the amygdala of rats in the model group were all lower than those in the normal control group (P <0.05 or 0.01).After moxibustion treatment,the protein expressions of MR,GR,and 11β-HSD1 in the amygdala of rats were higher than those in the model group (P <0.05).There were no significant differences between the CBX,moxi,and the moxi+CBX groups in the protein expressions of MR,GR,and 11β-HSD1 (P >0.05).(Figure 1A and 1B).The expression of 11β-HSD1 in the hypothalamus of rats in the model group was significantly lower than that in the normal control group (P <0.01).After treatment,the 11β-HSD1 protein expression in the hypothalamus of rats was significantly higher than that in the model group(P <0.01).There were no significant differences in the hypothalamic expression of 11β-HSD1 between the CBX,moxi,and the moxi+CBX groups (P >0.05)(Figure 1C and 1D).
3.5.mRNA expressions of MR,GR,11β-HSD1,and CRH in the amygdala and 11β-HSD1 and CRH expressions in the hypothalamus measured using in situ hybridization
As shown in Figures 2-4,in situhybridization-positive cells contained brown spots or particles,as indicated by the arrows.In the normal control group,considerable mRNA expressions of MR,GR,11β-HSD1,and CRH in the amygdala and 11β-HSD1 and CRH in the hypothalamus were observed (Figures 2-4).In the modeled rats,there were a few mRNA expressions of MR,GR,11β-HSD1,and CRH in the amygdala or 11β-HSD1 and CRH expressions in the hypothalamus.The IOD values of mRNA expressions of MR,GR,11β-HSD1,and CRH in the amygdala and 11β-HSD1 and CRH in the hypothalamus of rats in the model group were significantly lower than those in the normal control group (P <0.01) (Figure 2,Table 6).
Treatment with moxibustion increased the mRNA expressions of MR,GR,11β-HSD1,and CRH,and the IOD values of their positive cells in the amygdala of rats in the moxi group were significantly higher than those in the model and CBX groups (P <0.01 and 0.05) (Figures 2,3,Table 6).The IOD value of MR-positive cells in the moxigroup was significantly higher than that in the moxi+CBX group (P <0.05) (Figure 2,Table 6).The IOD value of 11β-HSD1-positive cells in the moxi+CBX group was higher than that in the CBX group (P <0.05).The IOD value of CRH-positive cells in the moxi+CBX group was higher than that in the model group (Figure 3,Table 6).Treatment with moxibustion increased the mRNA expression of 11β-HSD1.The IOD values of 11β-HSD1-positive cells in the hypothalamus of rats in the moxi and the moxi+CBX groups were significantly higher than those in the CBX group (P <0.01 or 0.05) but not those in the model group (P> 0.05).Treatment with moxibustion increased the mRNA expression of CRH.The IOD values of CRH-positive cells in the hypothalamus of rats in the moxi and the moxi+CBX groups were significantly higher than those in the model group (P <0.01 or 0.05) but not those in the CBX group(P >0.05) (Figures 4,Table 7).
Table 7 Comparison of the IOD values of 11β-HSD1 and CRH mRNA-positive cells in hypothalamus ()
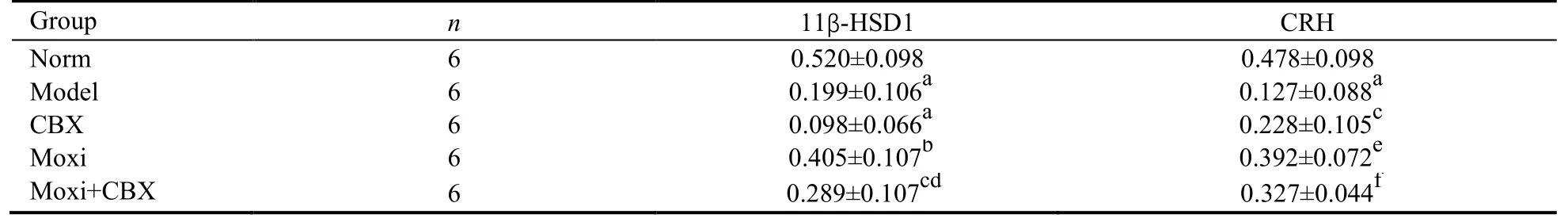
Table 7 Comparison of the IOD values of 11β-HSD1 and CRH mRNA-positive cells in hypothalamus ()
Notes: norm: normal control group;model: model group;CBX: carbenoxolone intraperitoneal injection group;moxi: moxibustion group;moxi+CBX: moxibustion plus carbenoxolone intraperitoneal injection group.IOD: integrated optical density;11β-HSD1: 11 beta-hydroxysteroid dehydrogenase;CRH: corticotropin-releasing hormone.aP < 0.01,cP < 0.05 versus norm;eP < 0.01,fP < 0.05 versus model;bP < 0.01,dP < 0.05 versus CBX.

Figure 3 mRNA expression of 11β-HSD1 and CRH in the amygdala following treatment (in situ hybridization,× 200)

Figure 4 mRNA expression of 11β-HSD1 and CRH in the hypothalamus following treatment (in situ hybridization,× 200)
Table 6 Comparison of the IOD values of MR,GR,11β-HSD1 and CRH mRNA-positive cells in amygdala ()

Table 6 Comparison of the IOD values of MR,GR,11β-HSD1 and CRH mRNA-positive cells in amygdala ()
Notes: norm: normal control group;model: model group;CBX: carbenoxolone intraperitoneal injection group;moxi: moxibustion group;moxi+CBX: moxibustion plus carbenoxolone intraperitoneal injection group.IOD: integrated optical density;MR: mineralocorticoid receptor;GR:glucocorticoid receptor;11β-HSD1: 11 beta-hydroxysteroid dehydrogenase;CRH: corticotropin-releasing hormone.aP < 0.01 versus norm;bP <0.01,eP < 0.05 versus model;cP < 0.01,fP < 0.05 versus CBX;dP < 0.05 versus moxi.

Figure 1 MR,GR and 11β-HSD1 activation in the amygdala and hypothalamus following treatment
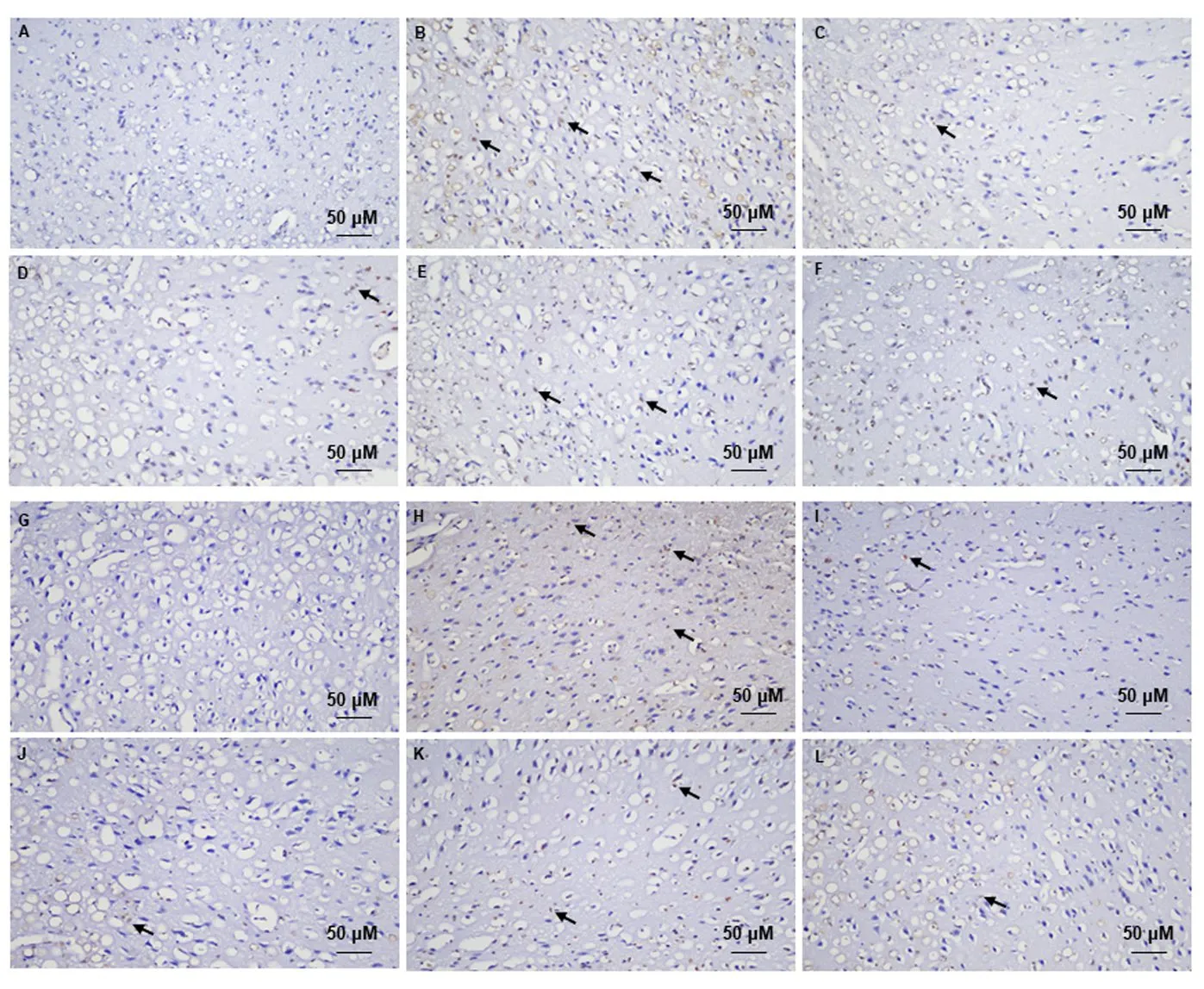
Figure 2 mRNA expression of MR and GR in the amygdala following treatment (in situ hybridization,× 200)
Table 4 Comparison of the relative mRNA expressions of MR,GR,11β-HSD1 and CRH in amygdala (/GAPDH) (‰,)
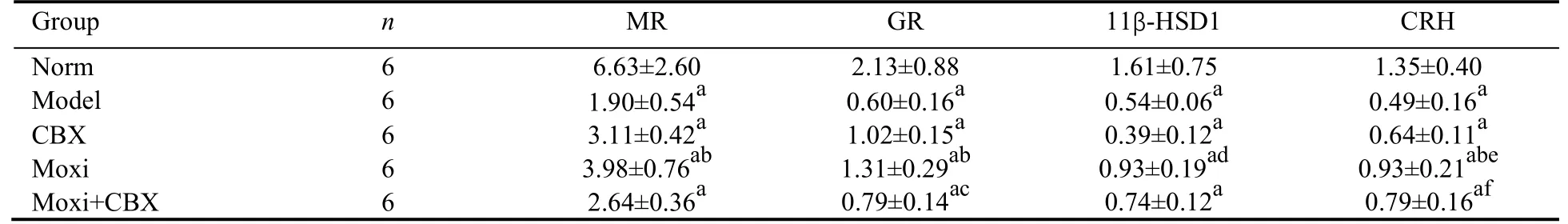
Table 4 Comparison of the relative mRNA expressions of MR,GR,11β-HSD1 and CRH in amygdala (/GAPDH) (‰,)
Notes: norm: normal control group;model: model group;CBX: carbenoxolone intraperitoneal injection group;moxi: moxibustion group;moxi+CBX: moxibustion plus carbenoxolone intraperitoneal injection group.MR: mineralocorticoid receptor;GR: glucocorticoid receptor;11β-HSD1: 11 beta-hydroxysteroid dehydrogenase type 1;CRH: corticotropin-releasing hormone;GAPDH: glyceraldehyde-3-phosphate dehydrogenase.aP < 0.01 versus norm;bP < 0.01,fP < 0.05 versus model;dP < 0.05,eP < 0.01 versus CBX;cP < 0.01 versus moxi.
Table 5 Comparison of the relative mRNA expressions of 11β-HSD1,CRH in hypothalamus and ACTH in pituitary (/GAPDH) (‰,)
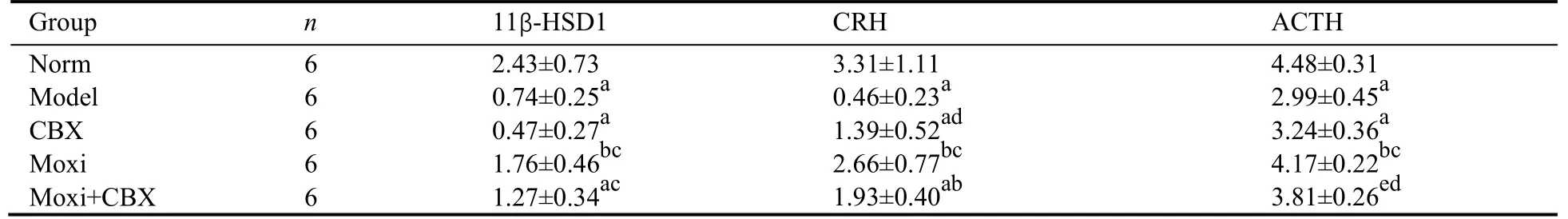
Table 5 Comparison of the relative mRNA expressions of 11β-HSD1,CRH in hypothalamus and ACTH in pituitary (/GAPDH) (‰,)
Notes: norm: normal control group;model: model group;CBX: carbenoxolone intraperitoneal injection group;moxi: moxibustion group;moxi+CBX: moxibustion plus carbenoxolone intraperitoneal injection group.11β-HSD1: 11 beta-hydroxysteroid dehydrogenase type 1;CRH:corticotropin-releasing hormone;ACTH: adrenocorticotrophic hormone;GAPDH: glyceraldehyde-3-phosphate dehydrogenase.aP < 0.01,eP <0.05 versus norm;bP < 0.01,dP < 0.05 versus model;cP < 0.01 versus CBX.
4.DISCUSSION
The KYDS model is the most widely used in the experimental study of TCM deficiencies.In the early 1960s,Kuang22established the first hydrocortisone animal model of KYDS.In the supplementary document,we show that modeling caused decreases in body weight and activity and interest index (interest in novel objects)scores,deterioration of the general physical condition,reduced urine 17-OHCS and serum testosterone,and increased serum estradiol levels.The aforementioned indicators were all ameliorated after treatment with Youguipills,proving that the KYDS model was successful.These methods and techniques are basically in line with the evaluation criteria of KYDS animal models.23Our previous research has verified the repeatability of the experimental animal model of KYDS induced by hydrocortisone injection.18,24
Stress can cause the body to produce large amounts of exogenous corticosteroids for prolonged periods,and the resultant GC leads to excessive activation of GR,which eventually damages nerve cells in upper regulatory centers such as the hippocampus and amygdala.Feedback from this inhibits the functions of the HPA axis.When the exogenous corticosteroid production ceases,the inhibition of the HPA axis becomes apparent.The adaptability of the body to changes in the external environment significantly decreases,and a series ofYangdeficiencies occur,including temperature dysregulation that causes the body to feel colder.25,26For example,the treatment for some autoimmune diseases requires longterm use of many hormones,when the disease abates or improves,the patient finds they have developed a dependency on the hormones,showing the symptoms ofYangdeficiency.27,28The rat model of KYDS is established on this same principle of hormone dependency.In the present study,after the model was established,the rats manifested numerous deficiencies such as withered hair,decreased body weight,slowed reactions,aversion to cold,weakness,a tendency to cluster,and decreased activity.Using PCR,Western blotting,andin situhybridization,we found lower expressions of MR,GR,11β-HSD1,and CRH in the amygdala of rats in the model group than those in the control group.A similar reduction was seen in the expressions of 11β-HSD1 and CRH in the hypothalamus of rats in the model group.Through feedback,GC can inhibit the generation of ACTH and CRH.In addition,the amygdala can also synthesize and secrete CRH,thus forming the feedback-regulating loop of the amygdala -HPA axis29-32In this study,we found that the levels of serum CORT,ACTH,and CRH and the mRNA expression of ACTH in the pituitary of model group rats were significantly lower than those of the normal control group.And a tendency also seen in our previous study.3311β-HSD has two isoenzymes,11β-HSD1 and 11β-HSD2.11β-HSD1 is a primary reductase that converts inactive 11-dehydrocorticosterone/cortisone into active CORT/cortisol.But the action of 11β-HSD2 is opposite to that of 11β-HSD1.14When the concentration or activity of GC is highin vivo,11β-HSD2 outweighs 11β-HSD1 in the role of conversion.In contrast,when GC is low,11β-HSD1 outweighs 11β-HSD2.Together,they assist MR and GR in the maintenance of stable hormone levels.CBX can inhibit both 11β-HSD1 and 11β-HSD2.15In this study,GC was low in the modeled rats.This caused activation of 11β-HSD1 and MR.Therefore,the mRNA expression of MR in the amygdala was approximately three times that of GR.As a blocking agent,CBX mainly inhibits 11β-HSD1,so 11β-HSD1 was selected as our observation index rather than 11β-HSD2.The expression of 11β-HSD1 in the amygdala and hypothalamus of rats in the CBX group was lower than that in the model group.As the effect of 11β-HSD1 was blocked by CBX,the concentration or activity of GC was lower in the CBX group,causing increased expression of MR in the amygdala through feedback regulation.Just as MR expression in the amygdala of rats in the CBX group was higher than that in the model group,correspondingly,CRH expression in the amygdala and hypothalamus and ACTH expression in the pituitary of the rats in the CBX group were all higher than those in the model group,and the levels of serum CORT,ACTH,and CRH in the CBX group were higher than those in the model group.A literature search found no similar studies reported.However,some research has used the expressions of CRH,MR,GR,etc.,in the amygdala and hypothalamus as observation indicators to study the effect of GC on the body.For example,when pregnant Wistar rats were injected with CBX (12.5 mg s.c.) daily throughout pregnancy,Welberget al34found that CBX treatment reduced birth weight and this reduced body weight persisted into adulthood.The offspring of the CBXtreated rats also engaged in less grooming and showed reduced mobility in a forced swim test.In addition,they found that these animals had increased basal CORT levels,increased CRH,reduced GR mRNA in the hypothalamus,and increased GR mRNA in the amygdala.Their MR mRNA expression levels were normal.The effect of CBX on the expressions of CRH,MR,and GR in these rats was inconsistent with the results of the present study.This maybe because the animal models were different in that experiment and this.
In our previous study,moxibustion on the Shenshu(BL23) and Guanyuan (CV4) acupoints was found to positively regulate the dysfunction of the pituitaryadrenal and pituitary-thyroid axes in KYDS model rats.18In the present study,there was significantly greater up-regulation of the mRNA and protein expressions of MR,GR,and 11β-HSD1 in the amygdala of rats after treatment of the Shenshu (BL23) and Guanyuan (CV4)points with suspended moxibustion than was seen in the model group.The mRNA expression of 11β-HSD1 and CRH in the hypothalamus;ACTH in the pituitary;and serum levels of CRH,ACTH,and CORT of rats were all significantly higher in the moxi group than those in the model group.According to TCM theory,Shenshu (BL23)is mainly used to repairYang,Guanyuan (CV4) is mainly used to repairQi,and the two acupoints are in harmony with each other,so as to cultivateYuan Qi,and to improve the function of tonifying kidney and warmingYang.35One possible reason is that,according to the theory fromYi Zong Jin Jian,36another classic work of TCM,Shenshu (BL23) point belongs to the bladder meridian,which is the back shu point of the kidney,and Guanyuan (CV4) point belonging to Ren meridian,is the small intestine collection point,and is the commonly used point for the treatment of all kinds of deficiency.Another reason may be that,according to modern neuroanatomical theory,the Shenshu (BL23) acupoint is innervated by the first to third lumbar ganglion segment,which is connected to the genitalia via the ilioinguinal nerve and the genital nerve of the lumbar plexus.It is closely connected to the kidneys,adrenal glands,and internal genitalia through the branches of the lumbar sympathetic trunk and the lumbar visceral nerve.18The Guanyuan (CV4) acupoint is connected to the liver,spleen,and kidneys through the 12th thoracic nerve and related small visceral nerves.Zhouet al37used horseradish peroxidase nerve tract-tracing technology and found that the afferent projections of the Guanyuan(CV4) and the uterus converge and overlap in the spinal ganglia between lumbar 3 and sacral 5.In the present study,the strong theoretical basis for treatment with suspended moxibustion over the Shenshu (BL23) and Guanyuan (CV4) was supported by our experimental findings.Zhaoet al38found that the symptoms of KYDS were improved after mild moxibustion treatment of the Guanyuan (CV4) and Shenshu (BL23) acupoints in elderly patients.Ren et al35found that the moxibustion of the Shenshu (BL23) and Guanyuan (CV4)significantly improved the inhibitory state of the HPA axis in KYDS rats.This was one of the reasons that Shenshu (BL23) and Guanyuan (CV4) were selected for the present study.
In this study,we demonstrated that suspended moxibustion can effectively improve serum levels of ACTH,CRH,and CORT and up-regulate the mRNA and protein expressions of MR,GR,11β-HSD1,CRH,and ACTH in the amygdala and hypothalamus of kidney-Yang,-deficient rats.The experimental model of KYDS used in this study shares pathologic similarities to the presentation of KYDS in humans.Thus,MR,GR,and 11β-HSD1 could represent novel therapeutic targets in the treatment of KYDS.
 Journal of Traditional Chinese Medicine2023年1期
Journal of Traditional Chinese Medicine2023年1期
- Journal of Traditional Chinese Medicine的其它文章
- Effects of the Huangkui capsule (黄葵胶囊) on chronic kidney disease: a systematic review and Meta-analysis
- Effectiveness of moxibustion alone on lumbar disc herniation: a Meta-analysis of randomized controlled trials
- Efficacy of acupuncture therapy for post-stroke fatigue: a systematic review and Meta-analysis
- Efficacy of luteolin on the human gastric cancer cell line MKN45 and underlying mechanism
- Tilianin extracted from Xiangqinglan (Herba Dracocephali Moldovicae) inhibits apoptosis induced by mitochondrial pathway and endoplasmic reticulum stress in H9c2 cells after oxygenglucose deprivation/reoxygenation
- Comparing the effects of three decoctions for coronavirus disease 2019 on severe acute respiratory syndrome coronavirus 2-related tolllike receptors-mediated inflammations
