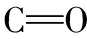Improved Sensitivity of Localized Surface Plasmon Resonance Using Silver Nanoparticles for Indirect Glyphosate Detection Based on Ninhydrin Reaction
XU Meng-lei,GAO Yu,ZHU Lin,HAN Xiao-xia,ZHAO Bing*
1.State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun 130012, China 2.College of Food Science and Engineering, Jilin University, Changchun 130062, China 3.College of Plant Protection Key Laboratory of Soybean Disease and Pest Control(Ministry of Agriculture and Rural Affairs),Jilin Agricultural University, Changchun 130118, China
Abstract Conventional pesticide residue detection still suffers from numerous steps, is long time-consuming, and insufficient delicacy.According to ninhydrin colouring and the principle of localized surface plasmon resonance(LSPR)enhancing absorption, glyphosate in water samples can be detected by ultraviolet-visible spectroscopy(UV-Vis).Furthermore, density functional theory is used to analyze the enhancement mechanism of absorption of purple color dye(PD)products.The PD product displays a maximum absorption of around 570 nm when glyphosate reacts with ninhydrin is detected by UV-Vis.There are slight shifts from 570 nm in the UV-Vis spectrum to 568 nm with a stronger peak when the PD product is absorbed on Ag NPs, and the limit of detection at 2.017 4×10-11 mol·L-1, which is much lower than 6.5×10-7 mol·L-1 limit of detection reported.Gaussian 09 software carried out that the PD product would attach to Ag NPs via an Ag—O bond through ninhydrin’s group vertically.MEP mapping provides group interaction with Ag NPs stable, group coupled C—N comprise a large π-conjugated system in their plane.The color of the C—N group is blue, which suggests that C—N coupled with the group are the chromophore in the PD product.Thus, an indirect detection method derived from ninhydrin can be used for glyphosate detection in water samples.The LSPR effect of Ag NPs enhances the absorption intensity with higher sensitivity than the conventional method.
Keywords Localized surface plasmon resonance; Purple color dye product; Ultraviolet and visible spectrophotometry; Density functional theory; Glyphosate; Ninhydrin
Introduction
Glyphosate(C3H8NO5P)is the highest volume herbicide used worldwide with a potential toxic pollutant in the environment[1].The common determination methods use chromatographs coupled to various detectors[2].These methods are sensitive.However, they still suffered with numerous steps for the purification and derivatization due to their highly polar and no fluorescence group[3].Therefore, developing a new analytical technique for monitoring glyphosate is necessary[4-5].
The colorimetric method has been found simple and economical for determining of glyphosate[6].Glyphosate reacts with ninhydrin from its amino group, and the product is purple, similar to Ruhemann’s purple product, while this ‘Purple Color Dye(PD)Product’ is slightly different.This conventional colorimetric method appeared to have a limit of quantification(LOQ)at 0.04 μg·mL-1in water samples in UV-Vis[7].Localized surface plasmon resonance(LSPR)is a collective oscillation of conduction band electrons in metal nanoparticles(NPs)driven by the electromagnetic field of the incident light, which could significantly increase the light absorption and the generation of the photon carriers.Metal NPs have been widely exploited as colorimetric sensors or probes for detecting a variety of analytes because of their high extinction coefficients and strong LSPR properties[8].In this paper, we describe a simple and cheap screening method for detecting glyphosate in water enhanced by LSPR of Ag NPs based on the ninhydrin reaction aim of high sensitivity and simplicity.
1 Material and Methods
1.1 Chemicals
Silver nitrate(AgNO3)was purchased from Sigma-Aldrich Chemical Co., Ltd.Glyphosate, sodium citrate(Na3C6H5O7·2H2O), sodium molybdate(Na2MoO4)and ninhydrin were obtained from Aladdin Industrial Corporation.Tap water was collected without further clean-up for real sample determination.
1.2 Apparatus
UV-Vis extinction spectra were measured with a lambda1050+ spectrophotometer(Perkinelmer).
1.3 Sample preparation
Ag NPs were prepared as follows, 36.0 mg AgNO3was added into 200.0 mL H2O first, and using sodium citrate(1%ω/V, 4 mL)as a reducing agent.A grey-green colloid formed and was naturally cooled at room temperature, after heating at 85 ℃ for 40 min.
Ninhydrin working reagent: Nninhydrin solution(5%,ω/V)+water+acetate buffer(0.4 mol·L-1, pH 5.5)(2∶1∶1,V/V/V).Mixed working solutions: Ninhydrin working reagent+5% Na2MoO4+sample/glyphosate solution(1∶1∶1,V/V/V)were heated in boiling water for 30 min; and mixed with Ag NPs(1∶1,V/V).
Water samples were only clean-up by a 0.45 μm microporous ultrafiltration membrane, and then added different concentrations of glyphosate.
1.4 Theoretical method
All geometries were optimized using the B3LYP exchange-correlation function.The 6-311++G(d,p)basis set was used for the H, C, O, N, P atoms, and LanL2DZ level for Ag NPs.All calculations were carried out using the Gaussian 09 software program.
2 Results
2.1 UV-Vis spectra of Ag NPs, PD product, and PD product adsorbed on Ag NPs
The Ag NPs used in this study have a maximum absorption of around 440 nm(Fig.1a).The PD product displays a maximum absorption of around 570 nm(Fig.1d).When the PD absorbed on Ag NPs, there are slight shifts from 570 nm in the UV-Vis spectrum to 568 nm with a stronger peak(Fig.1b).This should originate from the interaction between the Ag NPs and the PD product molecules.
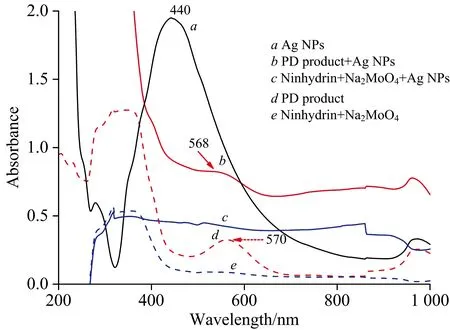
Fig.1 UV-Vis spectrum of Ag NPs(a), the PD product+Ag NPs(b), ninhydrin+Na2MoO4+Ag NPs(c), PD product(d), and ninhydrin+Na2MoO4(e)(glyphosate concentration of 1.0×10-4 mol·L-1)
2.2 Molecular geometry of PD product on Ag
The optimized geometry of the PD product[Fig.2(a)], its Ag[Fig.2(b)]and Ag3[Fig.2(c)]complexes were geometry-optimized.
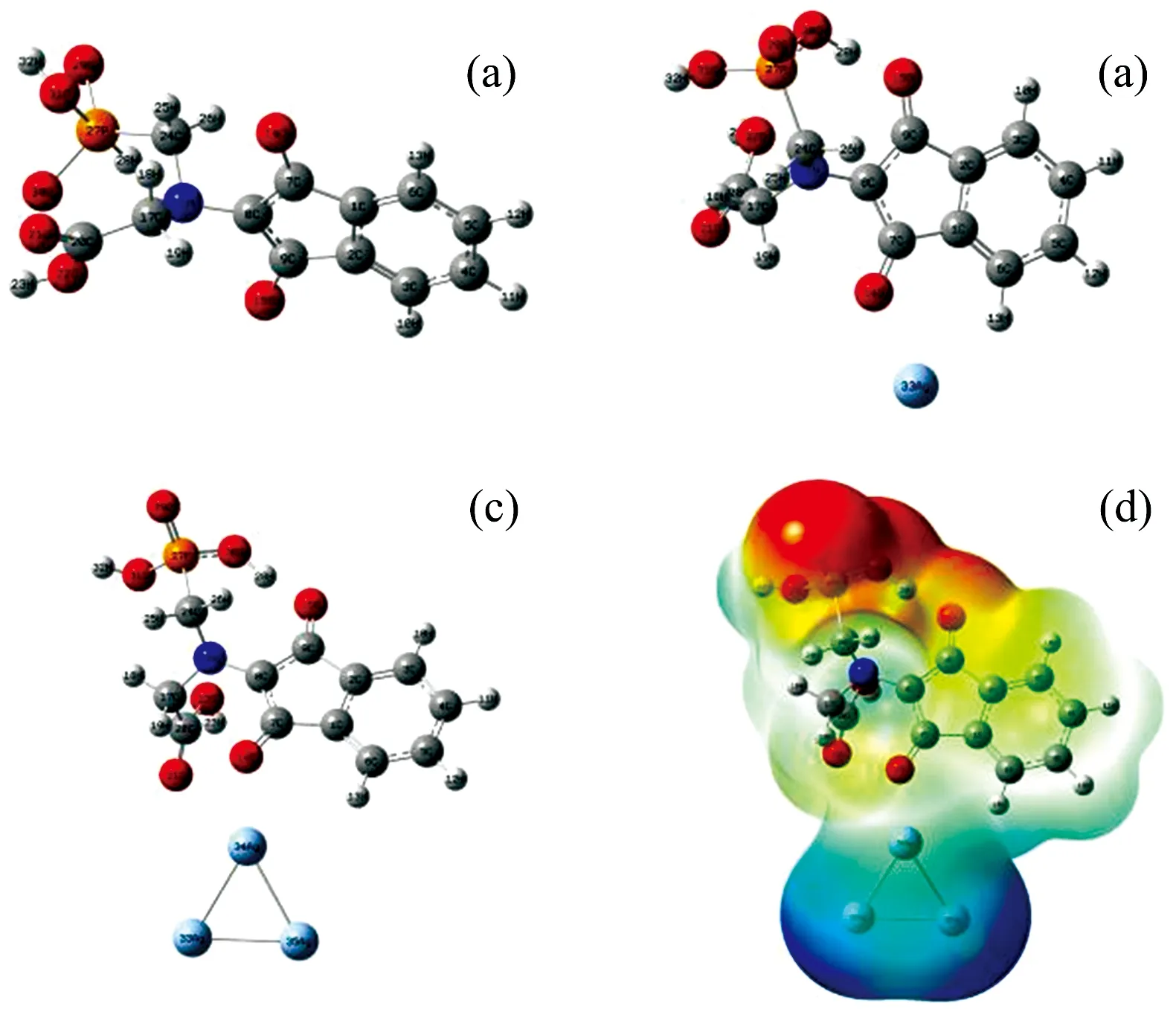
Fig.2 Molecular structures and electrostatic potential(MEP)mapping of purple color dye(PD)product and its Ag complexes
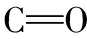
2.3 Quantitative analysis of glyphosate
Representative concentration-dependent UV-Vis spectra of the PD products were shown in Fig.3(a).The absorbance intensity at 568 nm shows a good linear relationship with the concentrations of PD product in the range of 1.00×10-9~1.00×10-4mol·L-1(y=1.000 3+0.053 23×lgc,R2=0.939 5)[Fig.3(b)].The LOD is thus calculated to be 2.017 4×10-11mol·L-1.
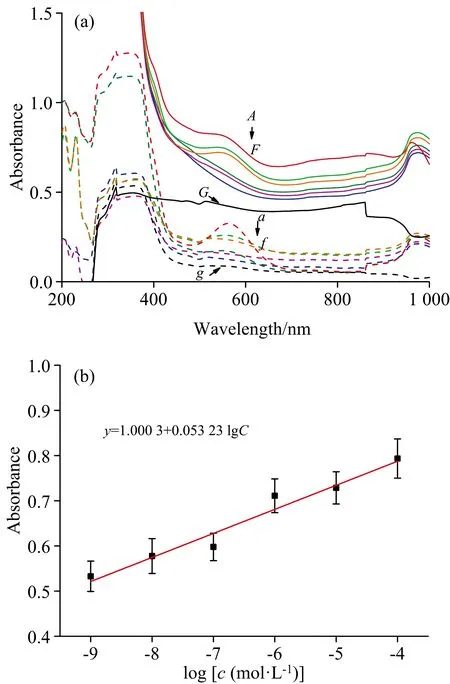
Fig.3 UV-Vis spectra of the PD products at different glyphosate concentrations(a)and representative concentration-dependentof the PD products(b)
Recovery experiments were performed in each batch analysis to validate the method by spiking samples in five replicates at 1.00×10-8, 1.00×10-7, 1.00×10-6and 1.00×10-5mol·L-1.The recoveries of water samples ranged from 71.26%±1.63% to 114.68%±11.30% without further clean-up.After further clean-up process, the recoveries ranged from 88.76%±4.81% to 98.51%±4.16%.It should be some unknown molecules in tap water that interfered with the adsorption of the target molecules on Ag NPs.
2.4 Influence of coexisting substance on PD product and UV-Vis spectra
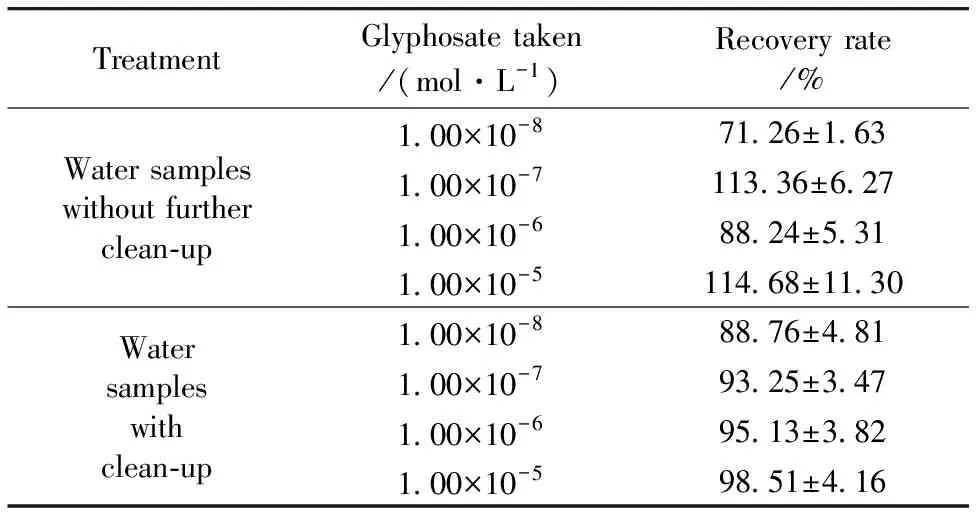
Table 1 Recoveries of glyphosate spiked into tap water
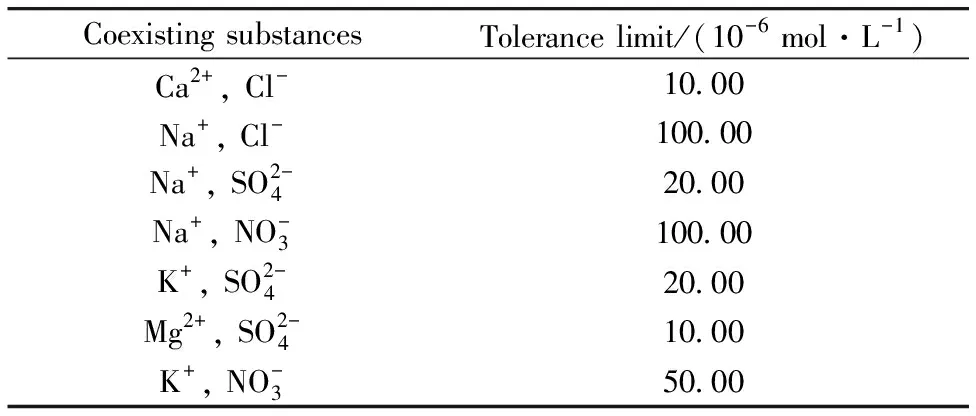
Table 2 Influence of coexisting substances on glyphosate detection in water samples
3 Discussion