Purif ication and immunoglobulin E epitopes identif ication of low molecular weight glutenin: an allergen in Chinese wheat
Yno Wng, Yihng Tong, Jinru Zhou, Dong Yng, Linglin Fu,*
a Food Safety Key Laboratory of Zhejiang Province, School of Food Science and Biotechnology, Zhejiang Gongshang University, Hangzhou 310018, China
b College of Food Science and Nutritional Engineering, China Agricultural University, Beijing 100083, China
Keywords:Triticum aestivum Allergen Low molecular weight glutenin (LMW-GS)Immunological tools
A B S T R A C T As one of the most important cereals, wheat (Triticum aestivum) has high nutritional value and is widely cultivated in China. However, wheat can cause severe allergic reactions, and a growing number of people are developing allergies to Chinese wheat. Low molecular weight glutenin (LMW-GS), an important allergen in susceptible populations, is responsible for celiac disease and wheat contacts dermatitis. In this study,LMW-GS was highly purif ied from Chinese wheat (Xiaoyan 6) and further identif ied and characterized. In addition, 8 peptides were predicted eff iciently by 5 immunological tools, among which 5 peptides showed signif icant immunoglobulin E binding abilities. Two specif ic epitopes were found to be in the non-conserved region of the amino acid sequence of LMW-GS, which was speculated to be the specif ic epitope of Chinese wheat. This systematic research of LMW-GS may provide new insights into the prevention of wheat allergy and development of hypoallergenic wheat products.
1. Introduction
Wheat (Triticum aestivum) is spread all over the world as one of the most important cereals, and wheat can be made into bread,noodles and cake. Moreover, wheat has a high nutritional value which is rich in B vitamins, protein, and minerals, and is widely used in processed foods such as noodles, bread, biscuits and so on.Unfortunately, wheat can induce various clinical types of food allergy,such as celiac disease, baker’s asthma, wheat contacts dermatitis and wheat-dependent exercise-induced anaphylaxis (WDEIA). According to epidemiological statistics, 0.2%-0.9% of adults and 0.4%-1.3%of children in the world are allergic to wheat [1,2]. In addition,t he prevalence of celiac disease which is mainly caused by wheat glutenin has gradually increased in recent decades and has reached 1% in the western population [3]. In China, adults account for the majority of the wheat allergic population, and wheat induces 57% of food severe allergic reactions [4]. So far, 28 proteins in wheat have been registered as allergens by the World Health Organization and International Union of Immunological Societies (WHO/IUIS, www.allergy.org). Due to their solubility, wheat allergens can be classif ied into soluble protein (soluble in water and salt), gliadin (soluble in 70% ethanol) and glutenin (soluble in weak acid or weak base) [5].Especially, low molecular weight glutenin (LMW-GS) is responsible for many clinical symptoms of wheat allergy, such as celiac disease and wheat contacts dermatitis [6,7].
LMW-GS accounts for nearly 40% of wheat proteins and is composed of 3 subunits with a molecular weight of 30-80 kDa [8].The extraction and separation of LMW-GS are diff icult because of its poor solubility and the similarity of molecular weight to gliadin. In addition, mass spectrometry may not provide suff icient information to identify LMW-GS, because its subunit contains many single proteins with similar sequences, including plentiful proline and glutamine.These proteins usually only produce one or two trypsin peptides [9].Numerous separations attempts in the past have focused on the selective precipitation technique such as dimethyl sulfoxide (DMSO) [10],acetone [11], andn-propanol precipitation [12]. However, these methods have an unideal separating effect because of the similar properties of gliadin and glutenin especially LMW-GS. In addition,Molecular size exclusion chromatography (SEC) was developed to purify LMW-GS [13]. However, the disadvantage of SEC was that urea can destroy the disulfide and hydrogen bonds in the glutenin,causing the separation of LMW-GS into individual subunits. As a result of obtaining high-purity LMW-GS, it is beneficial to produce protein standards and analyze the physicochemical property. On the basis of the above research status, purification of LMW-GS is worthy of further exploration.
The allergenicity of the allergen mainly depends on the epitopes [14],which are mostly located on the surface of antigenic substances and identified by corresponding antibodies or sensitized lymphocytes. In addition, the presence of similar epitopes may induce immunoglobulin E(IgE) cross-reactivity in clinical status of patients [15]. So far, various typical methods to map epitopes, such as overlapping peptides [16],phage display [17] and protein chip [18], are expensive and time-consuming. Bioinformatics is a quick and effective method that applies computer technology to perform statistical analysis on related properties of allergens, and predicts epitopes through different algorithms with an accuracy of over 80% [19]. It is worth mentioning that the different allergen-associated clinical symptoms from different wheat species or patients in different regions are not completely identical [20]. According to previous reports on WEDIA allergy patients [21], LMW-GS is the main allergen causing WEDIA in Japan, and celiac disease in French [22]. LMW-GS is responsible for most wheat-induced food allergies in Japan. In previous studies,epitopes of LMW-GS are mainly concentrated in Japanese wheat by phage display [23]. However, the epitopes of Chinese wheat allergens have not been systematically explored. One type of Chinese wheat,Xiaoyan 6, is representative wheat due to it has large output and pioneers the way of crop breeding, whose highest output can reach 450 kg per acre in China. It is important to analyze the epitopes of Xiaoyan 6 by bioinformatics methods to further compare the differences with other wheat cultivars.
In the present study, we established a simple method to highly purify LMW-GS with ion-exchange chromatography, and further identified by double enzyme (trypsin and chymotrypsin) digestion in liquid chromatography-tandem mass spectrometry (LC-MS/MS).Moreover, the secondary structure and allergenicity of purified LMW-GS were characterized. Finally, the IgE epitopes of LMW-GS in Chinese wheat (Xiaoyan 6) were predicted by bioinformatics methods and further identified by competitive inhibitory enzyme-linked immunosorbent reaction (icELISA) assay.
2. Materials and methods
2.1 Materials and human serum samples
Xiaoyan 6 wheat was obtained from Henan Academy of Agricultural Sciences, China. Human serums came from allergic patients with obvious clinical symptoms after taking wheat were purchased from PlasmaLab (USA) and listed in Table S1. The 10 patients, 4 females and 6 males, aged 16 to 50 and the average age was 35 years old. Allergy symptoms were related to IgE-mediated allergy,and the sIgE values of all sera were determined by ImunoCAP 100(Phadia, Sweden). All patients were in class 3 allergy (> 3.5 kU/L),and 2 patients were in class 4 allergy (> 17.5 kU/L). These serums were stored at -80 °C until further use.
2.2 Preparation of glutenin from wheat
According to previous studies [24,25], the wheat flour was firstly decreased by acetone at a 1:10 (m/V) ratio at 4 °C until the solution was clear and transparent. Then, defatted wheat was ultrasonically vibrated in 70% ethanol at a 1:5 (m/V) ratio for 30 min to remove gliadin. The extract was centrifuged at 7 000 ×gfor 20 min at 4 °C to collect the precipitate and then repeated 2 times. The precipitate was then mixed with extractant A (50%N-propanol, 1%dithiothreitol (DTT)) at a 1:5 (m/V) ratio for 30 min at 60 °C, and the supernatant was obtained after centrifugation at 10 000 ×gfor 30 min at 4 °C. After that, the supernatant was adjusted to 80%N-propanol with extractant B (100%N-propanol, 1% DTT), followed by stirring for 1 h at 4 °C, and the precipitate was collected after centrifugation at 10 000×gfor 30 min at 4 °C. Then, the precipitate was dissolved in 0.5 mol/L acetic acid, and analyzed by sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE).
2.3 Purification of LMW-GS
The precipitate was subjected to a cation exchange column (0.7 ×2.5 cm) using SP Sepharose Fast Flow (GE Healthcare) equilibrated in buffer (10 mmol/L glycine-acetate, 50% iso-propanol, 2 mmol/L DTT, pH 6.5) at a flow rate of 0.5 mL/min. Then, linear 0-0.3 mol/L sodium chloride gradient in the eluent was performed at a flow rate of 1 mL/min, and the collected fractions containing LMW-GS were analyzed by SDS-PAGE.
2.4 Mass spectrometry identification
2.4.1 In-gel digestion
The band of the LMW-GS on SDS-PAGE gel was excised and then destained in a destaining solution (50 mmol/L NH4HCO3, 50%acetonitrile). Then, the gel pieces were dehydrated with 100 µL of 100% acetonitrile for 5 min, rehydrated in 5 mmol/L dithiothreitol and incubated at 56 °C for 20 min after removing liquid. After that, the gel pieces were washed with 50 mmol/L NH4HCO3and dehydrated with 100% acetonitrile. Followed by rehydration with 10 ng/µL chymotrypsin resuspended in 100 mmol/L Tris-HCl and 10 mmol/L CaCl2, the gel pieces were then digested with chymotrypsin at 25 °C overnight. Finally, 10 ng/µL trypsin was added into the gel at 37 °C for 10 h.
2.4.2 LC-MS/MS analysis
The tryptic peptides were dissolved in solvent A (0.1% formic acid), and directly loaded onto a home-made reversed-phase analytical column. The gradient was comprised of an increase from 6% to 23% solvent B (0.1% formic acid in 98% acetonitrile) over 16 min. Then, 23% to 35% solvent B was eluted for 8 min, climbed to 80% for 3 min, and held at 80% for the last 3 min. All procedures were performed at a flow rate of 300 nL/min on an EASY-nLC 1000 UPLC system (Thermo Scientific, New York, USA). The mass spectrometry parameters were set as follows: electrospray voltage applied, 2.0 kV;m/zscan range, 350 to 1 800; resolution of Orbitrap,70 000; dynamic exclusion, 15 s, and automatic gain control (AGC).
2.4.3 Data analysis
The resulting MS-MS data were processed using MaxQuant.The search parameters were set as follows: database,uniprottriticum+aestivum+ldatabase; TOF MS-MS match tolerance, 0.02 Da;specified fixed modification, Carbamidomethyl on Cys; variable modification, Deamidation (NQ) and Acetyl (Protein N-term), and Min delta score for modified peptides.
2.5 Characterization of LMW-GS
The secondary structure of LMW-GS was determined by using circular dichroism (CD). The purified LMW-GS was dissolved in 0.5 mol/L acetic acid after nitrogen blowing. After dialysis with water and lyophilization, the sample was dissolved in water and diluted to 0.2 mg/mL. The scanning conditions of the CD instrument were set as follows: wavelengths, 190 nm to 260 nm; scanning speed,100 nm/min; bandwidth, 1 nm; and cuvette width, 1 mm. The CD was performed by JASCOJ-1500 (Jasco, Tokyo, Japan). The LMW-GS was then analyzed by UV-vis spectrum via a UV2600 UV-vis spectrophotometer (Shimadzu, Kyoto, Japan).
The allergenicity of LMW-GS was determined by indirect ELISA and Western blotting. For indirect ELISA, purified LMW-GS (0.25,0.50, 1.00, 5.00, 10.00 μg/mL) was immobilized in 96-well microtiter plates overnight at 4 °C. After that, the plate was washed three times and incubated with 100 µL of primary antibody (pooled serum from 10 patients at dilution of 1:100 (V/V) with a normal serum as a negative control) for 1.5 h at 37 °C. After washing 3 times, 100 µL of secondary antibody (horseradish peroxidase (HRP)-conjugated goat anti-human IgE) at dilution of 1:5 000 (V/V) was added into each well, followed by incubating for 1.5 h at 37 °C. After that, 100 µL of 3,3’,5,5’-tetramethylbenzidine (TMB) solution was added into the plate and the plates were incubated for 15 min at 37 °C, and the color development reaction was stopped by 50 µL of 2 mol/L H2SO4solution. The optical density of each well was measured at 450 nm with a microplate reader (Spectra Max i3x; Molecular Devices, USA).
For Western blotting, LMW-GS (3 mg/mL) was transferred to polyvinylidene fluoride (PVDF) membrane after being run in a polyacrylamide gel (5% and 12%). Then, the membrane was incubated with positive serums (pooled serum from 10 patients at dilution of 1:100 (V/V) with a normal serum as a negative control ) for 1.5 h at 37 °C. The IgE bound on LMW-GS were detected using ECL after incubation with HRP-conjugated goat anti-human IgE. Then, the membrane was scanned using AlphaView SA 3.4.0 software.
2.6 LMW-GS sequence analysis and epitopes prediction
The amino acid sequence (GenBank: ACP27643. 1) of LMW-GS from Xiaoyan 6 was obtained from NCBI (https://www.ncbi.nlm.nih.gov/), and the sequence was analyzed the hydrophilicity, solvent accessibility, flexibility and antigenicity using DNASTAR 11.0 and AnTheProt 5.0 software. To improve the prediction accuracy, IEDB(http://www.iedb.org/) was used to predict potential B cell epitopes and predicted items are mainly related to flexibility, antigenicity,accessibility and epitopes. Meanwhile, two online servers ABCpred(https://webs.iiitd.edu.in/raghava/abcpred/) and IMED (http://imed.med.ucm.es/Tools/antigenic.pI) were used to predict peptide segment of B cell epitopes.
2.7 Allergenicity analysis of epitope peptides by icELISA
The synthesized peptides were identified by high-performance liquid chromatography (HPLC), and electrospray ionization-mass spectrometry (ESI-MS). The allergenicity of synthesized peptides was determined by icELISA. Briefly, purified LMW-GS was immobilized in 96-well microtiter plates overnight at 4 °C. After that, 50 µL of the peptides (0.001, 0.01, 0.1, 1, 10 μg/mL) were incubated with 100 µL of pooled serum from 10 patients at dilution of 1:100 (V/V)and then added into the plate, followed by incubation for 1.5 h at 37 °C. After washing three times, 100 µL of secondary antibody(horseradish peroxidase (HRP)-conjugated goat anti-human IgE)was added into each well, followed by incubation for 1.5 h at 37 °C.After that, 100 µL of TMB solution was added to the plate and the plates were incubated for 15 min at 37 °C, and the color development reaction was stopped by 50 µL of 2 mol/L H2SO4solution. The optical density of each well was measured at 450 nm with a microplate reader(Spectra Max i3x; Molecular Devices, USA). The inhibition rate of the immune response was calculated according to 1-ODinhibitor/ODblank.The icELISA standard curve of peptides was obtained to calculate IC50(the concentration of peptides when the inhibition rate is 50%).The amino acid type and frequency of active peptides were analyzed by Bioediet 7.0 software.
3. Results
3.1 Purification and identification of LMW-GS
LMW-GS was mainly composed of subunits in three regions(B: 40-50 kDa, C: 30-40 kDa and D: 55-70 kDa) according to the coding of Glu-3 site [26]. To highly purify LMW-GS from wheat (Triticum aestivum), the crude glutenin was purified by SP-Sepharose. From the chromatographic profile of the purification process, two elution peaks were observed after crude glutenin was injected into a SP-Sepharose column (Fig. 1A). As shown in Fig. 1A,the fraction of elution peak 1 not only contained the target protein but also several contaminated proteins below 30 kDa. In the SDS-PAGE electrophoretogram of fraction in elution peak 2, eluted at a NaCl concentration of 0.2 mol/L, 7 bands of molecular weight ranging 30-70 kDa were observed, which were presumably as the target protein. As shown in Fig. S1, the relative content of LMW-GS in a fraction of elution peak 2 is 95.27%, while the eighth band represented impurities which possessed 4.73%.
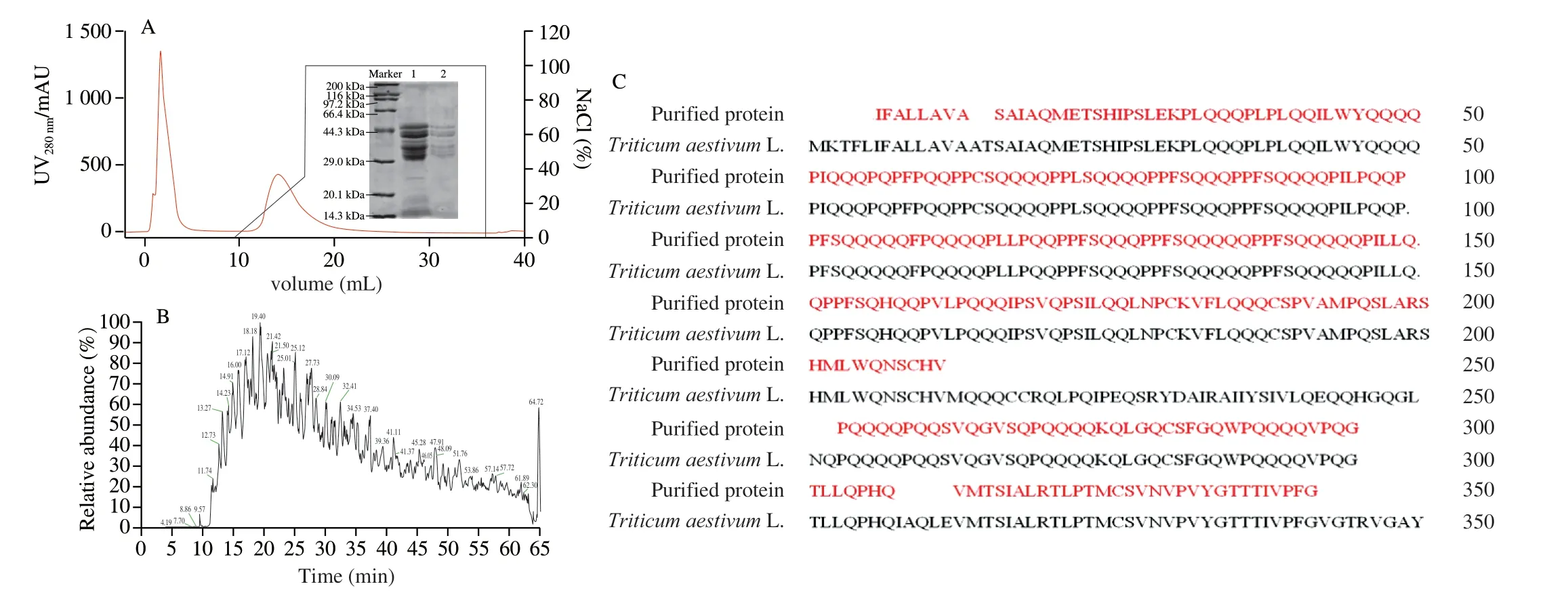
Fig. 1 The chromatographic profile of the purification process (A), total ion chromatogram (B) and amino acid sequences (C) of LMW-GS.
To confirm the identity of the purified protein, LC-MS/MS was employed after in-gel digestion with chymotrypsin and trypsin. The total ion chromatogram of the purified protein showed multiple peaks ranging from 0 to 65 min, showing a total of 545 peptides were detected by MS/MS (Fig. 1B). As shown in Fig. 1C, a total of 16 peptides matched LMW-GS from Xiaoyan 6 (ACP27643. 1), showing 83.3% identity with amino acid sequences. Hence, the purified protein can be confirmed as LMW-GS (Xiaoyan 6) with a molecular weight of 30-70 kDa, which corresponds to the results from SDS-PAGE.
3.2 Characterization of LMW-GS
The secondary structure of purified LMW-GS was analyzed by CD spectroscopy. We applied the method of Whitmore et al. [27]in DichroWeb on-line analysis to process secondary structure proportion. As shown in Fig. 2A, the contents were calculated to be 16.8%α-helix, 8.8%β-sheet, 32.6%β-turn, and 41.8% random curl of LMW-GS, indicating that the LMW-GS was rich inβ-turn and random curl structures [28]. This observation was supported by secondary structure prediction of LMW-GS according to its amino acid sequence using DNASTAR 11.0 and AnTheProt 5.0 software.Fig. S2 showed that LMW-GS contained 5.4%α-helix, 13.1%β-sheet,48.6%β-turn and 32.9% random curl. It was worth mentioning thatβ-turn and random curls were easier to form IgE epitope because they were generally distributed on the outside of protein.
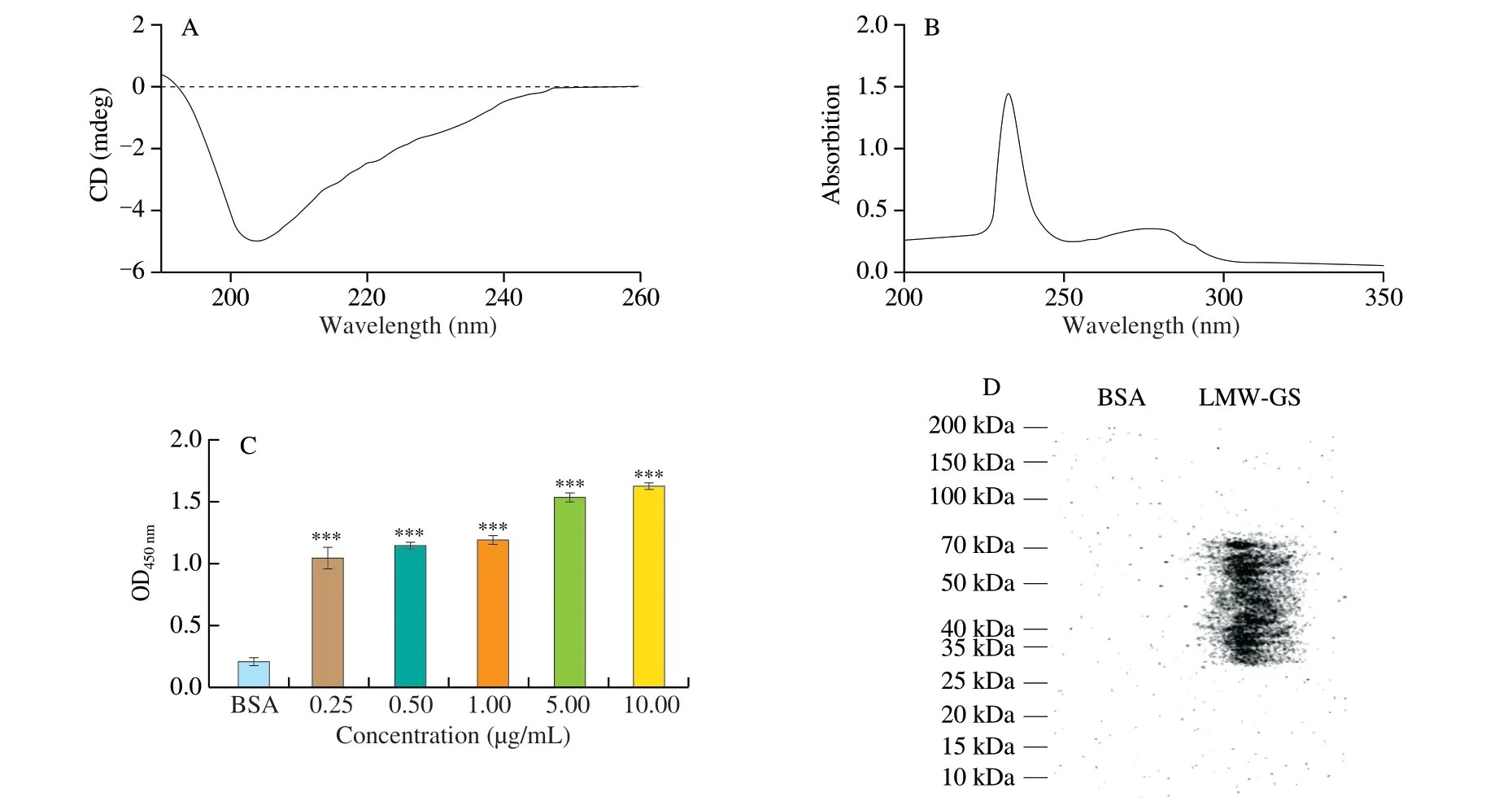
Fig. 2 The CD spectrum (A), UV spectrum (B), IgE-binding abilities (C), and IgE immunoblot banding patterns (D) of LMW-GS. ***P < 0.000 1.
The absorption peak of purified LMW-GS was determined by UV spectrum. As shown in Fig. 2B, in the scanning range of UV-visible light 200-350 nm, the absorption peaks of LMW-GS were located at 235 nm and 280 nm. In terms of the amino acid composition,LMW-GS mainly contained glutamine and proline. Thus, the appearance of a weak peak at 280 nm was due to the lack of aromatic amino acids (tyrosine, phenylalanine and tryptophan) in LMW-GS.In addition, the disulfide bonds presented in LMW-GS may cause the appearance of a distinct absorption peak at 235 nm.
Serum from 10 patients with reported allergies to wheat was tested for specific IgE binding to the purified LMW-GS. As shown in Fig. 2C, the IgE-binding abilities of purified LMW-GS with different concentrations were more than twice that of the BSA (negative control), with the OD values ranging from 0.998 1 to 1.521 6. Besides,the IgE-binding abilities of purified LMW-GS remarkably improved with the increasing of concentrations of LMW-GS, indicating that LMW-GS had strong immunogenicity. The IgE immunoblot banding patterns for purified LMW-GS were shown in Fig. 2D. The bands with molecular weight ranging 30-70 kDa exhibited high allergenicity, which was consistent with the results of SDS-PAGE, indicating that 3 regional subunits of LMW-GS all had prominent allergenicity.
3.3 Prediction of IgE epitopes in LMW-GS
The secondary structure, hydrophilic, solvent accessibility,flexibility, and antigenicity are the important parameters for predicting epitopes. Hydrophilic groups and areas with high solvent accessibility are usually exposed to the outside of protein, which can provide a possibility to form epitopes [29,30]. Moreover, amino acid sites with high flexibility were easier to form epitopes because they can bind to antibodies by changing their structure [31]. Therefore, we applied immunology tools such as DNASTAR 11.0 Protean module,AnTheProt 5.0 software, IEDB, IMED and ABCpred online website to predict IgE epitopes of LMW-GS according to its amino acid sequence. As shown in Table S2, a total of 36 peptides were predicted as potential allergenic epitopes in LMW-GS by 5 immunological tools. In addition, we selected 8 potential allergenic epitopes which were predicted by more than two methods as the candidate IgE epitopes (Table S3). These candidate peptides were named T-01 to T-08 and synthesized for further verification.
3.4 Verification of candidate epitopes
To verify the IgE-binding abilities of candidate peptides, an icELISA analysis was performed according to the IgE competitive action between the purified LMW-GS and synthesized epitope peptides. The synthesized candidate peptides were firstly identified by HPLC and mass spectrometry (MS). As shown in Fig. S3, the purity of synthetic candidate peptides was over 95%. Besides, the molecular weights of each synthesized candidate peptide determined by MS were basically consistent with their theoretical molecular weights (Fig. S4),indicating that these candidate peptides were correctly synthesized. In addition, the icELISA standard curves of each candidate peptide were described, in which the logarithm of the peptide concentration was set as the abscissa while competitive inhibition rate as the ordinate. The IC50of candidate peptides was calculated from their icELISA standard curves to express the IgE-binding abilities of candidate peptides. As shown in Fig. 3, 5 of the 8 candidate epitopes (T-01, T-02, T-03,T-06 and T-07) had a linear correlation of inhibition rate and the logarithm of the concentration, indicating they can bind to IgE and further effectively inhibit the reaction between purified LMW-GS and IgE. Among them, IC50of T-06 was the lowest, followed by T-03 and T-07, demonstrating these three peptides displayed strong IgEbinding abilities (Table 1). Therefore, the 5 candidate peptides (T-01,T-02, T-03, T-06 and T-07) were confirmed as the IgE peptides of LMW-GS from Chinese wheat (Xiaoyan 6). Next, we analyzed the amino acid composition of LMW-GS and epitopes. As shown in Fig. S5, the amino acid sequence of LMW-GS from Xiaoyan 6 contained plentiful glutamine, followed by proline, leucine and serine. The 5 epitopes were composed of 10 amino acids, among which glutamine, proline and serine appeared more frequently (B).Obviously, a lot of glutamine and proline can be found in both LMW-GS and epitopes of LMW-GS, which were predicted to be active amino acids and played a key role in epitopes.
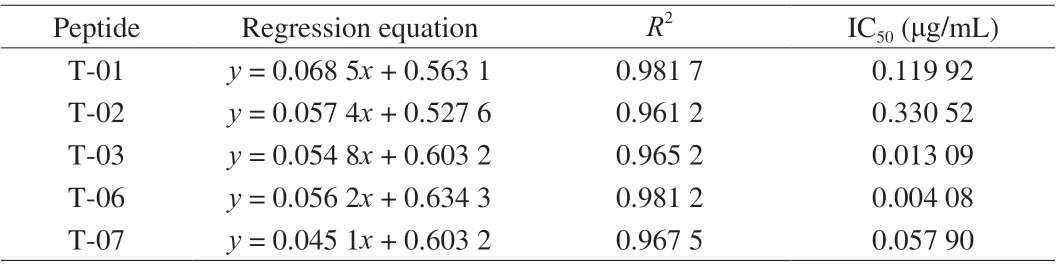
Table 1 The competition inhibition rate curve regression equation of IgE peptide of LMW-GS.
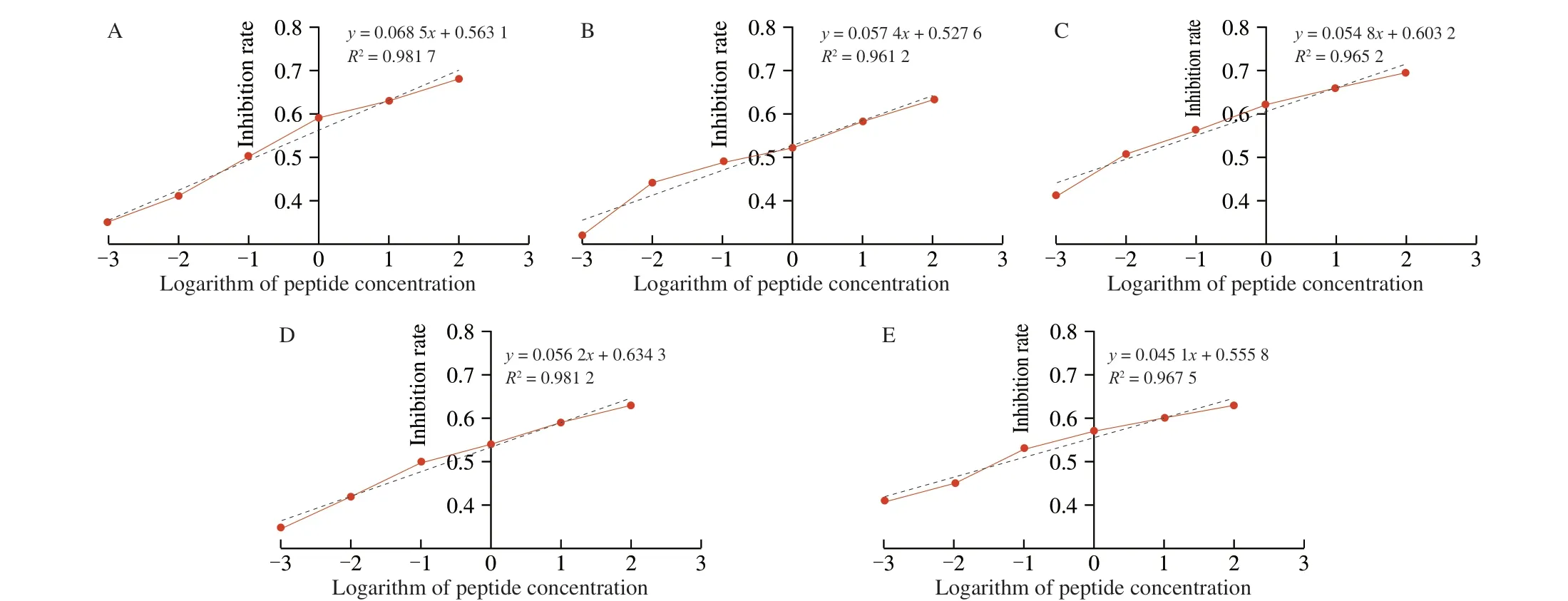
Fig. 3 The icELISA standard curves of candidate peptides. (A) T-01, (B) T-02, (C) T-03, (D) T-06, (E) T-07.

Fig. 4 The homology analysis of LMW-GS.
To analyze the homology of LMW-GS, four different Chinese wheat varieties were selected. As shown in Fig. 4, four wheat varieties(Xiaoyan 6, Chinese spring, Dongnong 101 and Lvhan 328) shared a sequence identity of more than 75%. Regions with high homology were mainly concentrated in the part of amino acid AA 20-40, AA 110-120,AA 200-280 and AA 310-322. Experimental linear epitopes of LMW-GS identified in the present research were labeled in aligned sequence using red boxes. Three epitope peptides (T-01, T-02 and T-06)were distributed in the conserved regions of amino acid sequences,which could explain the phenomenon for LMW-GS from different wheat varieties had cross-reactivity. Besides, the epitope peptides T-03 and T-07 located in the non-conserved region of the amino acid sequence were speculated to be specific epitopes of LMW-GS in Chinese wheat.
4. Discussion
In recent years, food allergies have increasingly become global concern due to the development of the social economy. Especially,wheat (Triticum aestivum), one of important cereals, is the major allergenic food in China. Glutenin accounted for about 40% of wheat protein, and it plays an important role in the flavor and quality of wheat products [32,33]. However, glutenin has been identified as a wheat allergen that is responsible for celiac disease and wheat contacts dermatitis [34,35]. Currently, many researchers are mainly focused on LMW-GS, while LMW-GS suffers from a lack of comprehensive review. Unfortunately, there is no simple and feasible method to highly pure LMW-GS because of its large number, small molecular weight, and similar properties of gliadin, which limited several research about LMW-GS. Therefore, we developed a simple and efficient method to successfully obtain LMW-GS with high-purity through ion-exchange chromatography, which can provide a basis for subsequent experimental research.
Glutenin is a polymer composed of a variety of proteins with different molecular weights. Thus, the composition of glutenin from different varieties is different, further affecting the flavor and quality of wheat products during processing. Especially, LMW-GS can form macromolecular polymeric protein, which can influence the strength of dough [36,37]. Besides, the secondary structure of glutenin can be influenced by its subunit composition. Fisichella et al. [38] employed CD spectroscopy to analyze the secondary structure of glutenin in Chinese spring wheat and Cheyenne wheat, showing that glutenin had similar weak positive peaks at 195 nm and the lowest peak appears at 205 nm. On this basis, we can preliminarily infer that the glutenin of different wheat varieties is rich inβ-turn and irregular curl. Besides,multipleβ-turn structures will form highly repetitive amino acid residues of glutenin have been identified [39], which will produce the strong hydrophobic segment in the middle of the glutenin and further support elasticity to wheat dough.
LMW-GS is highly conserved in N-terminal and C-terminal sequences, and contains generous Cys at N-terminal, which is beneficial to formβ-turn. Besides, non-repetitive regions of LMW-GS are prone toα-helix [40]. According to the report [41],linear epitopes were easily involved with allergic reactions during exposure to gastrointestinal food allergens. In a previous study,overlapping peptides were applied to identify the epitopes of LMW-GS in Japanese wheat [25]. However, the linear epitopes of LMW-GS in Chinese wheat have not been studied systematically. Hence, there is an urgent need to analyze the linear epitopes of LMW-GS in Chinese wheat to provide the basis for further studies about the treatment of wheat allergy and the development of hypoallergenic wheat products.We predicted and identified the epitope peptides in Chinese wheat(Xiaoyan 6). In 8 candidate epitopes, 5 epitopes showed significant IgE binding abilities, including T-01, T-02, T-03, T-06 and T-07.Among them, T-03, T-06 and T-07 had strong IgE binding abilities,which were speculated to be the main IgE epitopes of LMW-GS in Chinese wheat. Especially, T-01, T-02 and T-06 were in the conserved region of the sequences, which were speculated to be the common epitopes of LMW-GS in wheat. Moreover, the epitopes T-03 and T-07 located in the non-conserved region were speculated to be specific epitopes of LMW-GS in Chinese wheat. Denery-Papini et al. [23]reported 7 epitopes, which were all composed of 10 amino acids,and glutamine and proline account for 50%. Interestingly, among three subunits, the B subunit (40-50 kDa) of LMW-GS showed the strongest allergenicity and five epitope peptides all located on epitope peptides. In addition, the epitopes of LMW-GS contained abundant glutamine and proline which may be important amino acids in epitopes formation. Hence, we speculated three sequences with abundant glutamine and proline had the potential to form epitopes of LMW-GS, including QQPPFSQHQQP (in the part of amino acid AA 150-160), PSVQPSILQQ (AA 168-177) and PQIPEQ (AA221-226).
In conclusion, this work established a simple and efficient method to obtain highly purify LMW-GS after eliminating the interference of gliadin, which displayed abundantβ-turn and random curl structure and strong allergenicity. Moreover, the epitope peptides of LMW-GS were mapped and 5 critical epitope regions (T-01, T-02, T-03, T-06 and T-07) were identified as the IgE epitopes of LMW-GS in Chinese wheat (Xiaoyan 6). Given the relevance of LMW-GS as potential allergens of severe allergenic reactions, the highly purified LMW-GS and characterization of its epitopes may provide new insights into the prevention of wheat allergy and the development of hypoallergenic wheat products.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Acknowledgments
This study was financially supported by the State Key Research and Development Plan (2019YFC1605002).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.09.005.
- 食品科学与人类健康(英文)的其它文章
- Maternal obesity exacerbates the responsiveness of offspring BALB/c mice to cow’s milk protein-induced food allergy
- Au@Ag-labeled SERS lateral f low assay for highly sensitive detection of allergens in milk
- Impact of three different processing methods on the digestibility and allergenicity of Chinese mitten crab (Eriocheir sinensis) tropomyosin
- Effect of lipid peroxidation on the allergenicity and functional properties of soybean β-conglycinin (7S) and glycinin (11S)
- Comparative acetylome analysis reveals the potential mechanism of high fat diet function in allergic disease
- Therapeutic effects of epigallocatechin and epigallocatechin gallate on the allergic reaction of αs1-casein sensitized mice

