Metabolomics and network pharmacology reveal the mechanism of the Jiawei Yangshen pill in treatment of cyclophosphamide-induced dyszoospermia in mice
Jing-Ning Yan,Xiao-Qin Liu,Xiang-Long Meng,Xiao-Yan Zhang,Qi Sheng,Bo-Rui Guo,Yan Li,Kai-Han Li,Jia-Dong Chang
1Clinical College of Traditional Chinese Medicine,Shanxi University of Chinese Medicine,Jinzhong 030619, China. 2College of Chinese Materia Medica and Food Engineering,Shanxi University of Chinese Medicine,Jinzhong 030619,China. 3Shanxi Key Laboratory of Tradition Herbal Medicines Processing,Shanxi University of Chinese Medicine,Jinzhong 030619,China.
#Jing-Ning Yan and Xiao-Qin Liu are the co-first authors for this paper.
Abstract
Keywords:Jiawei Yangshen pill; dyszoospermia; metabolomics; network pharmacology
Background
According to the World Health Organization, the incidence of male reproductive dysfunction and infertility has been increasing annually,severely affecting the patient’s family harmony [1-3]. Currently reported causative factors include dyszoospermia, seminal obstruction, accessory gland dysfunction and other genetic or environmental factors. Dyszoospermia, also known as azoospermia or oligozoospermia, is the most common cause of reproductive dysfunction and infertility in males. It refers to the biological status of spermatogenic cell atrophy and degeneration, or cell division arrest and failure of sperm maturation and ejection, caused by testicular dysgenesis or injury, seminal tract obstruction, or epididymal inflammation [4]. Traditional Chinese medicine (TCM) is well known for its unique insights and potent efficacy in treating male dyszoospermia [5]. In this regard, the Jiawei Yangshen pill (JWYS) is a typical experiential prescription of Shang-Hua Zhao, an aged and famous TCM clinician awarded by the State Administration of Traditional Chinese Medicine of China. [6]. This formula adapts lamb testicles as its sovereign, nourishing the kidney and increasing sperm count and activity.Semen Cuscutae,Rhizoma Curculiginis,Folium Epimedii,Astragali Complanati Semen,Morinda Officinalis Radix,ActinoliteandCynomorii Herbaserve as ministers and enhance sperm abundance. The assistant and courier of JWYS, that isCornel,Lycii Fructus,Radix CodonopsisandSemen Coicis. In traditional Chinese medicine culture, it has the function of “Ziyin Jiangzao” [7-9]. The pharmacological mechanism of action of JWYS remains unclear. In the present study, dyszoospermia was induced in mice using cyclophosphamide to study the regulatory effects of JWYS on spermatogenesis. Metabolomics and network pharmacology were combined to verify the effects and explore the underlying mechanisms of action.
Methods
Experimental herbal medicine, reagents and devices
Lamb testicles,Cuscuta australisR.Br. (Cuscutae Semen),Curculigo orchioidesGaertn. (Curculiginis rhizoma),Epimedium brevicornuMaxim.(Epimedii Folium),Astragalus complanatusR.Br. (Astragali Complanati Semen),Morinda officinalisHow (Morinda OfficinalisRadix),Actinolite,Cynomorium songaricumRupr. (Cynomorii Herba),Cornus officinalisSieb.et Zucc. (Corni fructus),Lycium barbarumL. (Lycii fructus),Codonopsis pilosula(Franch.) Nannf. (Codonopsis radix) andCoix lacryma-jobiL. var. Mayuen (Roman.) Stapf (Coicis Semen) were ordered from Shanxi Herentang and identified by Professor Ying Jia of Shanxi University of Chinese Medicine (the plant names were checked at http://www.theplantlist.org on March 20, 2022). All voucher specimens (voucher numbers: SXTCM-Yan-2021001 to SXTCM-Yan-2021012) were preserved at the Herbarium of the Shanxi University of Chinese Medicine.
Reagents and kits used in this study were acetonitrile(1499230-935; Merck, Darmstadt, German), acetic acid (70221;Sigma, St. Louis, MO, USA), cyclophosphamide (20200621;PudePharma, Datong, China), Testosterone kit (AD20200508),luteinizing hormone (LH) kit (AD20200505), transforming growth factor-β1 (TGF-β1) (Cat: A2124) and nuclear factor kappa B (p65)(NF-κB (p65)) antibodies (Cat: A19653), anti-β-actin monoclonal antibody (Cat: AC038), horse radish peroxidase - conjugated goat anti-rabbit IgG (Cat: AS014, ABclonal Technology, China),anti-adenosine monophosphate-activated protein kinase (AMPK) (Cat:A00994-1), phosphorylated AMPK (p-AMPK) (Cat: BM4718), protein kinase B (AKT) (Cat: A00024-2), phosphorylated AKT (p-AKT) (Cat:BM4744), Bcl-2 (Cat: A00040-1), Bcl-2-associated X (Bax) (Cat:A00183), enhanced radio immuno precipitation assay lysis buffer,SDS-polyacrylamide gel electrophoresis preparation kit, broad spectrum phosphatase inhibitor (100×) (AR0102, AR1112, AR1195;Boster Biological Technol, Ltd., Wuhan, China) and Tris-HCL-buffered saline with tween (20200915; Solarbio, Beijing, China).
Devices included Centrifuge 5430R (Eppendorf, Germany),Excelsior ES automate tissue embedder (LEICA), RM2235 Rotary Slicer (LEICA), DM6000B Bioscope (LEICA), AB Triple TOF 6,600 mass spectrometer (Applied Biosystems, Milford, MA, USA), Agilent 1290 Infinity LC(Agilent,Sacramento,CA, USA) and chromatographic column (2.1 mm × 100 mm, 1.7 μm; ACQUITY UPLC BEH Amide)(Waters, Milford, MA, USA).
A JWYS suspension (1 g/mL) was prepared in normal saline (NS).High-performance liquid chromatography was performed according to our previous method to calculate the content of each fraction,including hyperin (0.68 mg/g) and icariin (0.57 mg/g)(Figure 1).
Experimental animals and modeling
Forty male C57BL/6 mice, 7 weeks old and weighing 20 ± 2 g, were obtained from Specific Pathogen Free (Beijing) Biotechnology Co.,Ltd. (SCXK-Jing-2019-0010). All mice were housed at 25 ±2°C and a relative humidity of 50 ± 10% under a 12/12 light/dark cycle. They were fed regular feed and were allowed to drink water freely. They were allowed to acclimatize for 1 week. All animal procedures were approved by the Ethics Committee of the Shanxi University of Chinese Medicine (Permit No. 2022DW273). All studies abided by and conformed to the requirements of thePrinciples of Laboratory Animal Care(Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC: The National Academies Press) .
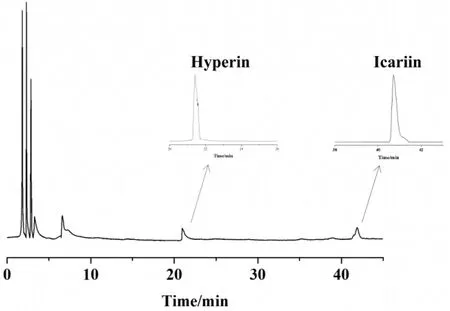
Figure1High-performanceliquidchromatographychromatogram of JWYS.JWYS, Jiawei Yangshen pill.
The 40 mice were then randomly divided into four groups: blank,model, JWYS and levocarnitine. Mice in the blank group were intraperitoneally administered NS, while the mice in the other three groups were intraperitoneally administered cyclophosphamide (50 mg/kg) of equal volume [10]. After injection for 7 consecutive days,intragastric administration was performed once daily for 4 consecutive weeks, with NS (1 mL/kg) administered in the blank and model groups, JWYS suspension (500 mg/kg) in the JWYS group, and levocarnitine (100 mg/kg) in the levocarnitine group. During treatment, the mice were fed regular feed and weighed weekly. The dose was determined according to the conversion of body surface area between mice and humans, and based on previous studies(unpublished).
Examination of biological and pathological indicators
After the last drug administration, the mice were fasted for 12 h,allowed to drink water freely, and anesthetized with isoflurane.Abdominal aortic blood (1-2 mL) was collected and centrifuged at 3,000 r/min for 10 min. The supernatant was collected to determine the levels of serum testosterone and LH using the corresponding enzyme linked immunosorbent assay kits. Bilateral testes and epididymis were sampled and weighed. The visceral index was calculated as the ratio of organ mass(mg) to body mass.The left testis was immediately preserved at -80 °C for further metabolomic analysis. The right testis was fixed with Bouin’s fixative for 24 h,paraffin-embedded, sectioned, and stained with hematoxylin and eosin. Seminiferous tubules and interstitial cells were observed under an optical microscope and photographed.
The left epididymis was transferred to a culture dish containing 1 mL of prewarmed (37 °C) stroke-physiological saline solution. The cauda epididymis was cut using surgical scissors, and seminal fluid was collected and diluted. Sperms were counted and observed under a high-magnification microscope. Abnormal sperm were defined as sperm that were amorphous, banana-shaped, fat-headed,double-tailed, double-headed, tailless or de-capped.
G组患儿的血清胆红素水平明显低于D组患儿,黄疸的消退时间也明显比D组患儿短,G组患儿在血清胆红素水平、黄疸消退时间方面明显优于D组患儿,且两组相比,差异有统计学意义(P<0.05)。见表1。
Metabolomic analysis
The left testis was thawed at 4 °C, and a pre-cooled aqueous solution of methanol/acetonitrile(2:2:1, v/v) was added to it. The mixture was vortexed, sonicated in an ice bath for 30 min, kept at-20 °C for 10 min, and then centrifuged at 14,000 × g at 4 °C for 20 min. The supernatant was collected and evaporated to dryness. Aqueous acetonitrile solution (100 μL, 1:1, v/v) was then added. The reconstituted mixture was vortexed and centrifuged at 14,000 × g and 4 °C for 15 min. The supernatant was then collected for further analysis. An Agilent 1290 Infinity LC was applied to the hydrophilic interaction chromatography column. Mass spectrometric detection was performed in positive ion mode (PIM) and negative ion mode(NIM) with electrospray ionization using a Triple TOF 6600 mass spectrometer. Metabolomic analysis was performed according to our previously reported method [11].
Network pharmacology analysis
The active ingredients of JWYS were retrieved from the Traditional Chinese Medicine Systems Pharmacology Database Analysis Platform(http://ibts.hkbu.edu.hk/LSP/tcmsp.php), with the following keywords as search terms: lamb testicles,Semen Cuscutae,Rhizoma Curculiginis,Folium Epimedii,Astragali Complanati Semen,Morinda Officinalis Radix,Actinolite,Cynomorii Herba,Cornel,Lycii Fructus,Radix CodonopsisandSemen Coicis. Active ingredients of JWYS and protein targets with oral bioavailability ≥30% and drug-likeness ≥0.18 were screened out [12]. Dyszoospermia-related genes were retrieved from the Online Mendelian Inheritance in Man(http://www.omim.org/) and GeneCards ®: the HumanGene Database (https://www.genecards.org/) using the following keywords as search terms: dyszoospermia, spermatogenesis dysfunction (alone and combined). The drug and disease target genes were uploaded to the Venn Program (http://bioinfogp.cnb.csic.es/tools/venny/index.Html) to identify overlapping genes associated with both JWYS and dyszoospermia. The gene names of the protein targets were retrieved from UniProt (https://www.uniprot.org/), PubMed (https://www.ncbi.nlm.nih.gov/pubmed) and HGNC (https://www.genenames.org/). Cytoscape 3.7.1 (http://cytoscape.org/) was used to construct a network of “ingredient-target-diseases”. The overlapping genes associated with both JWYS and dyszoospermia were projected onto STRING 11.0 (https://string-db.org) to generate a protein-protein interaction network. Finally, the overlapping genes were subjected to Gene Ontology and Kyoto Encyclopedia of Genes and Genomes enrichment analysis (https://www.genome.jp/kegg/pathway.html)using DAVID (https://david.ncifcrf.gov/home.jsp). Enrichment results were visualized using Cytoscape 3.7.1.
Western blotting
The testes were treated with tissue protein extraction reagent to extract total proteins. The Bradford method was used to determine the protein concentration. SDS-polyacrylamide gel electrophoresis was performed to separate the proteins, which were then transferred to a nitrocellulose membrane. The membrane was blocked for 1 h with 5%skim milk, followed by the addition of primary antibodies (AMPK,p-AMPK, AKT, p-AKT, Bcl-2, Bax, TGF-β1, NF-κB (p65) and β-actin)and secondary antibodies successively at room temperature. Protein bands were visualized using the GeneGnome system (Gene Company,Hong Kong, China) and quantitatively analyzed using ImageJ 2 (NIH,Bethesda,MD, USA).
Statistical analysis
Biological and pathological indicators: statistical analysis was performed using SPSS 22.0 (IBM, New York, NY, USA) software. All data are expressed asx ± s. Two means were compared using t-tests,whereas multiple means were compared using one-way analysis of variance.P<0.05 was considered statistically significant.
Metabolomic data: all raw data files were peak-processed using Compound Discoverer 2.0 data analysis suite (Thermo Fisher Scientific, Waltham, MA, USA). The normalized data were used for principal component analysis, partial least-squares discriminant analysis, and orthogonal partial least-squares discriminant analysis using SIMCA-P 14.1 (Umetrics, Malmö, Sweden). The most significant differential variable was identified by value in performance >1 in the S-plots andP<0.05 in the independent-sample t-test. Data of the differential variables were subjected to metabolic pathway enrichment analysis using Metabo Analyst 5.0.
Results
Effect of JWYS on testis tissue morphology and relevant biological and pathological indicators
Hematoxylin and eosin staining revealed a large area of shed cells and significantly decreased number of spermatogenic cells in the model group. In contrast, the spermatogenic cells in mice treated with JWYS were well ordered and showed an increased number in the testis tissue(Figure 2A).
Compared to the blank group, the testis index (Figure 2B,P<0.0001), epididymis index (Figure 2C,P<0.01), sperm count (Figure 2D,P<0.0001) and testosterone level (Figure 2F,P<0.0001) were remarkably lower in the model group, while the sperm mortality(Figure 2E,P<0.01) and LH levels (Figure 2G,P<0.0001) were significantly higher. Following treatment with JWYS, the testis index,epididymis index, sperm count (P<0.05) and testosterone level (P<0.05) increased remarkably, with concurrent decreases in sperm mortality (P< 0.01) and LH levels. Similar changes were also observed in mice treated with levocarnitine compared to those in the model group.
Effect of JWYS on testis tissue metabolomic profiling
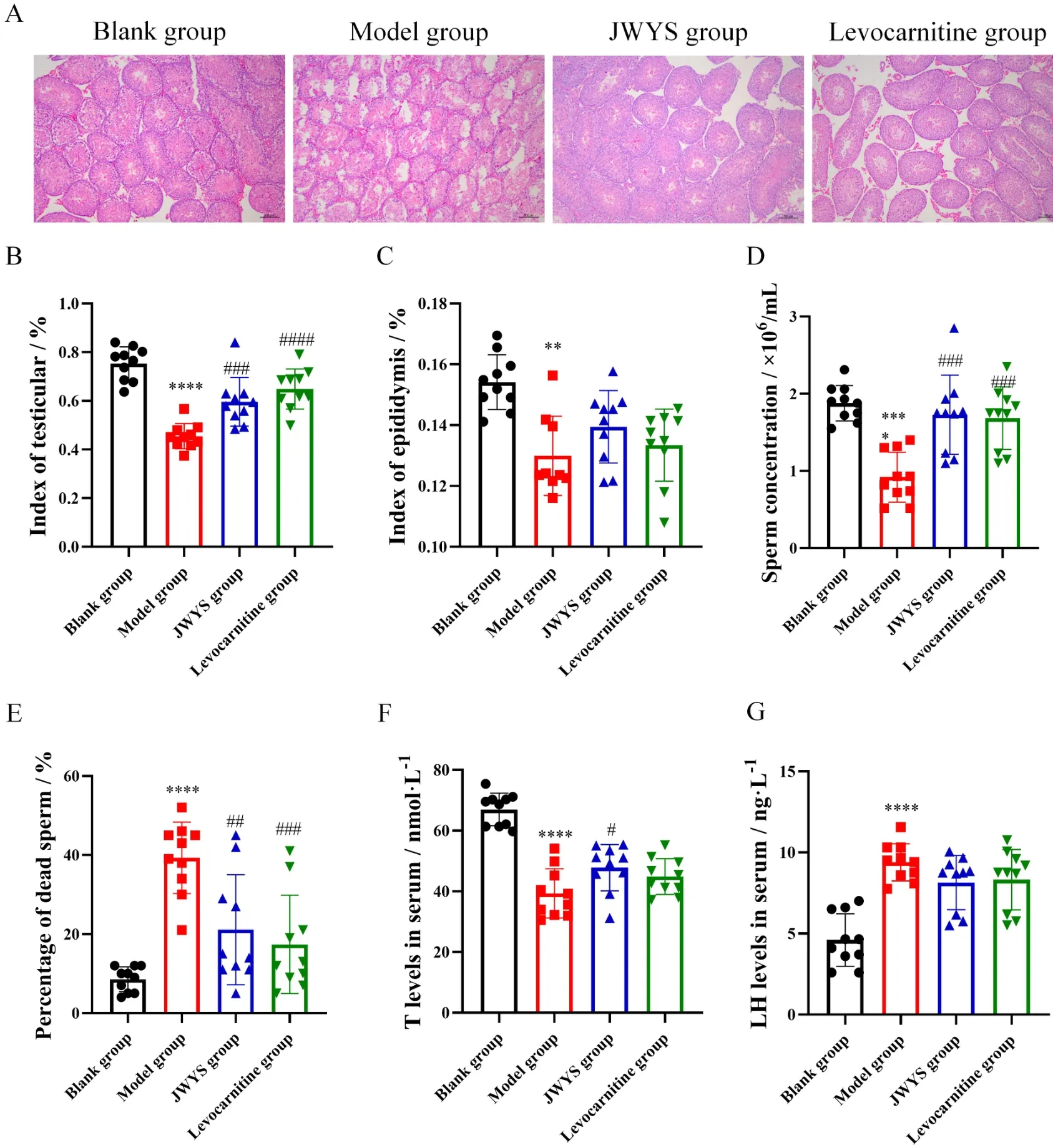
Figure 2 JWYS improves testis tissue morphology and relevant biological and pathological indicators. Mice model of dyszoospermia was established by cyclophosphamide (50 mg/kg, consecutive 7 days) and the mice were given JWYS (500 mg/kg) and levocarnitine (100 mg/kg),respectively, for 4 weeks. The testis tissue morphology (A) (H & E, 400×) was observed, and testis index (B), epididymis index (C), sperm count(D),sperm mortality(E),T level(F),LH level (G)were detected.**P <0.01,****P <0.0001 vs.blank group.#P <0.05,##P <0.01,####P <0.0001 vs. model group. JWYS, Jiawei Yangshen pill; LH, luteinizing hormone; T, testosterone.
A total of 378 metabolites were identified in the PIM (n = 238) and NIM (n = 208). Peaks extracted from all experimental and quality control samples were subjected to a multivariate analysis (Figure 3).
According to value in performance >1 andP<0.05, in the orthogonal partial least-squares discriminant analysis, there were 55 significantly different metabolites in the PIM (n = 41) and NIM (n =19) between the blank and model groups. Of the 41 differential metabolites in the PIM, 18 and 23 were upregulated and downregulated, respectively. Of the 19 differential metabolites in the NIM, 6 and 13 were upregulated and downregulated, respectively(Figure 3A-3F, Supplementary Table S1, Supplementary Table S2). In both PIM and NIM, levels of inosine, taurine and oxidized glutathione were upregulated in the model group, while levels of phenylalanine and 4-pyridoxic acid were downregulated. Correlational analysis revealed that arginine-glutamine and oxidized glutathione were highly positively associated with other metabolites, whereas acetylcarnitine and 2-oxoadipic acid were significantly negatively associated with other metabolites (Figure 4A, Figure 4B).
Compared with the model group, there were 34 significantly different metabolites in the PIM (n = 21) and NIM (n = 15) in the JWYS group. Of the 21 differential metabolites in the PIM, 8 and 13 were upregulated and downregulated, respectively. Of the 15 differential metabolites in the NIM, 5 and 10 were upregulated and downregulated, respectively (Figure 3G-3L, Supplementary Table S1,Supplementary Table S2). In both PIM and NIM, the levels of l-alanine and phosphorylcholine were downregulated in the JWYS group.Correlational analysis revealed that 20-hydroxyarachidonic acid and 2’-deoxyuridine were highly positively associated with other metabolites, whereas l-leucine and phenyllactic acid were significantly negatively associated with other metabolites (Figure 4C,Figure 4D).
Effect of JWYS on testis tissue metabolic pathways
Metabolic pathway enrichment analysis was performed for the differential metabolites in both PIM and NIM (Figure 4E).Between the blank and model groups, there were 11 differential pathways (impact>0.1), mainly multiple amino acid metabolic pathways (such as phenylalanine, tyrosine, tryptophan biosynthesis, glutamine and glutamate metabolism, and phenylalanine metabolism pathways),followed by energy metabolism, taurine and hypotaurine metabolism,and inositol phosphate metabolism pathways.
Between the model and JWYS groups, there were 7 differential pathways (impact > 0.1), mainly multiple amino acid metabolic pathways (glutamine and glutamate metabolism, histidine metabolism, glutamate, alanine and aspartate metabolism, arginine and proline metabolism, and arginine biosynthesis pathways),followed by energy metabolism, inositol phosphate metabolism,nucleotide metabolism and pyrimidine metabolism pathways.
Network pharmacology analysis results
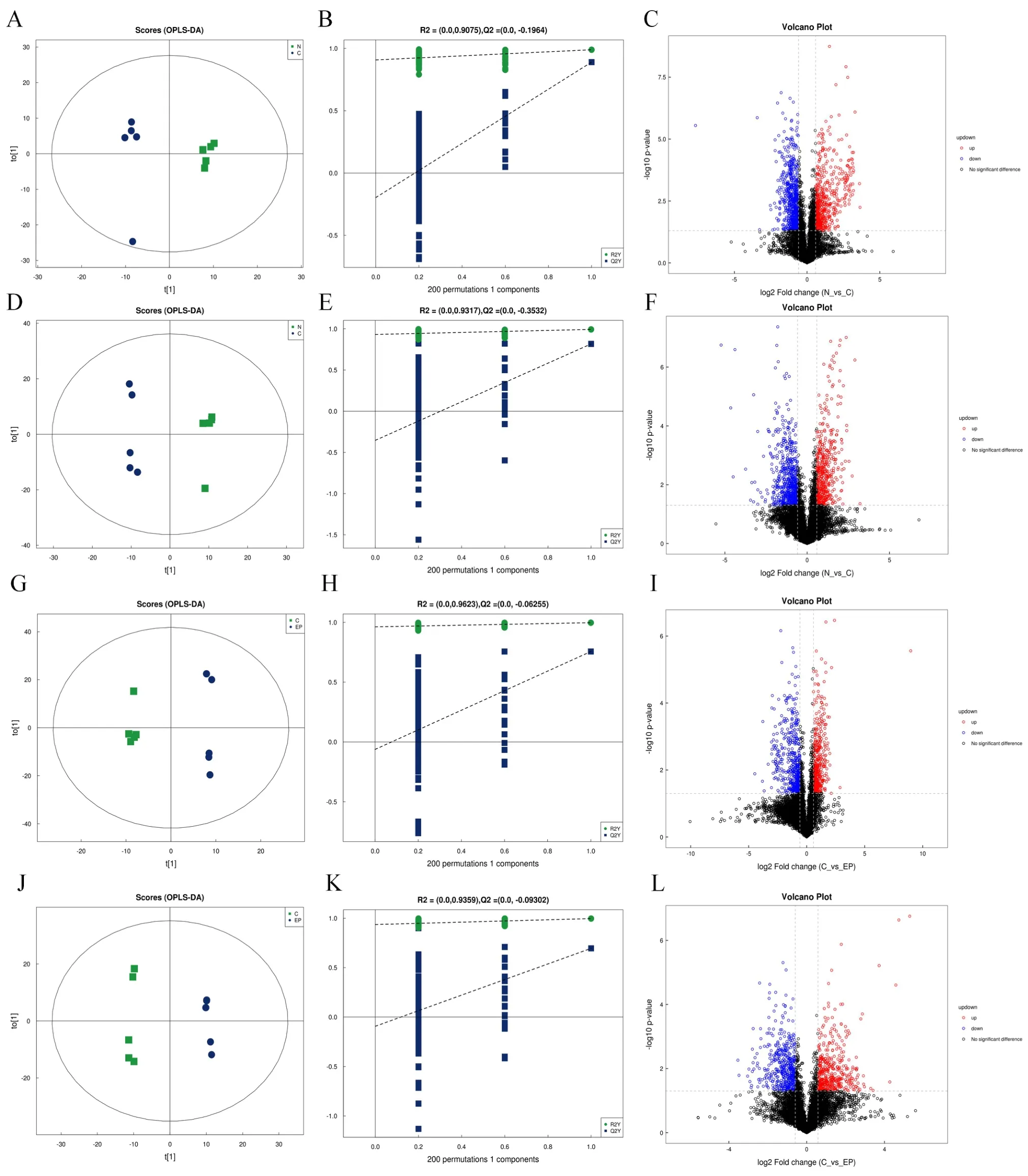
Figure 3 Testis tissue metabolomic profiling in mice of different groups. OPLS-DA score plots, 200-time permutations were performedin and plotted and differential metabolite volcanic map in positive and NIMs of blank group and model group (A-F); OPLS-DA score plots, 200-time permutations were performedin and plotted and differential metabolite volcanic map in positive and NIMs of JWYS group and model group (G-L).NIM, negative ion mode; OPLS-DA, orthogonal partial least-squares discriminant analysis.
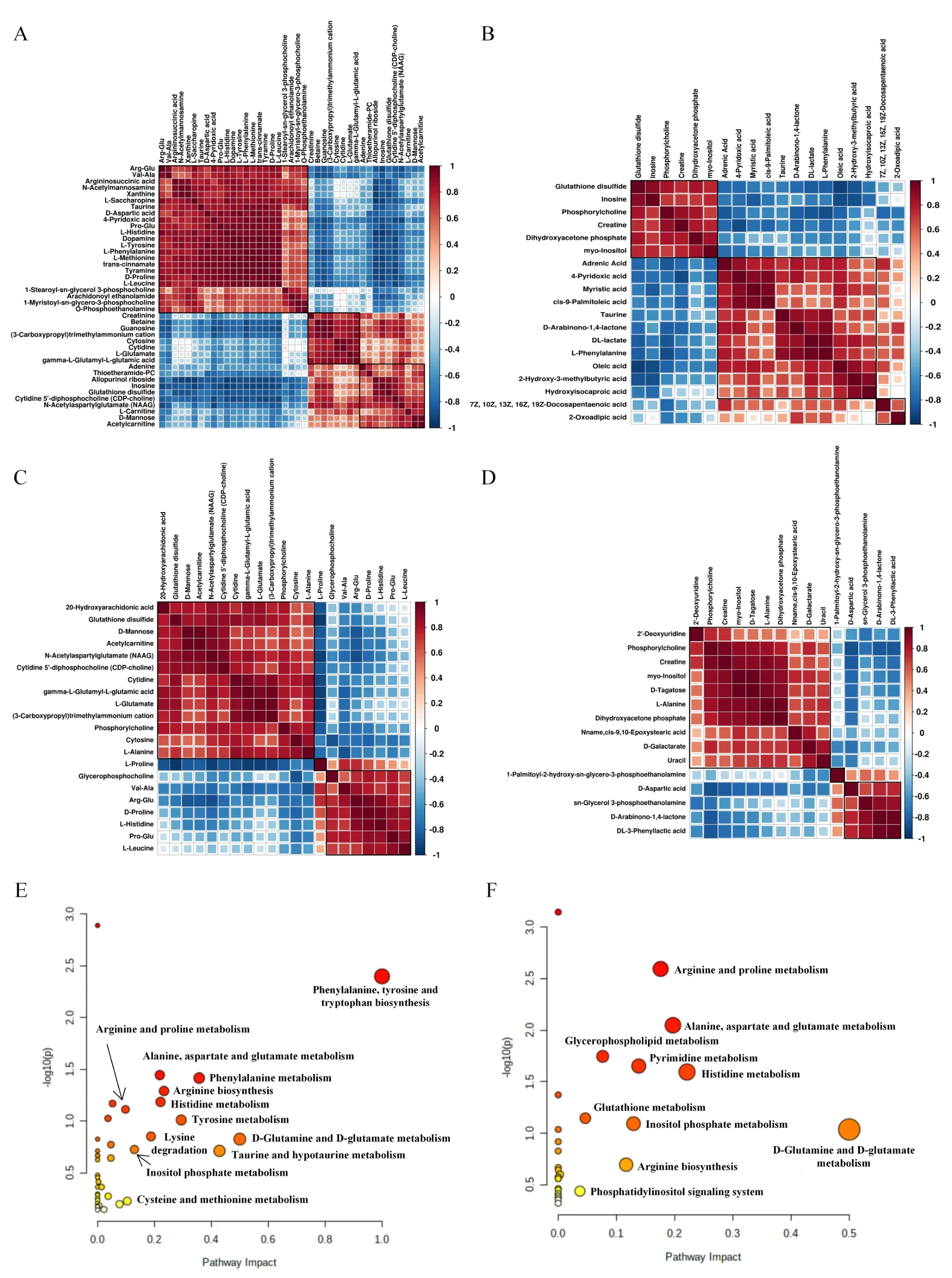
Figure 4 Correlation between differential metabolites and metabolic pathway enrichment analysis.(A, B)Correlation analysis of differential metabolites between blank group and model group in PIM; (C, D) correlation analysis of differential metabolites between JWYS group and model group under NIM; (E) metabolic pathway enrichment analyses between blank group and model group; (F) metabolic pathway enrichment analyses between JWYS group and model group. PIM, positive ion mode; JWYS, Jiawei Yangshen pill; NIM, negative ion mode.
The active ingredients of JWYS were retrieved. The protein targets of the active ingredients and dyszoospermia-related genes were identified (Table 1). A Venn diagram was plotted to obtain 97 intersecting genes associated with JWYS and dyszoospermia (Figure 5A). An “ingredient-target-disease” network was generated, where 32 common high-frequency ingredients were displayed (Figure 5B). A protein-protein interaction network of the intersecting genes centered on the estrogen receptor 1/α gene (ESR1) was constructed (Figure 5C,Figure 5D).Except for ESR1, nuclear receptor coactivator 1,specificity protein 1 (Sp1), beta-catenin, fructo-oligosaccharides (FOS) and mitogen-activated protein kinase 8(MAPK8) also appeared frequently.Further enrichment analysis of the intersected genes revealed 20 common high-frequency Gene Ontology entries, such as ubiquitin-like protein ligase binding, ubiquitin protein ligase binding and RNA polymerase II transcription factor binding (Figure 5E).In addition, the most enriched Kyoto Encyclopedia of Genes and Genomes pathways were involved in prostate cancer, colorectal cancer, Kaposi sarcoma-associated herpesvirus infection, lipid and atherosclerosis,and human cytomegalovirus infection (Figure 5F).
Effect of JWYS on expression of related proteins in testis tissue
As noted in the previous section,MAPK8 is involved in the therapeutic effect of JWYS in mice with dyszoospermia. It has been established that the MAPK8 signaling pathway can interact with Bax by increasing the phosphorylation of Bcl-2, which subsequently inhibits cell apoptosis, while the expression levels of Bcl-2 and Bax can be regulated by AKT and AMPK. Therefore, this study examined the expression levels of p-AMPK/AMPK, p-AKT/AKT, Bax/Bcl-2, TGF-β1,and NF-κB(p65) in the testes of mice using western blot analysis in an attempt to explore the mechanism of action by which JWYS improves cyclophosphamide-induced dyszoospermia in mice(Figure 6).
Compared to the blank group, the levels of p-AMPK/AMPK (P<0.01) and p-AKT/AKT (P<0.01) were significantly decreased in the model group, but the levels of Bax/Bcl-2 (P<0.01), TGF-β1 (P<0.01) and NF-κB (p65) (P<0.01) increased.
Compared to the model group, significantly increased levels of p-AMPK/AMPK (JWYS,P< 0.05; levocarnitine,P< 0.05) and p-AKT/AKT (JWYS,P<0.05; levocarnitine,P<0.01) were observed in the JWYS and levocarnitine groups. In contrast, reductions in Bax/Bcl-2 (JWYS,P< 0.05; levocarnitine,P< 0.01), TGF-β1(levocarnitine,P< 0.05) and NF-κB (p65) (JWYS,P< 0.05;levocarnitine,P<0.05) were observed.
Collectively, the results indicated that JWYS and levocarnitine could effectively improve cyclophosphamide-induced dyszoospermia in mice by altering levels of related proteins and there was no significant difference between them.
Discussion
Male infertility has become a significant issue nowadays because of the fast pace of life, different conceptions of sexuality and increasingly poor lifestyle habits. Oligozoospermia, asthenospermia and oligoasthenospermia are predominantly considered causative factors[13]. After cyclophosphamide is metabolized by the liver microsomal P450 enzyme system, toxic substances are generated, which decrease the activity of free radical scavenging enzymes and cause free radical accumulation, lipid peroxidation, cell membrane structure destruction, germ cell damage and eventually lead to spermatogenic dysfunction. Cyclophosphamide is commonly used to model dyszoospermia in animals [14, 15]. JWYS, a typical experiential prescription of Professor Zhao to treat male sexual dysfunction, can enhance kidney nourishment and sperm abundance [6]. In the current study, dyszoospermia was induced in mice via intraperitoneal injection of cyclophosphamide in an attempt to explore the regulatory effect of JWYS on spermatogenesis. Western blotting, metabolomics and network pharmacology were conducted to verify the effects and explore the underlying mechanisms of action. The results showed that JWYS could lead to a significantly increased testis index, epididymis index, sperm count and T level, and evident reductions in sperm mortality and LH levels. In addition, multiple amino acid metabolic pathways, energy metabolism and nucleotide metabolism changed during treatment. Furthermore, significantly increased levels of p-AMPK/AMPK and p-AKT/AKT and distinct reductions in Bax/Bcl-2,TGF-β1 and NF-κB(p65) were observed after administration of JWYS.
The etiology of male dyszoospermia remains elusive. TCM differentiation is effective in clinical practice.It has been reported thatTCM alone or in combination could regulate the secretion of follicle-stimulating hormone and LH in a bidirectional manner,thereby increasing sperm count and quality[15-19].

Table 1 Active ingredients and key targets of JWYS in treatment of dyszoospermia
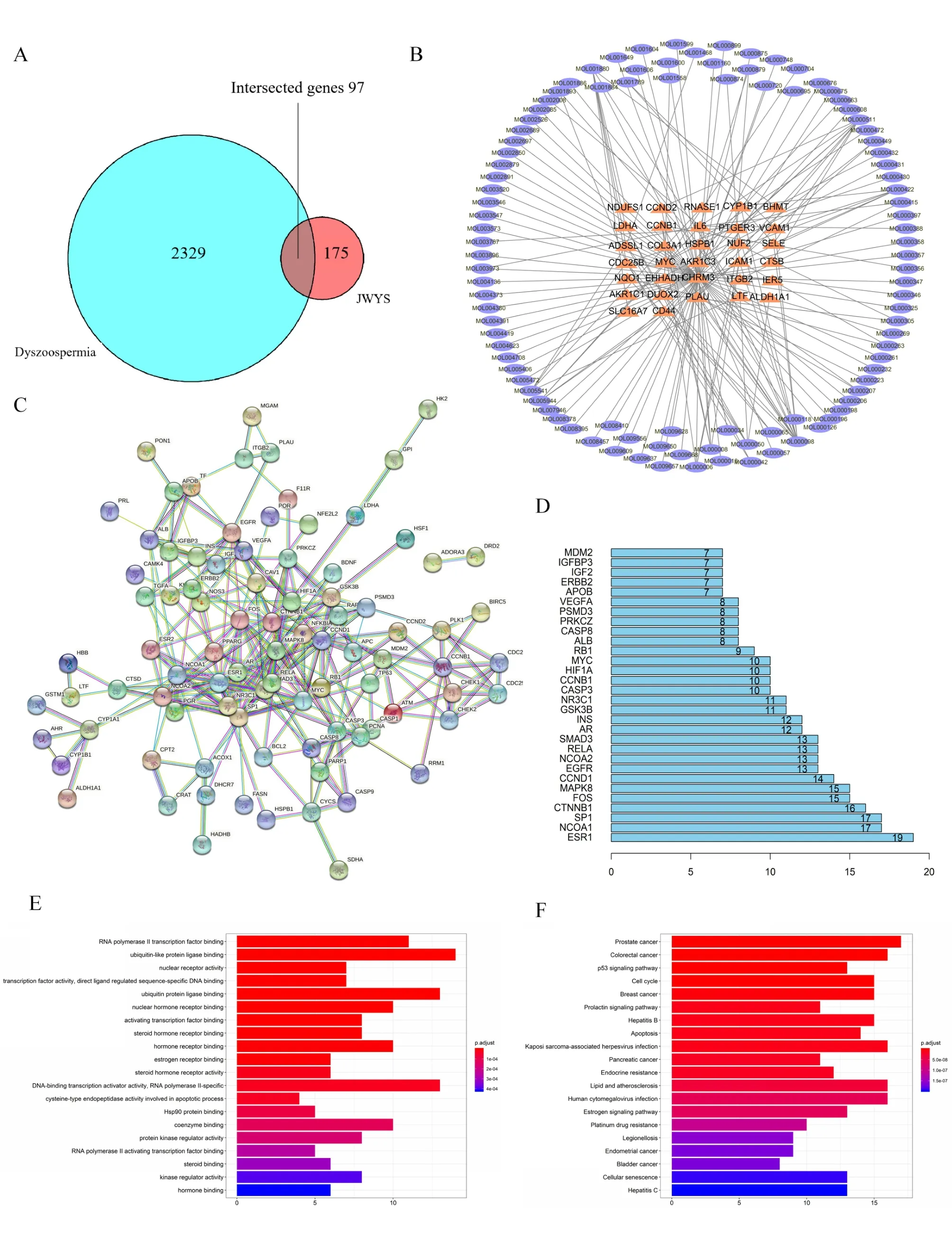
Figure 5 Network pharmacology analysis. (A) Venn; (B) potential active ingredient-target-disease network; (C) PPI network; (D) frequency analysis of protein targets; (E) GO function analysis; (F) KEGG pathway enrichment analysis. JWYS, Jiawei Yangshen pill; PPI, protein-protein interaction; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes.
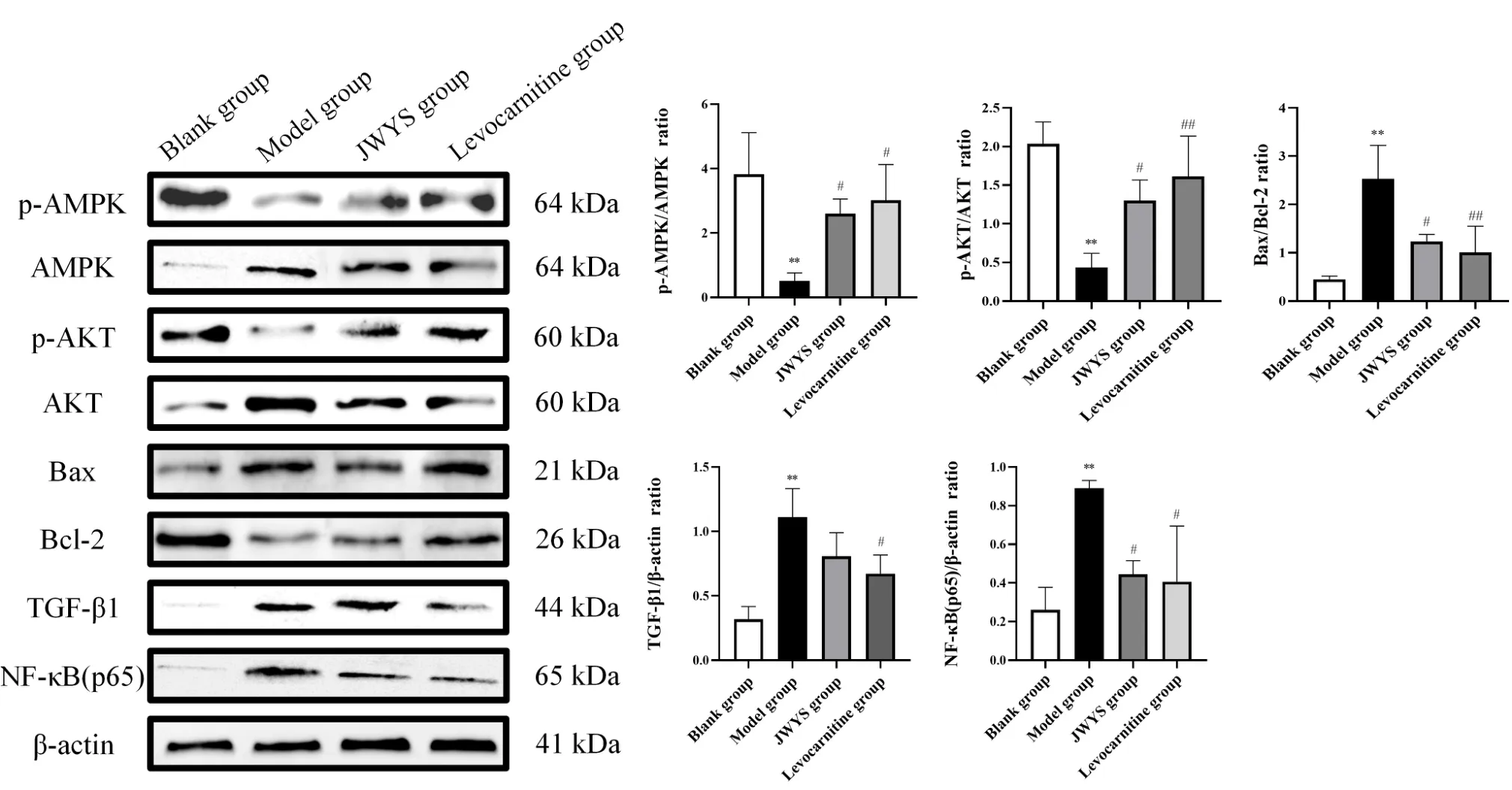
Figure 6 Expression of related proteins in testis tissue of mice with dyszoospermia. Relative levels of phosphorylated the AMPK, AKT, Bax,Bcl-2, TGF-β1 and NF-κB (p65) proteins were determined with total and phosphorylated forms or β-actin for normalization determined by western blotting.*P <0.05,**P <0.01,***P <0.001 vs. blank group.#P <0.05,##P <0.01,###P <0.001 vs. model group. p-AMPK, phosphorylated AMPK; AMPK, anti-adenosine monophosphate-activated protein kinase; p-AKT, phosphorylated AKT; AKT, protein kinase B; Bax, Bcl-2-associated X;Bcl-2, B-cell lymphoma-2; TGF-β1, transforming growth factor-β1; NF-κB,nuclear factor kappa B.
Amino acids are basic units of macromolecules involved in biological functions and play a crucial role in vital activities.Supplementation with an appropriate amount of branched-chain amino acids may maintain sperm function and promote testosterone secretion [20]. Glutamines are excitatory neurotransmitters that can stimulate T secretion to maintain the body in a synthetic status [21].Arginines are highly basic sperm-specific nuclear proteins involved in spermiogenesis [22]. Supplementation with arginine can improve sperm quality and increase testosterone levels, which can antagonize reproductive toxicity [23]. Amino acids (such as arginine and glutamate) and inositols are chemical components of seminal plasma that are necessary during sperm delivery as they provide energy and nutrients [24]. Dysregulation of amino acid metabolism may cause idiopathic infertility. It has been suggested that supplementation with energy required for sperm survival and activity could improve the quality of sperms [25].
In the present study, network pharmacology analysis was performed and revealed the important roles of ESR1, nuclear receptor coactivator 1, Sp1, beta-catenin, FOS and MAPK8 during the treatment of dyszoospermia with JWYS. ESR1 is critical for the development of testicular output tubules, epididymis and prostate in male fertility,and the loss of ESR1 impairs ion transport and water reabsorption,leading to sperm abnormalities [26]. Triptolide was found to inhibit GATA binding protein 4 glycolysis by inhibiting Sp1-dependent the platelet isoform of phosphofructokinase expression in synaptonemal comlex, resulting in testicular toxicity and spermatocyte apoptosis[27]. C-Fos can regulate the proliferation and differentiation of testicular tissue cells and promote the development of spermatogenic cells during the transitional period. It can also directly regulate testosterone secretion by Leydig cells, which can maintain spermatogenesis [28]. It has been established that the MAPK8 signaling pathway is involved in cell proliferation, transformation,differentiation, apoptosis and inflammatory responses. It can promote the phosphorylation of Bcl-2 to interact with Bax, thereby suppressing apoptosis. AKT and AMPK have been reported to regulate the expression of anti-apoptotic factors of the Bcl-2 family via the mammalian target of rapamycin signaling pathway [29, 30]. Bax has been identified to show high expression in spermatogenic cells of the testis, whereas downregulation of Bcl-2 can advance apoptosis and differentiation of these cells [31]. AKT is a direct target of PI3K and p-AKT induced by activated PI3K can directly phosphorylate mTOR to regulate the proliferation and differentiation of spermatogonia and spermiogenesis [32]. AMPK has been identified as a sensor of the cellular energy involved in energy metabolism. Upon phosphorylation, downstream mTOR signaling is suppressed, which can subsequently affect spermiogenesis [33]. These results suggest that the treatment of dyszoospermia with JWYS is related to the regulation of energy metabolism, expression of inflammatory factors,cell growth and differentiation and amino acid synthesis, which is consistent with the metabolomic pathways (amino acid-related metabolic pathways and energy metabolism-related pathways). It can be concluded that JWYS can improve spermiogenesis by regulating energy metabolism and decreasing cell apoptosis.
Conclusion
JWYS showed effectiveness as a treatment of cyclophosphamideinduced dyszoospermia in mice by alleviating testicular microcirculatory injury and improving overall tissue metabolism. This study provides experimental evidence and a reference for the scientificity of clinical experience in using JWYS for the treatment of dyszoospermia.
 Traditional Medicine Research2023年1期
Traditional Medicine Research2023年1期
- Traditional Medicine Research的其它文章
- Multi-component quantitative and feed-forward neural network for pattern classification of raw and wine-processed Corni Fructus
- Rutaecarpine attenuates monocrotaline-induced pulmonary arterial hypertension in a Sprague-Dawley rat model
- An updated study of traditional medicines to the era of 1,3,4 oxadiazole derivatives for malaria treatment
- Forget the past,there is no future
- Medicinal plants and phytomedicines are used to treat or prevent illnesses in Sudan:a review
