Clinical features of retinal amyloid angiopathy with transthyretin Gly83Arg variant
Gang Su, Xing-Wang Chen,4, Jun-Lin Pan, Hong Li,4, Bing Xie,4, Shan-Jun Cai,4
1Department of Ophthalmology, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China
2Guizhou Eye Hospital, Zunyi 563000, Guizhou Province, China
3Guizhou Provincial Branch of National Eye Disease Clinical Research Center, Zunyi 563000, Guizhou Province, China
4Special Key Laboratory of Ocular Diseases of Guizhou Province, Zunyi 563000, Guizhou Province, China
Abstract
● KEYWORDS: retinal amyloid angiopathy; hereditary transthyretin amyloidosis; fundus fluorescein angiography;vitrectomy; retinal photocoagulation
INTRODUCTION
Retinal amyloid angiopathy (RAA) was first reported in 1955 by Fallset al[1]. RAA is an uncommon retinal vasculopathy secondary to hereditary transthyretin amyloidosis (hATTR) that can lead to severe visual impairment. The common clinical manifestations of RAA are retinal microangiomas, recurrent vitreous hemorrhages, retinal neovascularization, and neovascular glaucoma[2]. The RAA findings of classic fundus fluorescein angiography (FFA)include focal arterial retinal obstruction and perivascular staining. Autofluorescence imaging showed hyper‐autofluorescence amyloid deposits along the retinal vessels[3].Histopathological studies confirmed the harmful effects of amyloid deposits on endothelial cells. These effects lead to capillary occlusion and loss of the integrity of the endothelial barrier, thus confirming the relevant vascular pathological components of this disease[2]. A variety of transthyretin (TTR)gene mutation types can cause RAA, and the currently reported mutation types are V30M, Y114C, E54G, R34K, and A36P[4‐8].TTRGly83Arg is a mutation hotspot in Southwest China, which can lead to hATTR (ATTRG83R amyloidosis)[9].The ATTRG83R amyloidosis mainly involves the eyes[10].However, there was no RAA has been found secondary to ATTRG83R amyloidosis in previous reports.
In this study, we report five patients with RAA caused by TTR Gly83Arg and analyze clinical features to supplement the available information on this disease and improve understanding.
SUBJECTS AND METHODS
Ethical ApprovalThis study was approved by the Medical Ethics Committee of Zunyi Medical University (approval number: 2018‐1‐060) and performed following the World Medical Association’s Declaration of Helsinki. Informed consent was obtained from all of the patients.
A consecutive interventional case series of seven eyes of fi ve patients seen at Affiliated Hospital of Zunyi Medical University from January 2010 to December 2021 with RAA is reported.The diagnosis was based on a detailed medical history inquiry,systemic and ophthalmic examinations, and gene sequencing.The DNA sequencing method and primer information were described previously[11]. The ocular examinations included best‐corrected visual acuity (BCVA) assessment with the logarithm of the minimum angle of resolution (logMAR),anterior segment slit‐lamp biomicroscopy (YZ5EⅢ; 66 Vision Tech Co., Suzhou, China and SL990N; C.S.O. SRL,Italy), gonioscopy (G‐1 Gonio; Volk, Ohio, USA), noncontact tonometry (CT‐80A; Topcon, Tokyo, Japan), and fundus evaluation by indirect ophthalmoscopy (All Pupil; Keeler,Windsor, UK), further underwent fundus photography (AFC‐330; Nidek, Japan), ocular ultrasound (SW‐2100; Suoer, Tianjin,China), and ultrasound biomicroscopy (UBM) examination (SW‐3200L; Suoer, Tianjin, China). All affected eyes were excluded from diseases such as vitreitis, peri retinal vasculitis, retinal vein occlusion, and diabetic retinopathy. For patients who underwent vitrectomy in our hospital, postoperative fundus photographs were documented, and visual acuity, intraocular pressure (IOP), and fundus examination were assessed at follow‐up. FFA (Spectralis; Heidelberg Engineering Inc.,Germany) was performed in patients 4 and 5.
RESULTS
Clinical CharacteristicsA total of fi ve patients (7 eyes) were diagnosed with RAA secondary to ATTRG83R amyloidosis in this study. These patients were identifi ed from 14 patients(28 eyes) with ATTR amyloidosis previously diagnosed in our department. The mean age of the patients was 52.00±7.23y.They had been diagnosed with ATTRG83R and had vitreous amyloidosis in all 10 eyes. The diagnosis of ATTRG83R amyloidosis was established by family history, Congo red staining, and genetic sequencing. Seven of the ten eyes were diagnosed with RAA 2 to 20y after the onset of ATTRG83R amyloidosis. Two eyes (29%) exhibited recurrent vitreous hemorrhage, two eyes (29%) had neovascular glaucoma,and one eye (14%) had neovascularization of the iris. FFA suggested that the incidence of retinal microangioma and retinal non‐perfusion area were 100% and 67%, while no cases of retinal neovascularization were identifi ed. Six eyes, except the left eye in case 5, had undergone vitrectomy for vitreous amyloidosis before being diagnosed with RAA. All patients had no abnormalities detected in the whole‐body examination.A summary of each case is presented in Table 1.
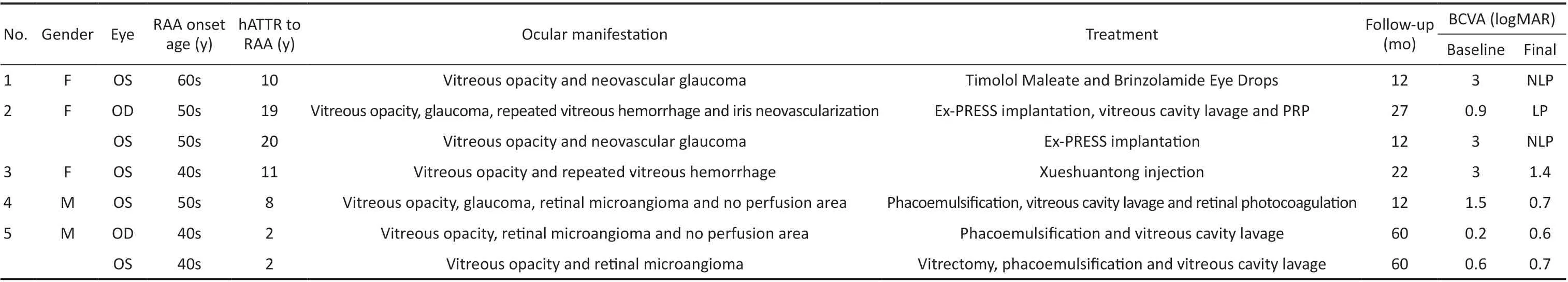
Table 1 Clinical data of 5 patients with retinal amyloid angiopathy

Figure 1 Photomicrographs of the anterior segment in a patient with RAA (case 2) A: Hyphema in the right eye, with a depth of about 1 mm; with an Ex-PRESS glaucoma drainage device visible in the supranasal atrial corner; B: Hyphema in the right eye with a depth of approximately 8 mm, with two Ex-PRESS glaucoma drainage devices visible in the supratemporal and supranasal atrial corners; C: Occlusion of pupil and iris neovascularization at 11 o’clock and two Ex-PRESS glaucoma drainage devices visible in the supratemporal and supranasal atrial corners; D: A medically dilated pupil in the left eye with a pupil diameter of approximately 9 mm; E: Neovascularization on the surface of the iris in the left eye with posterior iris synechia; F: Corneal clouding, corneal endothelial folds, neovascularization on the surface of the iris and abnormal pupillary shape. RAA: Retinal amyloid angiopathy.
Case 1A woman in her 60s presented with pain and decreased vision in the left eye. She had been diagnosed with ATTRG83R amyloidosis 3y earlier and had undergone vitrectomy and phacoemulsification for vitreous amyloidosis in the left eye.One month ago, the right eye of the patient also underwent vitrectomy due to vitreous amyloidosis. At the current presentation, a BCVA of 3 logMAR (hand motion equivalent)and IOP of 52.5 mm Hg in the left eye, a BCVA of 0.7 logMAR and an IOP of 10 mm Hg in the right eye. In the left eye, slit‐lamp examination revealed neovascularization on the surface of the iris, fundus examination and B‐ultrasound revealed vitreous opacity, but no obvious abnormality was found in the right eye. UBM for the left eye showed that the depth of the anterior chamber was normal, and the anterior chamber angle narrowed at all 360 degrees. Ex‐PRESS glaucoma drainage device implantation was recommended, but the patient refused the surgery. Timolol maleate and brinzolamide eye drops were prescribed. Regrettably, the vision after 12mo was no light perception.
Case 2A woman in her 50s presented with pain and decreased vision in the left eye. She had been diagnosed with ATTRG83R amyloidosis 11y earlier and had undergone a vitrectomy for vitreous amyloidosis in both eyes, followed by phacoemulsification in the right eye 5y later. At the current presentation, her right eye exhibited a BCVA of 0.9 logMAR and IOP of 38 mm Hg. Fundus examination and B‐ultrasound revealed vitreous hemorrhage. UBM for the right eye showed that the depth of the anterior chamber was normal, and the anterior chamber angle narrowed at 12 o’clock and 6 o’clock.The patient underwent Ex‐PRESS glaucoma drainage device implantation. Six weeks after surgery, the patient was hospitalized again because of pain and decreased vision in the right eye. The BCVA of the right eye was 3 logMAR (hand motion equivalent) and the IOP was 33.8 mm Hg. Hyphema in the right eye, with a depth of about 1 mm (Figure 1A),may be led to the blockage of the drainage device. Therefore,the patient underwent Ex‐PRESS glaucoma drainage device implantation again. BCVA at 12mo after surgery was 1.1 logMAR and IOP was 12 mm Hg. Totally 24mo after surgery, the patient was hospitalized again due to decreased vision in the right eye.The visual acuity was light perception and IOP was 8.6 mm Hg.Slit‐lamp examination showed an 8 mm depth of hyphema(Figure 1B), and B‐ultrasound revealed vitreous hemorrhage.The patient underwent anterior chamber irrigation, vitreous cavity lavage and panretinal photocoagulation. After 3mo, the visual acuity was light perception and the IOP was 10 mm Hg.Slit‐lamp examination revealed occlusion of the pupil and iris neovascularization at 11 o’clock (Figure 1C).
She presented with pain and decreased vision in her left eye,one year after similar symptoms in her right eye. At the current presentation, her left eye exhibited a BCVA of 3 logMAR(hand motion equivalent) and IOP of 40 mm Hg. Slit‐lamp examination showed neovascularization on the surface of the iris (Figure 1E), and UBM showed that posterior iris synechia,iris bombe, and the anterior chamber angle narrowed at all 360 degrees. The patient underwent Ex‐PRESS glaucoma drainage device implantation, intra‐ and postoperative courses were uneventful. The visual acuity at 12mo after surgery was no light perception and IOP was 22.3 mm Hg. Slit‐lamp examination showed corneal clouding, corneal endothelial folds, neovascularization on the surface of the iris and abnormal pupillary shape (Figure 1F).
Case 3A woman in her 40s presented with sudden‐onset decreased vision in the left eye. She had been diagnosed with ATTRG83R amyloidosis 7y earlier and had undergone a vitrectomy for vitreous amyloidosis in both eyes. Followed by vitreous cavity lavage for recurrence of vitreous amyloidosis in the left eye 5y later. At the current presentation, a BCVA of 3 logMAR (hand motion equivalent) and 0.3 logMAR in the left and right eye, respectively. Fundus examination and B‐ultrasound revealed vitreous hemorrhage in the left eye and normal in the right eye. The patient received an intravenous infusion of Xueshuantong injection for 1wk. BCVA at 12mo after treatment was 0.2 logMAR. Totally 48mo after treatment,the patient was hospitalized again because of sudden‐onset decreased vision in the left eye. The BCVA of the left eye was 3 logMAR (hand motion equivalent). Fundus examination and B‐ultrasound revealed vitreous hemorrhage. She received an intravenous infusion of Xueshuantong injection for 1wk again.BCVA at 22mo after the latest treatment was 1.4 logMAR.Fundus examination showed vitreous opacity, multiple white patches (recurrent amyloidosis) on the surface of the retina and hemorrhage focus beside the subtemporal branch vein of the retina.
Case 4A man in his 50s presented with decreased vision in the left eye. He had been diagnosed with ATTRG83R amyloidosis 7y earlier and had undergone a vitrectomy for vitreous amyloidosis in the left eye. At the current presentation,a BCVA of 1.5 logMAR and IOP of 34.2 mm Hg in the left eye, a BCVA of 1.4 logMAR and IOP of 15.3 mm Hg in the right eye. Slit‐lamp showed that lens opacity, and fundus examination showed that vitreous opacity. Optic cup/optic disc was approximately 0.3 in the left eye. Fundus examination showed that the fundus structure of the right eye was unclear due to vitreous opacity. UBM for the left eye showed that the depth of the anterior chamber was normal, and the anterior chamber angle narrowed at 12 o’clock, 9 o’clock, and 6 o’clock.The patient’s left eye underwent phacoemulsification and vitreous cavity lavage. Postoperative FFA suggested multiple end‐vessel fluorescein staining and microangiomas in the nasal, superior, and temporal peripheral retina of the left eye,with a large perfusion‐free area visible in the superior temporal area and late fluorescein leakage at the lesion. And retinal photocoagulation of the non‐perfused area was performed.BCVA at 12mo after surgery was 0.7 logMAR and IOP was 18 mm Hg.
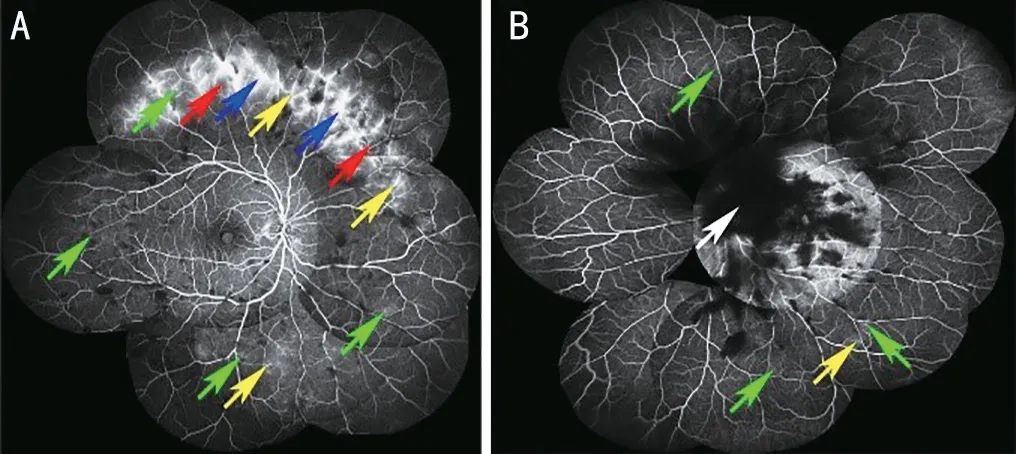
Figure 2 FFA images of a patient with RAA (case 5) A: Multiple fluorescein staining (yellow arrows) of the vascular canal wall and microangiomas (green arrows) in the right eye’s nasal, superior, temporal,and inferior peripheral retina; with small areas of non-perfusion (red arrows) above the nose and late fluorescein leakage (blue arrows) at the lesion; B: A large vitreous opacity (white arrow) in the posterior pole of the left eye obscured fluorescence, and three peripheral vascular fluorescein staining (yellow arrow) and microangiomas (green arrows) were seen in the peripheral retina above and below the temporal area, with late fluorescein leakage at the lesion. FFA: Fundus fluorescein angiography; RAA: Retinal amyloid angiopathy.
Case 5A man in his 40s presented with decreased vision in the left eye. He had been diagnosed with ATTRG83R amyloidosis 1y earlier and had undergone a vitrectomy for vitreous amyloidosis in the right eye. At the current presentation, his left eye exhibited a BCVA of 0.6 logMAR.Fundus examination and B‐ultrasound for the left eye revealed vitreous opacity. FFA suggested that multiple fluorescein staining of the vascular canal wall and microangiomas in the right eye’s nasal, superior, temporal, and inferior peripheral retina; with small areas of non‐perfusion above the nose and late fluorescein leakage at the lesion (Figure 2A). A large vitreous opacity in the posterior pole of the left eye obscured fluorescence, and three peripheral vascular fluorescein staining and microangiomas were seen in the peripheral retina above and below the temporal area, with late fluorescein leakage at the lesion (Figure 2B). The patient underwent a vitrectomy for his left eye. BCVA at 12mo after surgery was 0.3 logMAR.Totally 58mo after surgery, the patient was hospitalized again because of decreased vision in both eyes. Slit‐lamp showed that lens opacity, and B‐ultrasound showed vitreous opacity.The patient underwent phacoemulsification and vitreous cavity lavage for both eyes. The BCVA of the right eye and the left eye 12mo after surgery were 0.6 logMAR and 0.7 logMAR,respectively.
DISCUSSION
In this study, RAA occurred in five (7 eyes) out of 14 (28 eyes)TTR G83R carriers diagnosed with hATTR in our department,with a 25% (7/28) prevalence of RAA. Beirãoet al[5]found a 4% prevalence of RAA in a cross‐sectional study of 518 hATTR patients carrying V30M. The incidence of RAA was significantly higher in ATTRG83R than in patients carrying V30M. This is because of the significant correlation between RAA and vitreous amyloidosis[5]. hATTR due to ATTRV30M is dominated by peripheral nervous system involvement,with ocular involvement in only 24% of patients[12]. So far,most of the reported ATTRG83R amyloidosis patients havevitreous amyloidosis as the first symptom[11,13‐15]. This leads to differences in the incidence of RAA between the mutation types. Furthermore, Kakiharaet al[16]suggested that RAA correlates with the TTR synthesized by retinal pigment epithelial cells. However, similar results were not observed in the present study. Polyneuropathy was not observed in RAA patients caused by TTR G83R in this study. However, RAA patients caused by other TTR variants have been observed with other organs affected (Table 2)[4,6‐8].
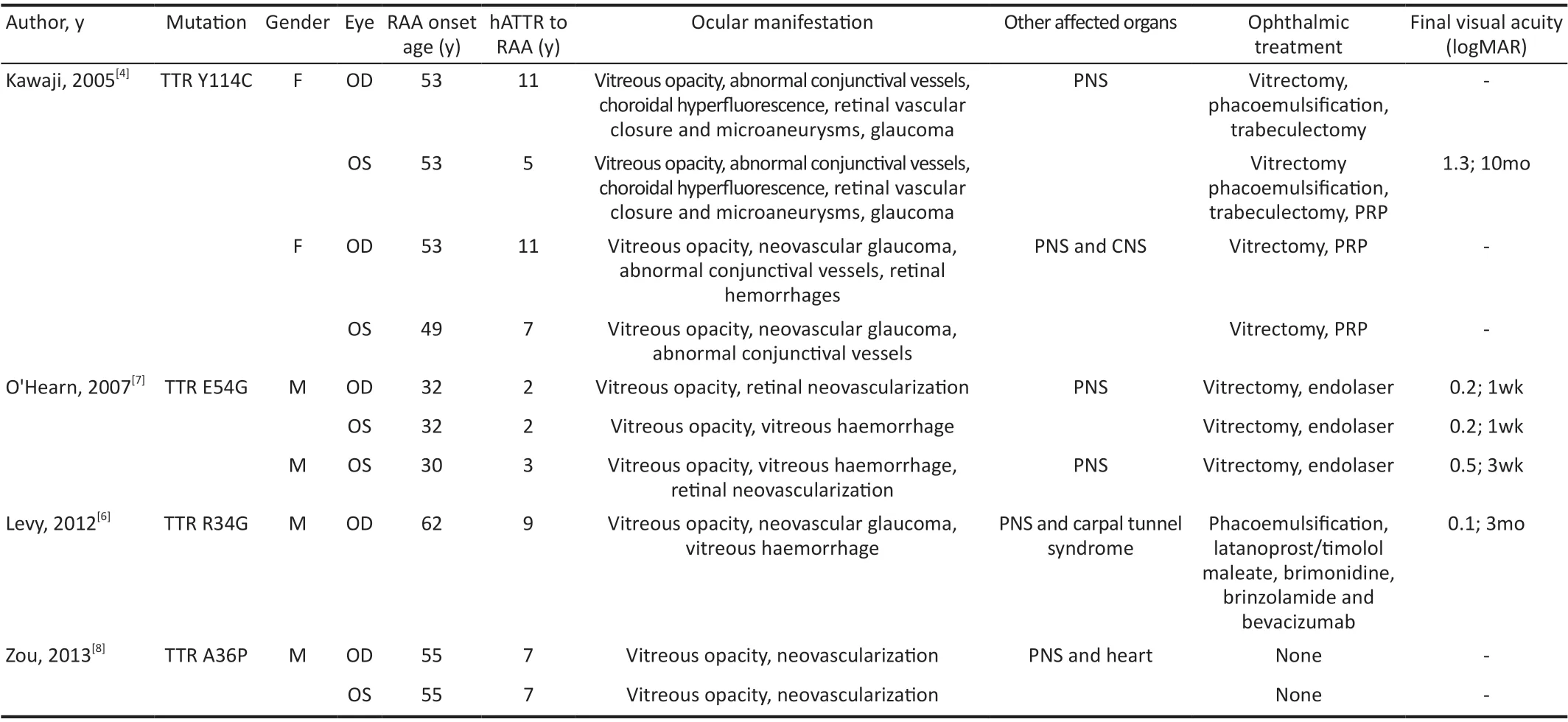
Table 2 Summary of clinical data provided in published case reports of retinal amyloid angiopathy
RAA presents with retinal microangioma, retinal hemorrhage,focal retinal ischemia, neovascular glaucoma, and retinal neovascularization[17]. The typical FFA manifestations of RAA are retinal microangioma, focal retinal artery occlusion,and segmental vascular staining[2‐4,18]. Among these, retinal microangiomas are the most common and are present in almost all patients with RAA[2]. Similarly, microangioma lesions were found in all affected eyes that underwent FFA in this group of cases. Microangiomas are the cause of recurrent retinal hemorrhage in patients with RAA[2]. Retinal vasculopathy,which can lead to retinal ischemia and thus neovascularization,usually occurs in the anterior segment of the eye and less frequently in the posterior segment[17]. Neovascularization is the most serious ocular complication of RAA and can lead to blindness[17]. The most fundamental cause of these pathologies is the aggregation of amyloid near the retinal vessels, which was observed in a histopathological study by Kawji[4]. Recent histopathological studies have shown that amyloid can cause vascular endothelial cell damage[19]. These injuries lead to capillary occlusion and loss of endothelial barrier integrity,initially forming microangiomas in the capillaries[19]. These microangiomas can lead to recurrent retinal hemorrhage[2].Further progression of the lesion disrupts retinal vascular wall integrity and forms localized vascular occlusions,leading to ischemic lesions[19]. These ischemic lesions can cause photoreceptor and macular dysfunction[3]. RAA,similar to other retinal ischemic diseases, eventually causes neovascularization with serious consequences. The incidence of neovascularization in this study was 43% (3/7), which contrasts with the incidence of neovascularization in another Japanese study (0/36)[16]. This suggests differences in the incidence of neovascularization in RAA, based on mutation type and geography[16].
Liver transplantation is a recognized hATTR treatment that improves patient survival and halts or slows the progression of peripheral neuropathy[20]. However, Beirãoet al[5]found that liver transplantation did not alter the progression of RAA, suggesting that the amyloid protein that causes RAA originates from retinal pigment epithelial cells[21]. It has been demonstrated that reducing the secretion of ocular TTR by panretinal photocoagulation lowers the recurrence rate of vitreous amyloidosis. Panretinal photocoagulation[22]and retinal photocoagulation in the non‐perfused area[17]were both equally effective in reducing the risk of neovascularization in RAA. Therefore, an intraoperative combination of panretinal photocoagulation was chosen based on the recurrence of vitreous amyloidosis, recurrent retinal hemorrhage,and blindness in the contralateral eye due to neovascular glaucoma in case 2. However, this patient still developed iris neovascularization postoperatively. In case 4, a perfusion‐free area of the retina with a more limited lesion was identified on postoperative FFA; hence, retinal photocoagulation of the perfusion‐free area was chosen as the treatment method.Whether the therapeutic goal can be achieved remains to be further observed. Minnellaet al[17]believed that vitrectomy accelerates the onset and progression of RAA. In this study,the course of hATTR was essentially the same in both eyes of case 5. His right eye, which had been treated with vitrectomy, exhibited significantly more severe RAA in the FFA one year after surgery compared with the left eye. This may be due to the aggravation of RAA caused by vitrectomy.However, it is not ruled out that it is caused by two eyes suffering from diseases successively. Therefore, further,observation is required to confirm. Patients with hATTR are also at risk of developing glaucoma after vitrectomy[10,22];therefore, the timing of surgery should be carefully managed,and postoperative FFA should be routinely performed. It is necessary to perform FFA once a year in the follow‐up after vitrectomy. And, FFA should also be performed in time if the hATTR patients have clinical manifestations such as vitreous hemorrhage and high intraocular pressure. Ophthalmologic follow‐up of hATTR patients is essential. Patients with hATTR should undergo an ophthalmologic examination at the time of diagnosis, regardless of whether the first symptoms involve the eye. Thereafter, ophthalmologic follow‐up should be performed every two years for asymptomatic carriers and once a year for symptomatic patients. If the patient has an ocular lesion, follow‐up is based on the different types of ocular lesions: annual for conjunctival vascular abnormalities; semi‐annual for dry eyes, amyloid deposits on the pupil rim or crystal surface; and every 3mo for fan iris, glaucoma, vitreous amyloidosis, and RAA[21,23].
In summary, this is the first time that RAA has been described in ATTRG83R amyloidosis patients. Complications such as RAA and glaucoma will seriously affect the visual prognosis of patients. Thereafter, regular ophthalmic follow‐up of patients with hATTR is essential. And FFA examination after vitrectomy is very important, which can help ophthalmologists detect RAA earlier and treat it in time.
ACKNOWLEDGEMENTS
The authors would like to thank the patients in this study.
Authors’ contributions:Conception or design of the work:Cai SJ, Chen XW; Data collection: Pan JL; Data analysis and interpretation: Su G, Li H, Xie B; Drafting the article: Su G,Chen XW; Critical revision of the article: Cai SJ, Chen XW.All authors have read and approved the published version of the manuscript.
Foundations:Supported by the National Natural Science Foundation of China (No.31871261); the Guizhou Science and Technology Cooperation Foundation [No.ZK (2021) general 423]; the Research Initiation Fund for Masters in Affiliated Hospital of Zunyi Medical University (No.2016‐43).
Conflicts of Interest: Su G,None;Chen XW, None;Pan JL,None;Li H,None; Xie B, None;Cai SJ,None.
 International Journal of Ophthalmology2023年1期
International Journal of Ophthalmology2023年1期
- International Journal of Ophthalmology的其它文章
- Instructions for Authors
- Morphological and functional changes in the macular area in diabetic macular edema after a single intravitreal injection of aflibercept
- Macular vascularisation changes analysed using OCT angiography after successful rhegmatogenous retinal detachment repair
- Comparison of success rate and intraocular pressure spikes between selective laser trabeculoplasty and micropulse laser trabeculoplasty in African American and Hispanic patients
- Efficacy of custom-made soft keratoconus lenses on corneal aberrations and photic phenomena in patients with keratoconus: a corneal topography imaging based study
- Clinical observation of recombinant human nerve growth factor in the treatment of neurotrophic keratitis
