Inkjet 3D bioprinting for tissue engineering and pharmaceutics
Deng-ke ZHAO, He-qi XU, Jun YIN, Hua-yong YANG
Review
Inkjet 3D bioprinting for tissue engineering and pharmaceutics

1The State Key Laboratory of Fluid Power and Mechatronic Systems, School of Mechanical Engineering, Zhejiang University, Hangzhou 10058, China2Key Laboratory of 3D Printing Process and Equipment of Zhejiang Province, School of Mechanical Engineering, Zhejiang University, angzhou 310058, China3These authors contributed equally to this study
3D bioprinting has the capability to create 3D cellular constructs with the desired shape using a layer-by-layer approach. Inkjet 3D bioprinting, as a key component of 3D bioprinting, relies on the deposition of cell-laden droplets to create native-like tissues/organs which are envisioned to be transplantable into human body for replacing damaged ones. Benefiting from its superiorities such as high printing resolution and deposition accuracy, inkjet 3D bioprinting has been widely applied to various areas, including, but not limited to, tissue engineering and drug screening in pharmaceutics. Even though inkjet 3D bioprinting has proved its feasibility and versatility in various fields, the current applications of inkjet 3D bioprinting are still limited by the printing technique and material selection. This review, which specifically focuses on inkjet 3D bioprinting, firstly summarizes the techniques, materials, and applications of inkjet 3D bioprinting in tissue engineering and drug screening, subsequently discusses the major challenges that inkjet 3D bioprinting is facing, and lastly summarizes potential solutions to those challenges.
Inkjet 3D bioprinting; Biomaterials; In vitro tissue models; In vivo tissue substitutes; Drug screening
1 Introduction
The concept of 3D printing was firstly presented in 1986 when Charles W. Hull was able to form a 3D construct by curing photo cross-linkable materials using an early ultraviolet light-based stereolithography technique layer-by-layer (Murphy and Atala, 2014). Since then, 3D printing technology which can create 3D constructs with desired structures has drawn much attention (Zhang et al., 2019). For example, only two years after Charles W. Hull, Klebe attempted to print collagen and fibronectin using an HP thermal inkjet printer which relied on heat bubbles to squeeze the ink and form droplets with controllable size (Klebe, 1988). Even though the first commercial inkjet 3D printer was reported to come out in 2000, the development of the normal inkjet printer, with its first practical patent approved in 1951, was long before the proposal of the concept of 3D printing (Gudapati et al., 2016). With the consistent development of printing techniques, the application areas of 3D printing have been broadened into various fields such as electronic devices (Li et al., 2020). In recent years, with advances in materials science and biology, the application of 3D printing, including inkjet printing, has been further extended into tissue engineering by 3D printing the bioink composed of biological materials, living cells, and biomolecules. The concept of 3D bioprinting, with its aim to artificially create 3D functional tissues/organs to replace damaged ones (Mandrycky et al., 2016; Santoni et al., 2022), has thus been proposed. Also, 3D bioprinting has been used in a number of fields including drug delivery and screening (Ma et al., 2018), and cancer research (Knowlton et al., 2015).
The concept of inkjet 3D bioprinting was firstly presented by Boland after successfully depositing living cells using a modified inkjet printer (Mironov et al., 2003). One early application, using inkjet 3D bioprinting to fabricate poly (lactic-co-glycolic acid) (PLGA)-based scaffolds, on which living cells such as endothelial cells were seeded, can be traced back to 1997 (Griffith et al., 1997). Since then, inkjet 3D bioprinting, as one of the pioneering 3D bioprinting technologies, has been extensively used due to its low cost, high printing resolution, high deposition accuracy, and negligible harmfulness on the living cells during the printing process (Gudapati et al., 2016; Wu and Xu, 2018; Takagi et al., 2019). However, inkjet 3D bioprinting also has disadvantages such as the restriction in bioink viscosity because of the nozzle size (Xu et al., 2022a). Considering the broad impact of inkjet 3D bioprinting, it is critical to present a specialized review focusing on inkjet 3D bioprinting. The aim of this review is to list the main techniques of inkjet 3D bioprinting, summarize its typical applications in tissue engineering and pharmaceutics, discuss its limitations and key challenges, and present its future research directions so as to address the existing key challenges and promote the technological advances that will enable its broader impact.
2 Overview of inkjet 3D bioprinting technologies
Various inkjet printing technologies have experienced remarkable improvement during the past few decades. Depending on the droplet generating and dispensing mechanism, the most impactful ones can be divided into three categories, namely continuous inkjet (CIJ) printing, drop-on-demand (DOD) inkjet printing, and electrohydrodynamic (EHD) inkjet printing (Wijshoff, 2010; Gudapati et al., 2016; Shah et al., 2021). Schematic diagrams of these representative inkjet printing techniques are shown in Fig. 1. To the best of our knowledge, DOD and EHD inkjet printing technologies have already been used for 3D bioprinting whereas CIJ printing has yet to be used in inkjet 3D bioprinting. In this section, the principles of these three groups of inkjet printing technologies will be discussed and the characteristics of DOD and EHD inkjet 3D bioprinting will be summarized.
2.1 CIJ printing
In CIJ printing, as depicted in Fig. 1a, pressurized ink is ejected through a tiny nozzle to form an energetically unstable jet, which subsequently breaks up into a stream of droplets owing to Rayleigh-Plateau instability (Eggers and Villermaux, 2008). The rupture of the jet is stimulated via a piezoelectric vibration (produced by a piezoelectric transducer) exerted on the ink upstream of the nozzle, which regulates the breakup position of the jet and the velocity and size of the droplets (Basaran et al., 2013). After detaching from the end of the continuous liquid jet, the formed droplets are charged while passing through the electric field of the charging electrode, and are steered with a set of deflecting electrodes to be deposited at the pre-designed locations on the receiving substrate. The non-printed droplets are collected by a gutter and recycled back to the nozzle (Derby, 2010; Hoath, 2016).
CIJ printers are prevalent in industry thanks to their long drop throw distance (>10 mm) which is favored for printing on cardboard and curved surfaces (Hoath, 2016). Even though nozzle clogging is not frequently observed in CIJ printing due to its relatively large nozzle size (Derby, 2010), the recycling of the ink significantly increases the chance of contamination which would be detrimental for the encapsulated cells during 3D bioprinting. In addition, the complex composition of a CIJ printing system further restricts its applications (Li et al., 2020). For these reasons, CIJ printing has not been used in 3D bioprinting despite its significant contribution to industrial printing (Godleman et al., 2021; Phung and Kwon, 2022).
2.2 DOD inkjet 3D bioprinting
DOD inkjet printing, unlike CIJ printing, emits droplets only when the ejection signal is provided, enabling better controllability of the formed droplets. Compared to CIJ printers, DOD inkjet printers are simpler (Derby, 2010; Wijshoff, 2010). Furthermore, a DOD inkjet printer can be equipped with multiple printheads to enable multi-material printing. The current DOD inkjet 3D printing system can produce droplets with a volume as small as 1 pL (Yang et al., 2021), and for that reason, DOD inkjet printing is favored as one of the most promising 3D bioprinting technologies. According to the mechanism of the formation of droplets in DOD inkjet printing, different types of current DOD inkjet printing can be categorized. Typically, the bioink inside the fluid chamber is held in place at the nozzle orifice by surface tension and back pressure. A droplet is ejected when the controlled pressure pulse inside the nozzle exceeds a certain threshold, where the pressure pulse has been induced by means of a thermal, a piezoelectric or an electrostatic actuator, referring to thermal inkjet (TIJ) printing (Hoath, 2016), piezoelectric inkjet (PIJ) printing (Li et al., 2019; Kwon et al., 2020), electrostatic inkjet printing (Nishiyama et al., 2009; Gudapati et al., 2016; Li et al., 2020), respectively. In addition, microvalve inkjet printing where the droplets are expelled from the nozzle filled with positively pressurized bioink via the opening and shutting of a microvalve can also be seen as a modified version of DOD inkjet printing (Blaeser et al., 2016; Ng et al., 2017). All these four types of DOD inkjet printing have proved their feasibility in 3D bioprinting.
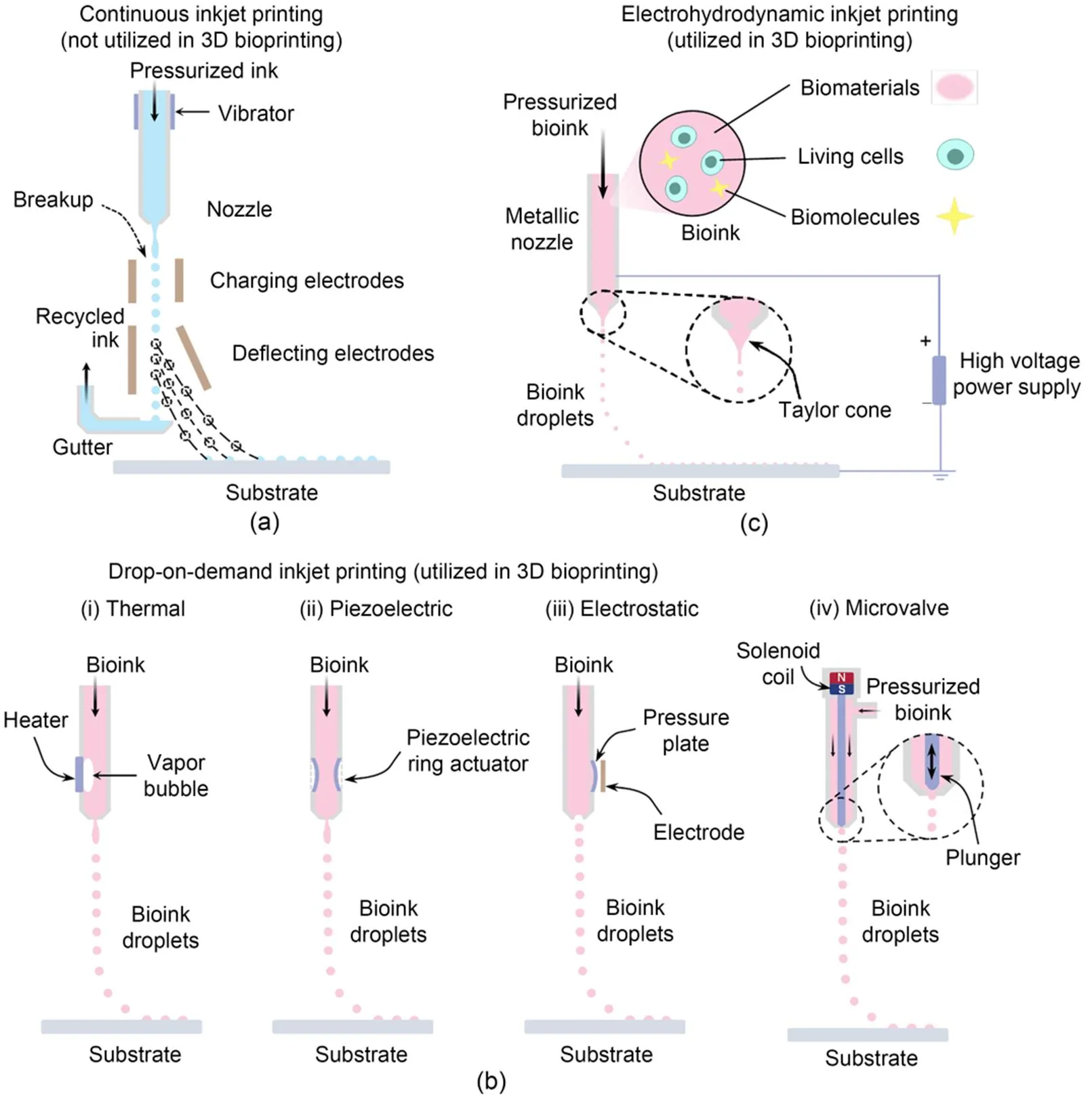
Fig. 1 Depiction of typical inkjet printing technologies: (a) CIJ printing, a liquid jet from a nozzle breaks up into a stream of droplets which are deflected by electric fields onto a substrate to conduct printing; (b) DOD inkjet 3D bioprinting: (ⅰ) TIJ 3D bioprinting where droplets are ejected by thermal bubble expansion, (ⅱ) PIJ 3D bioprinting where droplets are produced via fast piezoelectric ceramic deformation, (ⅲ) electrostatic inkjet 3D bioprinting where droplets are expelled through rapidly deforming the pressure plate, and (ⅳ) microvalve inkjet 3D bioprinting where droplets are generated with the rapid opening and shutting of the nozzle orifice; (c) EHD inkjet 3D bioprinting, which relies on electric force to expel the bioink from the nozzle to form droplets
2.2.1TIJ 3D bioprinting
In TIJ 3D bioprinting, as illustrated in Fig. 1b(i), a small thin-film heater is placed in the nozzle chamber to trigger thermal bubbles which expel a small amount of bioink from the nozzle to form droplets. During the droplet ejection process, upon receiving a current signal, the heater immediately boils the surrounding bioink for several microseconds at a greater than 107K/s heating rate to form the vapor bubble which expands rapidly to the point of collapse and generates the required pressure pulse to eject the ink (Hoath, 2016). The heating temperature usually rises to nearly 300 ℃; however, the temperature of the bioink within the chamber has been reported to increase no more than 4–10 ℃ because of the extremely short boiling time (Cui et al., 2012). Even though the thermal and shear stresses during printing might jeopardize cell viability and alter the phenotype of the encapsulated cells (Campbell et al., 2020), some studies have reported a greater than 90% cell viability by optimizing the printing parameters (Li et al., 2020; Ng et al., 2022; Suntornnond et al., 2022).
TIJ 3D bioprinting is favored for its rapid printing speed and low cost. However, it also has several disadvantages which restrict its broader applications. First of all, its lifetime is relatively low for several reasons, such as the electromigration of the heater, damage by bubble cavitation, and thermal stress-induced cracks, even though the lifetime can be prolonged by improving the thickness and shape of the heater (Lim et al., 2005). Kogation, which is caused by the accumulation of bioink particles on the heater, is another main issue in TIJ and affects the formation of bubbles and droplet ejection (Shah et al., 2021). Furthermore, it is challenging to maintain a steady formation process of droplets (Li et al., 2020). Lastly, the nozzle with a tiny orifice is prone to clogging, and the material choices of TIJ 3D printing are quite limited.
2.2.2PIJ 3D bioprinting
In PIJ 3D bioprinting, the pressure pulses for droplet ejection are introduced via direct mechanical deformation of a piezoelectric transducer. PIJ technologies can be further categorized into squeeze, bend, shear, and push modes depending on the configuration of the transducer (Kwon et al., 2020; Shah et al., 2021). Fig. 1b(ii) demonstrates the PIJ 3D inkjet bioprinting with a squeeze mode. During the droplet formation process, the actuator expands or contracts due to the voltage waveform, which thereby changes the volume of the bioink chamber to cause a pressure pulse for bioink ejection and droplet formation (Li et al., 2019; Kwon et al., 2020; Shah et al., 2021). The printing performance of PIJ 3D bioprinting can be mediated by optimizing the printing parameters, such as the parameters of driving voltage signal (Zhao et al., 2021) and the material composition of the bioink (Yin et al., 2019).
It is relatively simple to regulate the droplet formation during PIJ 3D bioprinting by adjusting the driving voltage waveform. Compared to TIJ 3D bioprinting, PIJ 3D bioprinting has broader choices of nozzle diameter (10 to 120 μm), and nozzle cleaning is easy to conduct (Li et al., 2020). Ideally, PIJ 3D bioprinting could deposit a wide variety of inks onto the substrate for the formation of well-defined patterns or structures. However, the instability of the droplet formation process and the entrainment of bubbles (Wijshoff, 2010) in the ink channel are two big challenges yet to be overcome. In addition, the generated ultrasonic and shear stresses during PIJ 3D bioprinting are also adverse to the viability and functionality of printed cells (Murphy and Atala, 2014; Shi et al., 2018). The cell viability in PIJ inkjet 3D bioprinting has been reported to lie in the range from 75% to 95% depending on the printed cell type, printing parameters, and bioink composition (Gudapati et al., 2016; Li et al., 2020; Kang et al., 2021).
2.2.3Electrostatic inkjet 3D bioprinting
The electrostatic inkjet 3D bioprinting process shown in Fig. 1b(iii) is similar to PIJ, with the pressure pulse for emitting the bioink also induced by the mechanical deformation of chamber wall. The pressure plate on the nozzle wall is attracted to the electrode owing to the electrostatic forces when a voltage pulse is applied between them, and the pressure plate subsequently regains its original shape in the absence of the voltage signal, so as to trigger a pressure pulse in the chamber to squeeze the bioink out for droplet formation (Wijshoff, 2010; Gudapati et al., 2016).
The nozzle selected for electrostatic inkjet 3D bioprinting typically has a diameter of 10–60 μm, resulting in the formation of droplets with a diameter ranging from 24 to 67 μm, while the droplet spacing between adjacent droplets during printing can be as small as 8.8 μm (Nakamura et al., 2005). Electrostatic inkjet printing is not selected for graphic and text printing onto paper because of its poor printability and reliability. However, it has been utilized for other applications owing to its low cost and easy implementation (Wijshoff, 2010). A representative application in the 3D bioprinting field was reported by Nishiyama et al. (2009), where they employed an Epson SEA-Jet printhead to print microbeads, fibers, sheet structure, and 3D tube structures with or without cells encapsulated. More than 70% of the cells were found to be alive (Nakamura et al., 2005, 2010; Nishiyama et al., 2009).
2.2.4Microvalve inkjet 3D bioprinting
The key element of a microvalve inkjet 3D bioprinting system is the normally-closed microvalve nozzle, with the chamber of the nozzle filled with bioink by positive back pressure. The outlet of the nozzle is governed by a plunger, and the bioink can be expelled to form a droplet when the plunger executes one open-shut action of the orifice within a short duration (about 100 μs). The movement of the plunger can be accurately driven by a solenoid coil, a piezoelectric-stack actuator or air pressure (Gudapati et al., 2016; Shah et al., 2021). Fig. 1b(iv) presents the schematic configuration of a solenoid microvalve inkjet printer. Once a voltage pulse is provided to the solenoid coil, the plunger is pulled upwards with the magnetic field generated by the coil resulting in the ejection of the bioink and generation of a droplet under the high positive back pressure. Afterwards, the plunger returns to its initial position to block the nozzle using a mechanical spring.
Even though sonication and heat are not major concerns in microvalve inkjet 3D bioprinting, the shear stress can still injure the cells (Blaeser et al., 2016) despite the larger nozzle orifice (about 100 to 600 μm in diameter) compared to TIJ, PIJ, and electrostatic inkjet 3D bioprinting (Blaeser et al., 2016; Ng et al., 2017; Bedell et al., 2022; Dufour et al., 2022). Even though a larger nozzle reduces the probability of nozzle clogging, the printing resolution is somewhat compromised. Microvalve inkjet 3D bioprinting is capable of handling bioink up to a viscosity of 200 mPa·s which is a unique advantage compared to the DOD printing processes described above (Ng et al., 2017). There have been several reported studies on the effects of process parameters on cell viability during microvalve inkjet 3D bioprinting (Lee et al., 2009; Gurkan et al., 2014; Faulkner-Jones et al., 2015). A cell viability greater than 86% has been broadly reported (Blaeser et al., 2016; Duarte Campos et al., 2019). However, studies reporting the utilization of microvalve inkjet 3D bioprinting on the fabrication of complex 3D tissue constructs are still missing (Xu CX et al., 2012; Christensen et al., 2015).
2.3 EHD inkjet 3D bioprinting
As illustrated in Fig. 1c, EHD inkjet 3D bioprinting relies on an electric force to emit bioink for droplet formation instead of the pressure pulse in DOD 3D bioprinting. This electric force is generated with a high-voltage (0.5 to 20.0 kV) power supply connected to the nozzle and substrate whose typical standoff distance is hundreds of microns (Gasperini et al., 2015; Gao and Zhou, 2019). In EHD inkjet 3D bioprinting, bioink in the metallic nozzle is charged and distorted into a Taylor cone, which is governed by the interplay of electric force, inertial force, capillary force, viscous force, and/or elastic force. Under a specific controlled voltage, the Taylor cone is stretched as the electric force exceeds the surface tension, which results in the ejection of a thin bioink filament to form droplets which are precisely deposited onto the substrate to construct structures (Zhang et al., 2016; Cai et al., 2021; Mkhize and Bhaskaran, 2022).
The nozzle diameter can be as small as 2 μm due to the Taylor-cone (Cai et al., 2021). Therefore, the diameter of the formed droplets in EHD inkjet 3D bioprinting is smaller than that in DOD inkjet 3D bioprinting. With the support of the electrostatic stresses, EHD inkjet 3D bioprinting is capable of providing higher printing resolution, with the highest resolution approaching 10 μm (Poellmann et al., 2011; Li et al., 2020). EHD bioprinters do not rely on a substantially high pressure to form the droplets and can print the bioink with a viscosity of up to 2000 mPa·s (Workman et al., 2014). However, it is challenging for EHD inkjet 3D bioprinting to print in a DOD manner, and thus it is not suitable for applications where high-accuracy delivery of cells and materials is required. In addition, although the cell viability and genomic expression are not significantly impacted immediately after bioprinting by the printing parameters (e.g., the applied voltage, cell concentration, and bioink compositions), long-term post-bioprinting cell viability has been reported to be adversely affected (Workman et al., 2014). A comparison of the typical characteristics of each inkjet 3D bioprinting technique is summarized in Table 1.

Table 1 Characteristics of representative inkjet 3D bioprinting technologies
3 Bioink for Inkjet 3D bioprinting
3.1 Biomaterials for inkjet 3D bioprinting
As mentioned above, the bioink, as a key element in inkjet 3D bioprinting, is typically composed of biomaterials and living cells. Biomolecules such as growth factor (Freeman et al., 2020) and drugs (Peng et al., 2017) are sometimes encapsulated within the bioink for specific applications. With recent advances in materials science, the choice of materials suitable for 3D bioprinting is continuously broadening. Like the other types of biomaterials which are widely utilized in 3D bioprinting, biomaterials which are suitable for inkjet 3D bioprinting should also have attributes such as good biocompatibility and biodegradability, suitable rheological properties, fast and stable cross-linking mechanisms, and satisfying mechanical properties that mimick the extracellular matrix (ECM) for cell adhesion, proliferation, and differentiation (Guvendiren et al., 2016).
Unlike the biomaterials which are used in extrusion-based bioprinting, biomaterials used for inkjet 3D bioprinting should have a relatively low viscosity or, better, a shear-thinning characteristic, to ensure the smooth flow inside the inkjet nozzle and successful droplet formation (Xu et al., 2022b). Therefore, there are limited sources of materials suitable for inkjet 3D bioprinting, among which, hydrogels with high water content are widely selected as proper materials for inkjet 3D bioprinting (Zhu et al., 2016). Hydrogels can be mainly categorized into naturally derived and synthetic hydrogels depending on their source. Natural hydrogels such as collagen and gelatin which are frequently found in human tissues, have proved their suitability for inkjet 3D bioprinting by providing the environment for cellular activities (Gudapati and Ozbolat, 2020; Bedell et al., 2022). In addition, alginate is another commonly used natural hydrogel in inkjet 3D bioprinting due to its good biocompatibility and fast ionic crosslinking mechanism (Xu et al., 2014). Synthetic hydrogels such as U.S. Food and Drug Administration (FDA)-approved polyethylene glycol (PEG) with low molecular weight have also been selected for applications using inkjet 3D bioprinting due to their enhanced mechanical properties (Gao et al., 2015). In addition to hydrogels, polymers such as poly (ε-caprolactone) (PCL), poly (lactic acid) (PLA), and PLGA have also been widely reported to be used in inkjet 3D bioprinting for various applications (Lee et al., 2012; Scoutaris et al., 2016). These polymers are used by being dispersed in organic solvents such as ethanol and dimethylformamide, which might be harmful for cells. Therefore, before cells are seeded on the scaffolds, it is necessary to guarantee that the solvents have fully evaporated to ensure cell viability and functionality during the post-printing culturing and maturation process.
3.2 Cells for inkjet 3D bioprinting
As the other key element in the bioink, there are many available choices of cells for inkjet 3D bioprinting depending on the applications. It is critical for the cells, especially those vulnerable cells such as stem cells, to remain alive and functional both during and after the bioprinting process to ensure their proliferation and differentiation ability and to guarantee the functionality of the 3D bioprinted constructs. Generally, the sources of cells can be divided into two types: primary cells and stem cells. There are several types of primary cells including, but not limited to, human microvascular endothelial cells (HMVECs) (Cui and Boland, 2009), human dermal fibroblasts (Lee et al., 2009), and porcine Schwann cells (Tse et al., 2016), which have been widely reported to be selected for inkjet 3D bioprinting. Meanwhile, due to the negligible injury of cells during inkjet 3D bioprinting process, stem cells, such as human mesenchymal stem cells (hMSCs) (Gao et al., 2015) and mouse embryonic stem cells (mESCs) (Yumoto et al., 2020), which maintain their pluripotent ability to differentiate into the desired cell type under specific guidance, have also been selected for inkjet 3D bioprinting demonstrating the potential of using inkjet 3D bioprinting to biofabricate 3D native-like constructs, which have the potential to be transplanted into the human body.
4 Inkjet 3D bioprinting for tissue engineering and pharmaceutics
Inkjet 3D bioprinting has been reported to be broadly used as a functional tool in various fields. Although it remains challenging for current inkjet bioprinted constructs to be used for clinical trials, inkjet 3D bioprinting has been widely used in in vitro tissue/organ reconstruction and the formation of in vivo tissue substitutes and high throughput drug screening models. In this section, the typical applications in various fields will be summarized.
4.1 In vitro biomimetic tissue models
In vitro biomimetic 3D tissue models enable the investigation of cell-cell interactions as well as of the cell response to changes in the microenvironment. They are therefore valuable tools for basic and clinical research, drug screenings or toxicological analyses (Moroni et al., 2018; Taymour et al., 2022). The functionality of living tissues is dictated by their highly specialized and hierarchical architecture. Meanwhile, tissues and organs are composed of many types of cells and ECM components and, with few exceptions, are infiltrated with vascular and innervation networks (Levato et al., 2020). There is a major challenge in recapitulating the native tissue-like architectures structurally and functionally. To address it, a fabrication method capable of precisely depositing various materials and cells in pre-defined locations in the 3D space is highly desirable (Shapira and Dvir, 2021). Inkjet 3D bioprinting, as one of the most effective biofabrication technologies, could endow us with that capability. Indeed, some intriguing research and fascinating developments in inkjet 3D bioprinting of in vitro tissue models have been witnessed. The bioprinted biomimetic tissues are sharing more and more structural and functional features with their natural counterparts.
At its early stage, inkjet 3D bioprinting of in vitro tissue model focused on the fabrication of 3D acellular structure to mimic the geometry and macro structure of various native tissues and organs (Derby, 2012; Gudapati et al., 2016; Li et al., 2020). Concurrently, the feasibility of using inkjet 3D bioprinting to deposit the bioink composed of various cell types and materials was checked (Xu et al., 2009, 2013; Herran and Huang, 2012; Kim et al., 2016; Kumar et al., 2021). A distinguished work that elegantly demonstrated the reconstruction of acellular biomimetic tissue structure was accomplished by Blaeser et al. (2016). In this work, the researchers developed a multi-nozzle inkjet 3D bioprinting system with a bi-phasic support liquid serving as a supporting and cross-linking bath. The liquid bath consisted of perfluorocarbon (PFC) and 50-mg/mL calcium chloride solution, where CaCl2solution floated on top of the PFC, forming a thin CaCl2aqueous layer on top. A droplet of 5-mg/mL sodium alginate ejected from a microvalve nozzle was cross-linked upon contact with the CaCl2solution. Besides, the bath could be cooled to print biomaterials, like gelatin, with a reversible thermal cross-linking mechanism. These biomaterials can be introduced as fugitive materials to support the printed structure, especially when a soft hydrogel is selected for 3D bioprinting. On the basis of this printing strategy, the authors successfully printed hollow acellular structures with a thin wall thickness, as shown in Fig. 2a, and demonstrated the possibility of constructing those native tubular tissues in human body. Using the same biomaterial, Christensen and his colleagues provided an excellent example of inkjet bioprinting of a bionic cellular structure (Christensen et al., 2015). In their study, the NIH 3T3 mouse fibroblasts with a cell density of 5 million cells/mL were suspended in 1% (in mass) sodium alginate to form the bioink, which was printed into a liquid bath of 2% (in mass) CaCl2solution to build a thin-walled tubular structure with multiple bifurcations in both horizontal and vertical directions. As illustrated in Fig. 2b, the printed structure containing 3T3 fibroblasts resembled native vasculature and maintained a more than 90% post-printing cell viability. Both the cellular and acellular bifurcated tubes were found to exhibit good fidelity.
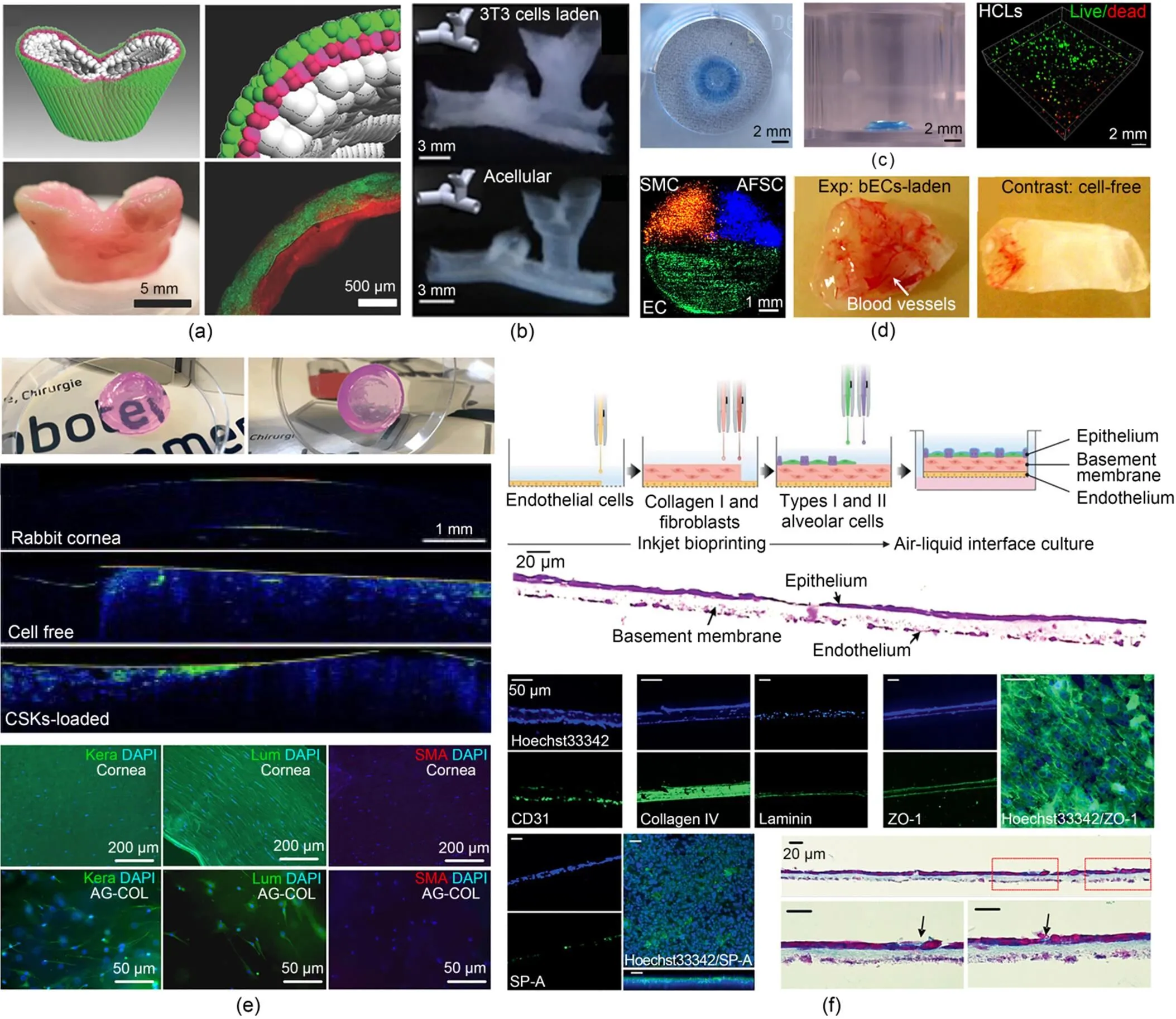
Fig. 2 Inkjet 3D bioprinting of in vitro biomimetic 3D tissue structures: (a) A hollow acellular structure with a thin wall thickness to mimic the shape of tubular tissue (reprinted from (Blaeser et al., 2016), Copyright 2016, with permission from Wiley); (b) Thin-walled tubular structures with bifurcations in horizontal and vertical directions representing vasculature models (reprinted from (Christensen et al., 2015), Copyright 2015, with permission from Wiley); (c) A 3D alginate ring structure with staining of the embedded HLCs derived from hESCs using live/dead assays (reprinted from (Faulkner-Jones et al., 2015), Copyright 2015, with permission from Institute of Physics); (d) 3D heterogeneous pie-shaped tissue constructs containing patterned hAFSCs, dSMCs, and bECs and 3D cuboidal homogenous tissue constructs with/without bECs after 8-week implantation (reprinted from (Xu et al., 2013), Copyright 2013, with permission from Elsevier); (e) Functional 3D biomimetic corneal models with optical properties similar to native corneal tissue with the encapsulated CSKs maintaining their native keratocyte phenotypes after 7-d in vitro culture (reprinted from (Duarte Campos et al., 2019), Copyright 2019, with permission from Wiley); (f) Three-layered alveolar barrier model recapitulating the structure, morphologies, and functionality of lung tissue (reprinted from (Kang et al., 2021), Copyright 2021, with permission from Wiley). HLCs: hepatocyte-like cells; hESCs: human embryonic stem cells; hAFSCs: human amniotic fluid-derived stem cells; dSMCs: canine smooth muscle cells; bECs: bovine aortic endothelial cells; CSKs: corneal stromal keratocytes; Kera: human keratocan; Lum: lumican; SMA: smooth muscle actin; DAPI: 4',6-diamidino-2-phenylindole; AG-COL: agarose-collagen; SP-A: surfactant protein A
After inkjet printing of living cells was proved feasible, researchers started to pay more attention to study the effects of process parameters on cells both during and after printing. Apart from cell viability, cellular behaviors such as the biomarker and gene expressions are also taken into consideration, pushing the in vitro tissue model from shape-centered to cell-centered. A typical work was conducted by Faulkner-Jones et al. (2015), where the first study using 3D bioprinting of human induced pluripotent stem cells (hIPSCs) was reported. In this study, hepatocyte-like cells (HLCs) differentiated from hIPSCs, and human embryonic stem cells (hESCs) were bioprinted, and the hepatic markers were examined to validate the feasibility of using microvalve 3D bioprinting to bioprint sensitive cells such as stem cells. It was found that the stem cells had successfully differentiated into hepatocytes. There was no evidence that the viability and pluripotency of hESCs and hIPSCs were affected by the bioprinting process. The authors also printed hESCs-derived HLCs in a 3D alginate matrix as shown in Fig. 2c. The viability and secretion of albumin were tested during differentiation. It was reported that a 40-layer construct containing HLCs reached peak albumin secretion at day 21. To fully imitate the heterogeneity of cell types in native tissue, Xu et al. (2013) pioneered multi-material and multiple cell type bioprinting. In their work, human amniotic fluid-derived stem cells (hAFSCs), canine smooth muscle cells (dSMCs), and bovine aortic endothelial cells (bECs) were separately suspended in a CaCl2solution to formulate three types of bioink. A 3D heterogeneous pie-shaped tissue construct as shown in Fig. 2d was printed by individually depositing these bioinks on a sodium alginate-collagen composite. 3D constructs with homogeneous cells of each cell type were also biofabricated for comparison. The formed constructs containing both one cell type and multiple cell types were cultured for one week and subcutaneously implanted into outbred athymic nude mice for up to 18 weeks. Afterwards, the constructs were surgically retrieved every 4 or 8 weeks. The experimental results showed that cell viability and cellular activities such as cell proliferation of the bioprinted cells of each type were not affected significantly both in vitro and in vivo. It is noted that the vascularization level of the constructs containing bECs was much higher compared to the control group.
As the focus of inkjet 3D bioprinting of in vitro tissue construction turns from shape to function, two exemplary works were recently published by Duarte Campos et al. (2019) and Kang et al. (2021). In the study presented by Duarte Campos et al. (2019), the primary corneal stromal keratocytes (CSKs) derived from human were bioprinted in collagen-based bioink to fabricate 3D biomimetic corneal models. The shape fidelity as well as the functionality of the bioprinted samples was evaluated after in vitro culture. In their study, CSKs mixing 0.5% agarose and 0.2% Type I collagen hydrogel were used as bioink, and the functional 3D corneal structure was directly printed using a solely freeform microvalve inkjet 3D bioprinting system without additional supporting bath. As illustrated in Fig. 2e, the bioprinted 3D corneal models were highly transparent, and the optical density of the constructs both with and without CSKs was similar to that measured for rabbit corneas (blue color intensity). In addition, the bioprinted CSKs were kept alive after the bioprinting process and maintained their native keratocyte phenotypes after a 7-d in vitro culture. In another elegant work presented by Kang et al. (2021), a 3D biomimetic alveolar barrier model was fabricated by inkjet printing of four human alveolar cell lines, namely, types I and II alveolar cells (NCI-H1703 and NCI-H441), lung fibroblasts (MRC5), and lung microvascular endothelial cells (HULEC-5a). Benefiting from the high resolution and good controllability of PIJ 3D bioprinting, a three-layered alveolar barrier model with an unprecedented thickness of about 10 µm was formed, as shown in Fig. 2f. Immunofluorescence and histology images showed that the 3D in vitro model better recapitulated the structure, morphology, and functionality of the lung tissue, compared to the conventional 2D cell culture model and 3D non-structured model of a homogeneous mixture of the alveolar cells and collagen. Moreover, it was found that this thin multilayered model could reproduce practical tissue-level responses to influenza infection, showing the great potential of using this 3D in vitro model for pathological and pharmaceutical applications.
Overall, these meticulously engineered tissues are definitely an important step in promoting the development of 3D bioprinting, especially inkjet 3D bioprinting. However, in vitro tissue reconstruction is still in its infancy; more efforts are needed to facilitate the development of inkjet bioprinting to enable better similarity of the engineered constructs as native tissues/organs.
4.2 In vivo tissue substitutes
Inkjet bioprinted constructs have not only been used for in vitro models, but also been used as in vivo substitutes. Several engineered tissues/organs fabricated using inkjet 3D bioprinting technique have been transplanted into human/animal body. Even though it remains challenging to implant engineered tissues/organs with complex geometries and heterogeneous cell/material types into human body to fully replace damaged native tissues/organs, simple structures such as bone, cartilage, and skin have reached the level of implantation (Chen et al., 2021). For example, as shown in Fig. 3a, Cooper et al. (2010) successfully created bones which can be transplanted into a mouse calvarial defect model for in vivo tests. It was found that the bone formation in vivo was similar to that in in vitro tests, and bio-patterning formed using inkjet 3D bioprinting could guide cell differentiation and facilitate tissue formation in vivo. Xu et al. (2009) presented an innovatory approach to simultaneously transfect genes and deliver cells using inkjet 3D bioprinting technique. In this study, porcine aortic endothelial (PAE) cells with genes modified were mixed with pmaxGFP plasmid and directly delivered to the desired locations on the printed fibrin gel in the subcutaneous tissues of athymic mice. The fibrin gel containing the PAE cells was retrieved from the mouse one week after the implantation. The printed tube shape was still found to match with the designed shape, and vasculature was observed in this printed fibrin tube as shown in Fig. 3b. Later, the same group (Xu T et al., 2012) selected a hybrid printing system by combining inkjet 3D bioprinting and electrospinning to fabricate the engineered cartilage tissues as shown in Fig. 3c. The cartilage-like constructs formed with PCL and fibrin-collagen were found to exhibit enhanced mechanical properties compared to those formed directly using an inkjet 3D bioprinting system. In addition, the cells encapsulated within the formed constructs were found to maintain the ability to proliferate, and the five-layered cellular cartilage-like constructs implanted into immunodeficient mice were found to be similar to native cartilage by detecting the successful deposition of type II collagen and glycosaminoglycans. Albanna et al. (2019) proposed a new concept by precisely inkjet printing the bioink containing dermal fibroblasts and epidermal keratinocytes onto the injured area of skin to replicate the native skin structure and facilitate wound healing. Their experimental results showed that both wound closure and re-epithelization were speeded up. In addition, these engineered skins shown in Fig. 3d showed similarities to healthy skin demonstrating the great potential of using inkjet 3D bioprinting for healing skin wounds. Currently, despite lacking successful transplantation of the complex engineered tissues/organs due to lacking sufficient mechanical properties and structural integrity, it can be expected that more and more engineered tissues/organs will be gradually implanted into human body to fully replace damaged ones as the bioprinting techniques become more and more mature.
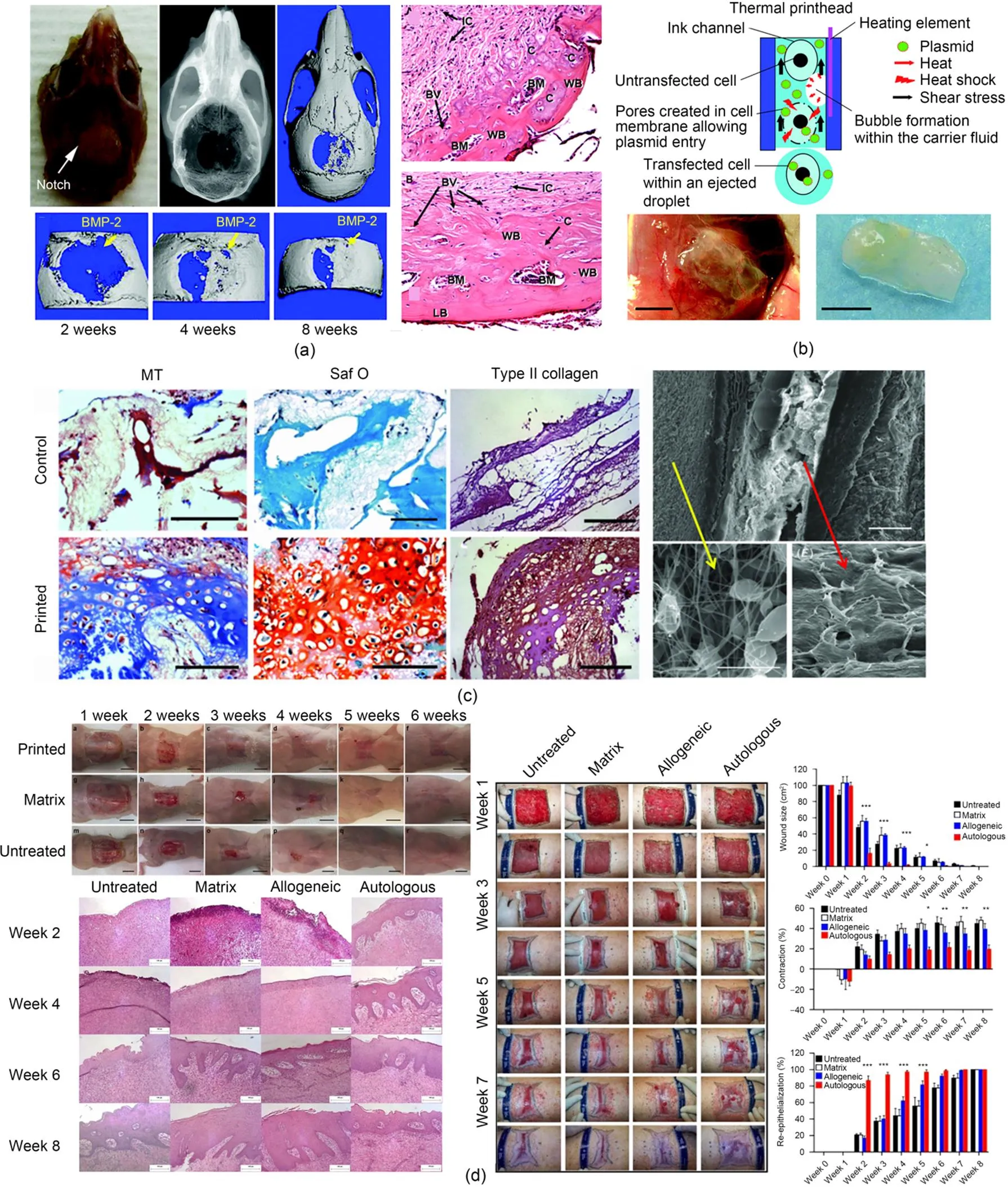
Fig. 3 (a) Engineered bones with bio-patterning capable of guiding cell differentiation in vivo (reprinted from (Cooper et al., 2010), Copyright 2010, with permission from Mary Ann Liebert); (b) Printed tube with vasculature formed 1 week after implantation into mice (reprinted from (Xu et al., 2009), Copyright 2009, with permission from Mary Ann Liebert); (c) Cartilage-like constructs implanted into mice resulting in successful deposition of type II collagen and glycosaminoglycans (reprinted from (Xu T et al., 2012), Copyright 2013, with permission from Institute of Physics); (d) Inkjet-printed dermal fibroblasts and epidermal keratinocytes onto the skin wound facilitating skin wound closure (reprinted from (Albanna et al., 2019), Copyright 2019, with permission from Springer Nature). BMP-2: bone morphogenetic protein-2; WB: woven bone; BM: bone marrow space; BV: blood vessel; C: endochondral bone; IC: invading undefined cells; LB: lamellar bone; MT: Masson's trichrome; Saf O: Safranin O
4.3 High throughput drug screening model
Drug screening, which is an essential step during drug development, refers to the evaluation process of the pharmacological function when selecting drug candidates. The appearance of 3D bioprinting provides a new solution for solving the current dilemma of drug screening (He et al., 2020; Ramezani et al., 2020; Zhang and Khademhosseini, 2020). For example, inkjet 3D bioprinting with a non-contact delivery mechanism provides a more efficient and accurate way to create an environment for chemical reactions since small volumes of droplets can be deposited at pre-defined locations with a high frequency and without contamination. The utilization of inkjet 3D bioprinting in drug screening has been found to significantly increase the efficiency and reduce material consumption. For example, Arrabito and Pignataro (2010) presented a non-contact and rapid way for drug screening by inkjet dispensing in a micro-array format as shown in Fig. 4a. By controlling the volume of the formed droplets, picolitre droplets containing a model substrate/inhibitor couple could be precisely deposited onto the desired location containing the enzymatic target. The experimental results showed that this method had the potential to be broadly utilized in drug screening. As shown in Fig. 4b, Rodríguez-Dévora et al. (2012) proposed an innovational method to assemble a miniature drug-screening platform with the help of an inkjet 3D bioprinting system to replace the normal expensive therapeutic process during drug development and evaluation. By screening the antibiotic inhibition on bacteria, this platform has validated its effectiveness and exhibited its superiority to the traditional drug evaluation process. Matsusaki et al. (2013) selected inkjet 3D bioprinting to rapidly form simplified micro-tissue chips representing simplified liver, as shown in Fig. 4c, on which 400 micro-arrays of multilayered structures were integrated. These chips were found to have great potential for tailored drug screenings and toxicological evaluations replacing high-cost and time-consuming animal experiments. Drug screening of antitumor drugs is a rapidly growing market (Ma et al., 2020; Xie et al., 2020), and understanding the underlying physics of invasion and metastasis of cancer cells is of great interest to tumor and cancer research. Therefore, in vitro 3D tumor models are necessary to study the cell-cell/matrix interactions and for anticancer drug screening. Recently, benefiting from 3D bioprinting, Jung et al. (2022) developed a sophisticated in vitro model as shown in Fig. 4d using a DOD 3D bioprinting system for better understanding cancer cell invasion and metastasis and for high throughput anticancer drug screening. With consistent technological advances, the printing resolution of the multi-nozzle inkjet 3D bioprinting system can be further increased resulting in higher deposition accuracy and efficiency, and the material usage can be further saved. Therefore, the applications of inkjet 3D bioprinting in drug screening can be further extended.
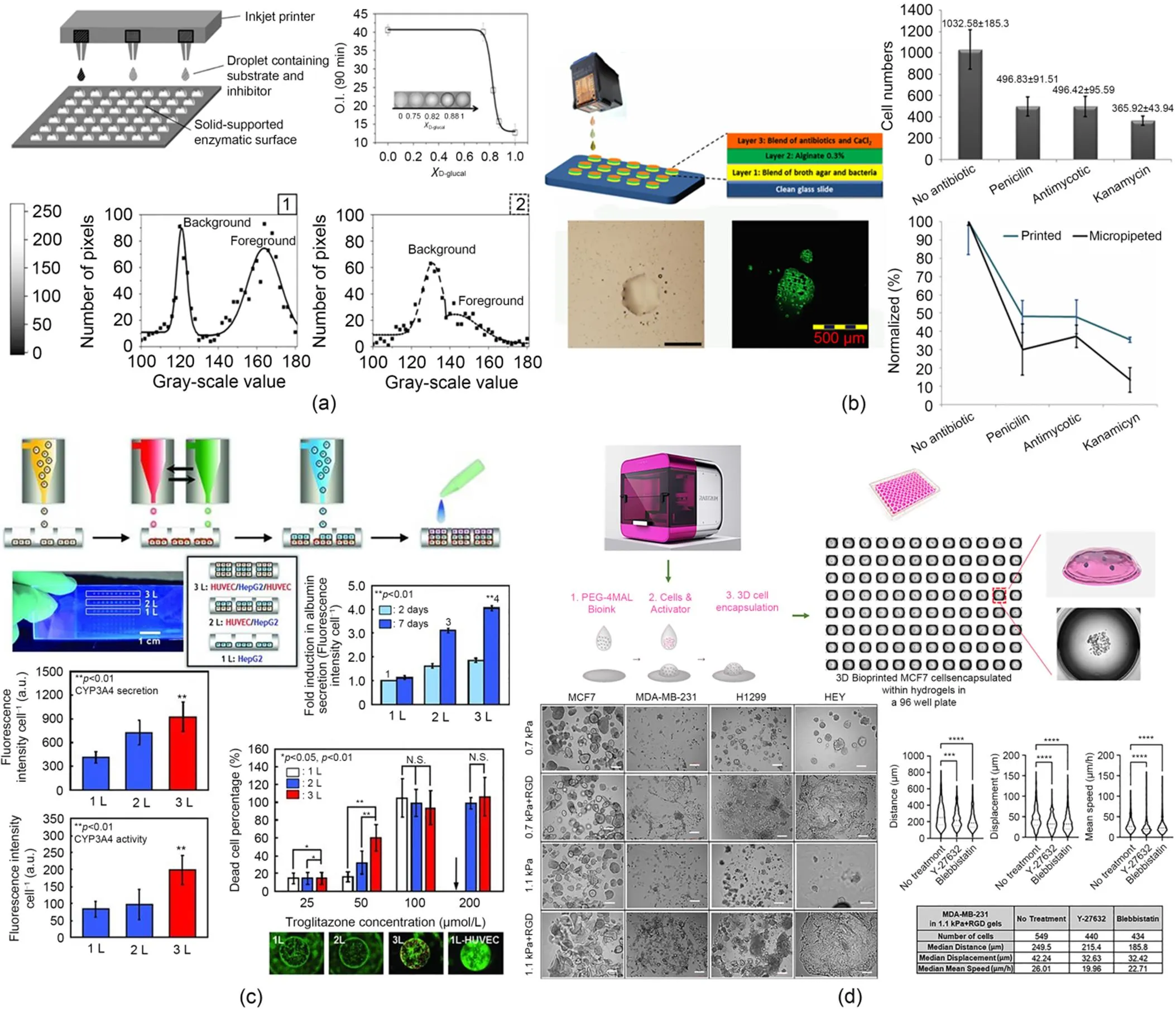
Fig. 4 (a) Inkjet-assisted micro-arrays for rapid drug screening (reprinted from (Arrabito and Pignataro, 2010), Copyright 2010, with permission from ACS); (b) A miniature drug-screening platform fabricated using inkjet 3D bioprinting demonstrating superior advantages over traditional drug screening process (reprinted from (Rodríguez-Dévora et al., 2012), Copyright 2012, with permission from Institute of Physics); (c) Micro-tissue chips fabricated with inkjet 3D bioprinting system for drug screening (reprinted from (Matsusaki et al., 2013), Copyright 2013, with permission from Wiley); (d) An in vitro model for studying cancer cell invasion and metastasis and high throughput anticancer drug screening (reprinted from (Jung et al., 2022), Copyright 2022, with permission from Royal Society of Chemistry). HUVEC: human umbilical vein endothelial cells; HepG2: human hepatocellular carcinoma; RGD: arginine-glycine-aspartic acid
5 Discussions and future perspectives
By summarizing the techniques, material selections, and typical applications of inkjet 3D bioprinting in tissue engineering and pharmaceutics, it is obvious that inkjet 3D bioprinting is versatile and can be envisioned as an effective tool for fabricating native-like tissues/organs which are transplantable to human body. However, there are still many challenges that inkjet 3D bioprinting is currently facing. These challenges restrict the application areas of inkjet 3D bioprinting. Generally, the challenges mainly come from the printing and materials side, and there could be several directions, listed as follows, to address them.
From the printing side, better designing the printing system might be helpful for improving the printing performance using inkjet 3D bioprinting. For example, it has been reported that the shear stress imposed on the cells during the printing process can be reduced by simply changing the shape of the inkjet nozzle (Liu et al., 2017). Using the substrate coating with low-absorptivity or low thermal conductivity materials has also been found to be effective in better protecting the cells during the landing of the droplets (Hopp et al., 2012; Talbot et al., 2012). In addition, better understanding of the physics during inkjet 3D bioprinting process will also be helpful for better designing the printing system. However, some underlying physics during the printing process is still unknown. For example, current inkjet 3D bioprinting of bioink usually causes splashes when the cell-laden droplets are collected on the substrate, especially when the droplet velocity is high (Yarin, 2006). This collision between the droplets and the container not only increases the shear stress imposed on the encapsulated cells, but also sacrifices the printing resolution (Tsai et al., 2011; Li et al., 2020). In addition, bubbles are sometimes entrapped within the nozzle during the droplet formation process (Liu et al., 2022) and wetting on the inkjet nozzle is frequently observed. These phenomena have significant effects on the stability of the formed cell-laden droplets and on the printing quality and functionality of the final constructs. However, these phenomena which are detrimental to the printing performance are still not yet understood.
The utilization of a multi-nozzle inkjet 3D bioprinting system/multi-method 3D bioprinting system is an alternative for improving the printing performance, especially for the fabrication of heterogeneous native-like constructs with complex geometries and different material compositions. Inkjet 3D bioprinting can be combined with other techniques such as microfluidic devices, extrusion-based bioprinting, and electrospinning to break the barriers and broaden the application areas (Zhou et al., 2022). For instance, as mentioned in this review, inkjet 3D bioprinting has been accompanied with electrospinning to fabricate cartilage-like constructs, and the experimental results have shown that their mechanical properties are far higher compared to those of the constructs formed purely using inkjet 3D bioprinting. However, multi-material/method 3D bioprinting may bring some side effects, such as an increase in the printing time because of the different cross-linking mechanisms of different materials, the complexity in planning the trajectory of the nozzles to avoid the interference, and the potential decrease in the printing resolution at the interface connecting the two layers formed with different bioprinting techniques.
Inkjet 3D bioprinting system relies on the ejection of the bioink from the nozzle orifice to form cell/drug-laden droplets as the basic building block. It is well known that the largest restriction of inkjet 3D bioprinting comes from the material choice of a low-viscosity bioink due to the limitation of the small nozzle size (tens of microns). Currently, even though there have been several materials which are suitable for 3D bioprinting, only few of them can be selected to be used in inkjet 3D bioprinting. For example, it is noted that gelatin methacrylate (GelMA) is a frequently used biological material in various bioprinting techniques due to its easily tunable properties and acceptable cell viability (Pepelanova et al., 2018). However, since the development of GelMA, it has seldomly been reported to be used for inkjet 3D bioprinting. One main reason is that natural light can potentially crosslink the GelMA-based bioink with the presence of the photoinitiator at the nozzle orifice and thus block the nozzle. Therefore, a better design of the nozzle is necessary. For example, coating on the exterior region of the nozzle might be helpful for avoiding nozzle clogging. Alternatively, an inkjet 3D bioprinting system with multiple nozzles connected with different bioink reservoirs could also be considered. In this case, photo cross-linkable materials can be held separate from the photoinitiator in different bioink reservoirs, and the photo cross-linking process cannot occur within the nozzle protecting the nozzle from blocking. With better protection of the nozzle, other photo cross-linkable materials with shear-thinning characteristics can also be selected for inkjet 3D bioprinting, enriching the material choices and expanding its applications. Besides, current 3D bioprinting is always a tradeoff between high cell viability and high printing shape fidelity since materials (e.g., synthetic hydrogels) providing sufficient mechanical strengths during and after the printing process are always lacking sufficient biocompatibility, while materials with superior biocompatibility (e.g., natural hydrogels) are unable to self-support the structures and thus reduce the shape fidelity (Unagolla and Jayasuriya, 2020). For these reasons, it is critical to seek for the next-generation materials which simultaneously hold several biomaterial superiorities and are more likely to imitate the environment of human body and provide better conditions for cellular activities.
To conclude, with a better physical understanding of the printing process, a better design of the printing system, a combination with other bioprinting techniques, and broader material choices, inkjet 3D bioprinting, as a key component in 3D bioprinting, has great potential to achieve the goal of 3D bioprinting in successfully replacing damaged tissues and organs in human body and becoming more involved in tissue engineering and pharmaceutics.
6 Conclusions
Recently, inkjet 3D bioprinting, as a key element in 3D bioprinting, has received much attention. This review focusing on inkjet 3D bioprinting firstly summarizes the techniques, materials, and applications of inkjet 3D bioprinting, subsequently discusses the major challenges that inkjet 3D bioprinting is currently facing, and lastly proposes potential solutions for addressing those challenges. Overall, inkjet 3D bioprinting has proved its feasibility and versatility in tissue engineering and pharmaceutics. However, despite the continuous advances in bioprinting techniques and materials science, inkjet 3D bioprinting still needs significant improvements in several aspects, such as its printing resolution and speed. In addition, the materials which are suitable for inkjet 3D bioprinting are still quite limited. Therefore, interdisciplinary collaboration between experts from mechanical engineering, materials science, and biology is necessary to promote the development of inkjet 3D bioprinting. Moreover, inkjet 3D bioprinting, combined with other printing techniques and relying on multi-method to 3D bioprint multi-material, will be the future trend of 3D bioprinting and lead 3D bioprinting to the next step towards the clinical transplantation of 3D engineered tissues and organs.
Acknowledgments
This work is supported by the Starry Night Science Fund of Zhejiang University Shanghai Institute for Advanced Study (No. SN-ZJU-SIAS-004) and the National Natural Science Foundation of China (No. 52075482).
Author contributions
Deng-ke ZHAO and Jun YIN designed the research. Deng-ke ZHAO and He-qi XU wrote the first draft of the manuscript. Jun YIN and Hua-yong YANG helped to organize the manuscript. He-qi XU revised and edited the final version. All the authors have approved the final manuscript.
Conflict of interest
Deng-ke ZHAO, He-qi XU, Jun YIN, and Hua-yong YANG declare that they have no conflict of interest.
Albanna M, Binder KW, Murphy SV, et al., 2019. In situ bioprinting of autologous skin cells accelerates wound healing of extensive excisional full-thickness wounds. Scientific Reports, 9(1):1856. https://doi.org/10.1038/s41598-018-38366-w
Arrabito G, Pignataro B, 2010. Inkjet printing methodologies for drug screening. Analytical Chemistry, 82(8):3104-3107. https://doi.org/10.1021/ac100169w
Basaran OA, Gao HJ, Bhat PP, 2013. Nonstandard inkjets. Annual Review of Fluid Mechanics, 45:85-113. https://doi.org/10.1146/annurev-fluid-120710-101148
Bedell ML, Torres AL, Hogan KJ, et al., 2022. Human gelatin-based composite hydrogels for osteochondral tissue engineering and their adaptation into bioinks for extrusion, inkjet, and digital light processing bioprinting. Biofabrication, 14(4):045012. https://doi.org/10.1088/1758-5090/ac8768
Blaeser A, Duarte Campos DF, Puster U, et al., 2016. Controlling shear stress in 3D bioprinting is a key factor to balance printing resolution and stem cell integrity. Advanced Healthcare Materials, 5(3):326-333. https://doi.org/10.1002/adhm.201500677
Cai SX, Sun YL, Wang Z, et al., 2021. Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing. Nanotechnology Reviews, 10(1):1046-1078.
Campbell A, Mohl JE, Gutierrez DA, et al., 2020. Thermal bioprinting causes ample alterations of expression of LUCAT1, IL6, CCL26, and NRN1L genes and massive phosphorylation of critical oncogenic drug resistance pathways in breast cancer cells. Frontiers in Bioengineering and Biotechnology, 8:82. https://doi.org/10.3389/fbioe.2020.00082
Chen EP, Toksoy Z, Davis BA, et al., 2021. 3D bioprinting of vascularized tissues for in vitro and in vivo applications. Frontiers in Bioengineering and Biotechnology, 9:664188. https://doi.org/10.3389/fbioe.2021.664188
Christensen K, Xu CX, Chai WX, et al., 2015. Freeform inkjet printing of cellular structures with bifurcations. Biotechnology and Bioengineering, 112(5):1047-1055. https://doi.org/10.1002/bit.25501
Cooper GM, Miller ED, DeCesare GE, et al., 2010. Inkjet-based biopatterning of bone morphogenetic protein-2 to spatially control calvarial bone formation. Tissue Engineering Part A, 16(5):1749-1759. https://doi.org/10.1089/ten.tea.2009.0650
Cui XF, Boland T, 2009. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials, 30(31):6221-6227. https://doi.org/10.1016/j.biomaterials.2009.07.056
Cui XF, Boland T, D’Lima DD, et al., 2012. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Patents on Drug Delivery & Formulation, 6(2):149-155. https://doi.org/10.2174/187221112800672949
Derby B, 2010. Inkjet printing of functional and structural materials: fluid property requirements, feature stability, and resolution. Annual Review of Materials Research, 40:395-414. https://doi.org/10.1146/annurev-matsci-070909-104502
Derby B, 2012. Printing and prototyping of tissues and scaffolds. Science, 338(6109):921-926. https://doi.org/10.1126/science.1226340
Duarte Campos DF, Rohde M, Ross M, et al., 2019. Corneal bioprinting utilizing collagen-based bioinks and primary human keratocytes. Journal of Biomedical Materials Research Part A, 107(9):1945-1953. https://doi.org/10.1002/jbm.a.36702
Dufour A, Gallostra XB, O’Keeffe C, et al., 2022. Integrating melt electrowriting and inkjet bioprinting for engineering structurally organized articular cartilage. Biomaterials, 283:121405. https://doi.org/10.1016/J.BIOMATERIALS.2022.121405
Eggers J, Villermaux E, 2008. Physics of liquid jets. Reports on Progress in Physics, 71(3):036601. https://doi.org/10.1088/0034-4885/71/3/036601
Faulkner-Jones A, Fyfe C, Cornelissen DJ, et al., 2015. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication, 7(4):044102. https://doi.org/10.1088/1758-5090/7/4/044102
Freeman FE, Pitacco P, van Dommelen LHA, et al., 2020. 3D bioprinting spatiotemporally defined patterns of growth factors to tightly control tissue regeneration. Science Advances, 6(33):eabb5093. https://doi.org/10.1126/sciadv.abb5093
Gao DJ, Zhou JG, 2019. Designs and applications of electrohydrodynamic 3D printing. International Journal of Bioprinting, 5(1):172. https://doi.org/10.18063/ijb.v5i1.172
Gao GF, Yonezawa T, Hubbell K, et al., 2015. Inkjet‐bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnology Journal, 10(10):1568-1577. https://doi.org/10.1002/biot.201400635
Gasperini L, Maniglio D, Motta A, et al., 2015. An electrohydrodynamic bioprinter for alginate hydrogels containing living cells. Tissue Engineering Part C: Methods, 21(2):123-132. https://doi.org/10.1089/ten.tec.2014.0149
Godleman J, Leroux F, Reynolds S, et al., 2021. Aromatic poly (fluorocarbinol)s: soluble, hydrophobic binders for inkjet formulations. Progress in Organic Coatings, 158:106378. https://doi.org/10.1016/j.porgcoat.2021.106378
Griffith LG, Wu B, Cima MJ, et al., 1997. In vitro organogenesis of liver tissue. Annals of the New York Academy of Sciences, 831(1):382-397. https://doi.org/10.1111/j.1749-6632.1997.tb52212.x
Gudapati H, Ozbolat IT, 2020. The role of concentration on drop formation and breakup of collagen, fibrinogen, and thrombin solutions during inkjet bioprinting. Langmuir, 36(50):15373-15385. https://doi.org/10.1021/acs.langmuir.0c02926
Gudapati H, Dey M, Ozbolat I, 2016. A comprehensive review on droplet-based bioprinting: past, present and future. Biomaterials, 102:20-42. https://doi.org/10.1016/j.biomaterials.2016.06.012
Gurkan UA, El Assal R, Yildiz SE, et al., 2014. Engineering anisotropic biomimetic fibrocartilage microenvironment by bioprinting mesenchymal stem cells in nanoliter gel droplets. Molecular Pharmaceutics, 11(7):2151-2159. https://doi.org/10.1021/mp400573g
Guvendiren M, Molde J, Soares RMD, et al., 2016. Designing biomaterials for 3D printing. ACS Biomaterials Science & Engineering, 2(10):1679-1693. https://doi.org/10.1021/acsbiomaterials.6b00121
He Y, Nie J, Xie M, et al., 2020. Why choose 3D bioprinting? Part III: printing in vitro 3D models for drug screening. Bio-Design and Manufacturing, 3:160-163. https://doi.org/10.1007/s42242-020-00067-7
Herran CL, Huang Y, 2012. Alginate microsphere fabrication using bipolar wave-based drop-on-demand jetting. Journal of Manufacturing Processes, 14(2):98-106. https://doi.org/10.1016/j.jmapro.2011.11.001
Herran CL, Coutris N, 2013. Drop-on-demand for aqueous solutions of sodium alginate. Experiments in Fluids, 54(6):1548. https://doi.org/10.1007/s00348-013-1548-9
Hoath SD, 2016. Fundamentals of Inkjet Printing: the Science of Inkjet and Droplets. John Wiley & Sons, Weinheim, Germany.
Hopp B, Smausz T, Szabó G, et al., 2012. Femtosecond laser printing of living cells using absorbing film-assisted laser-induced forward transfer. Optical Engineering, 51(1):014302. https://doi.org/10.1117/1.OE.51.1.014302
Jung MS, Skhinas JN, Du EY, et al., 2022. A high-throughput 3D bioprinted cancer cell migration and invasion model with versatile and broad biological applicability. Biomaterials Science, 10(20):5876-5887. https://doi.org/10.1039/D2BM00651K
Kang D, Park JA, Kim W, et al., 2021. All‐inkjet‐printed 3D alveolar barrier model with physiologically relevant microarchitecture. Advanced Science, 8(10):2004990. https://doi.org/10.1002/advs.202004990
Kim YK, Park JA, Yoon WH, et al., 2016. Drop-on-demand inkjet-based cell printing with 30-μm nozzle diameter for cell-level accuracy. Biomicrofluidics, 10(6):064110. https://doi.org/10.1063/1.4968845
Klebe RJ, 1988. Cytoscribing: a method for micropositioning cells and the construction of two-and three-dimensional synthetic tissues. Experimental Cell Research, 179(2):362-373. https://doi.org/10.1016/0014-4827(88)90275-3
Knowlton S, Onal S, Yu CH, et al., 2015. Bioprinting for cancer research. Trends in Biotechnology, 33(9):504-513. https://doi.org/10.1016/j.tibtech.2015.06.007
Kumar P, Ebbens S, Zhao XB, 2021. Inkjet printing of mammalian cells–theory and applications. Bioprinting, 23:e00157. https://doi.org/10.1016/J.BPRINT.2021.E00157
Kwon KS, Rahman MK, Phung TH, et al., 2020. Review of digital printing technologies for electronic materials. Flexible and Printed Electronics, 5(4):043003. https://doi.org/10.1088/2058-8585/ABC8CA
Lee BK, Yun YH, Choi JS, et al., 2012. Fabrication of drug-loaded polymer microparticles with arbitrary geometries using a piezoelectric inkjet printing system. International Journal of Pharmaceutics, 427(2):305-310. https://doi.org/10.1016/j.ijpharm.2012.02.011
Lee W, Debasitis JC, Lee VK, et al., 2009. Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials, 30(8):1587-1595. https://doi.org/10.1016/j.biomaterials.2008.12.009
Levato R, Jungst T, Scheuring RG, et al., 2020. From shape to function: the next step in bioprinting. Advanced Materials, 32(12):1906423. https://doi.org/10.1002/adma.201906423
Li HY, Liu JK, Li K, et al., 2019. Piezoelectric micro-jet devices: a review. Sensors and Actuators A: Physical, 297:111552. https://doi.org/10.1016/j.sna.2019.111552
Li XD, Liu BX, Pei B, et al., 2020. Inkjet bioprinting of biomaterials. Chemical Reviews, 120(19):10793-10833. https://doi.org/10.1021/acs.chemrev.0c00008
Lim JH, Kuk K, Shin SJ, et al., 2005. Failure mechanisms in thermal inkjet printhead analyzed by experiments and numerical simulation. Microelectronics Reliability, 45(3-4):473-478. https://doi.org/10.1016/j.microrel.2004.12.009
Liu JC, Shahriar M, Xu HQ, et al., 2022. Cell-laden bioink circulation-assisted inkjet-based bioprinting to mitigate cell sedimentation and aggregation. Biofabrication, 14(4):045020. https://doi.org/10.1088/1758-5090/ac8fb7
Liu WJ, Heinrich MA, Zhou YX, et al., 2017. Extrusion bioprinting of shear‐thinning gelatin methacryloyl bioinks. Advanced Healthcare Materials, 6(12):1601451. https://doi.org/10.1002/adhm.201601451
Ma L, Li YT, Wu YT, et al., 2020. The construction of in vitro tumor models based on 3D bioprinting. Bio-Design and Manufacturing, 3(3):227-236. https://doi.org/10.1007/s42242-020-00068-6
Ma XY, Liu J, Zhu W, et al., 2018. 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modeling. Advanced Drug Delivery Reviews, 132:235-251. https://doi.org/10.1016/j.addr.2018.06.011
Mandrycky C, Wang ZJ, Kim K, et al., 2016. 3D bioprinting for engineering complex tissues. Biotechnology Advances, 34(4):422-434. https://doi.org/10.1016/j.biotechadv.2015.12.011
Matsusaki M, Sakaue K, Kadowaki K, et al., 2013. Three‐dimensional human tissue chips fabricated by rapid and automatic inkjet cell printing. Advanced Healthcare Materials, 2(4):534-539. https://doi.org/10.1002/adhm.201200299
Mironov V, Boland T, Trusk T, et al., 2003. Organ printing: computer-aided jet-based 3D tissue engineering. Trends in Biotechnology, 21(4):157-161. https://doi.org/10.1016/S0167-7799(03)00033-7
Mkhize N, Bhaskaran H, 2022. Electrohydrodynamic jet printing: introductory concepts and considerations. Small Science, 2(2):2100073. https://doi.org/10.1002/smsc.202100073
Moroni L, Burdick JA, Highley C, et al., 2018. Biofabrication strategies for 3D in vitro models and regenerative medicine. Nature Reviews Materials, 3(5):21-37. https://doi.org/10.1038/s41578-018-0006-y
Murphy SV, Atala A, 2014. 3D bioprinting of tissues and organs. Nature Biotechnology, 32(8):773-785. https://doi.org/10.1038/nbt.2958
Nakamura M, Kobayashi A, Takagi F, et al., 2005. Biocompatible inkjet printing technique for designed seeding of individual living cells. Tissue Engineering, 11(11-12):1658-1666. https://doi.org/10.1089/ten.2005.11.1658
Nakamura M, Iwanaga S, Henmi C, et al., 2010. Biomatrices and biomaterials for future developments of bioprinting and biofabrication. Biofabrication, 2(1):014110. https://doi.org/10.1088/1758-5082/2/1/014110
Ng WL, Lee JM, Yeong WY, et al., 2017. Microvalve-based bioprinting–process, bio-inks and applications. Biomaterials Science, 5(4):632-647. https://doi.org/10.1039/c6bm00861e
Ng WL, Huang X, Shkolnikov V, et al., 2022. Controlling droplet impact velocity and droplet volume: key factors to achieving high cell viability in sub-nanoliter droplet-based bioprinting. International Journal of Bioprinting, 8(1):424. https://doi.org/10.18063/ijb.v8i1.424
Nishiyama Y, Henmi C, Iwanaga S, et al., 2008. Ink jet three-dimensional digital fabrication for biological tissue manufacturing: analysis of alginate microgel beads produced by ink jet droplets for three dimensional tissue fabrication. Journal of Imaging Science and Technology, 52(6):060201. https://doi.org/10.2352/J.ImagingSci.Technol.(2008)52:6(060201)
Nishiyama Y, Nakamura M, Henmi C, et al., 2009. Development of a three-dimensional bioprinter: construction of cell supporting structures using hydrogel and state-of-the-art inkjet technology. Journal of Biomechanical Engineering, 131(3):035001. https://doi.org/10.1115/1.3002759
Peng WJ, Datta P, Ayan B, et al., 2017. 3D bioprinting for drug discovery and development in pharmaceutics. Acta Biomaterialia, 57:26-46. https://doi.org/10.1016/j.actbio.2017.05.025
Pepelanova I, Kruppa K, Scheper T, et al., 2018. Gelatin-methacryloyl (GelMA) hydrogels with defined degree of functionalization as a versatile toolkit for 3D cell culture and extrusion bioprinting. Bioengineering, 5(3):55. https://doi.org/10.3390/bioengineering5030055
Phung TH, Kwon KS, 2022. Improved continuous inkjet for selective area coating using high‐viscosity insulating inks. Advanced Engineering Materials, 24(8):2101527.
Poellmann MJ, Barton KL, Mishra S, et al., 2011. Patterned hydrogel substrates for cell culture with electrohydrodynamic jet printing. Macromolecular Bioscience, 11(9):1164-1168. https://doi.org/10.1002/mabi.201100004
Ramezani H, Zhou LY, Shao L, et al., 2020. Coaxial 3D bioprinting of organ prototyps from nutrients delivery to vascularization. Journal of Zhejiang University-SCIENCE A (Applied Physics & Engineering), 21(11):859-875. https://doi.org/10.1631/jzus.A2000261
Rodríguez-Dévora JI, Zhang BM, Reyna D, et al., 2012. High throughput miniature drug-screening platform using bioprinting technology. Biofabrication, 4(3):035001. https://doi.org/10.1088/1758-5082/4/3/035001
Santoni S, Gugliandolo SG, Sponchioni M, et al., 2022. 3D bioprinting: current status and trends—a guide to the literature and industrial practice. Bio-Design and Manufacturing, 5(1):14-42. https://doi.org/10.1007/s42242-021-00165-0
Scoutaris N, Chai F, Maurel B, et al., 2016. Development and biological evaluation of inkjet printed drug coatings on intravascular stent. Molecular Pharmaceutics, 13(1):125-133. https://doi.org/10.1021/acs.molpharmaceut.5b00570
Shah MA, Lee DG, Lee BY, et al., 2021. Classifications and applications of inkjet printing technology: a review. IEEE Access, 9:140079-140102. https://doi.org/10.1109/ACCESS.2021.3119219
Shapira A, Dvir T, 2021. 3D tissue and organ printing—hope and reality. Advanced Science, 8(10):2003751. https://doi.org/10.1002/advs.202003751
Shi J, Wu B, Li SH, et al., 2018. Shear stress analysis and its effects on cell viability and cell proliferation in drop-on-demand bioprinting. Biomedical Physics & Engineering Express, 4(4):045028. https://doi.org/10.1088/2057-1976/aac946
Suntornnond R, Ng WL, Huang X, et al., 2022. Improving printability of hydrogel-based bio-inks for thermal inkjet bioprinting applications via saponification and heat treatment processes. Journal of Materials Chemistry B, 10(31):5989-6000. https://doi.org/10.1039/D2TB00442A
Takagi D, Lin WK, Matsumoto T, et al., 2019. High-precision three-dimensional inkjet technology for live cell bioprinting. International Journal of Bioprinting, 5(2):208. https://doi.org/10.18063/ijb.v5i2.208
Talbot EL, Berson A, Brown PS, et al., 2012. Evaporation of picoliter droplets on surfaces with a range of wettabilities and thermal conductivities. Physical Review E, 85(6):061604. https://doi.org/10.1103/physreve.85.061604
Taymour R, Chicaiza-Cabezas NA, Gelinsky M, et al., 2022. Core–shell bioprinting of vascularized in vitro liver sinusoid models. Biofabrication, 14(4):045019. https://doi.org/10.1088/1758-5090/ac9019
Tirella A, Vozzi F, de Maria C, et al., 2011. Substrate stiffness influences high resolution printing of living cells with an ink-jet system. Journal of Bioscience and Bioengineering, 112(1):79-85. https://doi.org/10.1016/j.jbiosc.2011.03.019
Tsai P, Hendrix MHW, Dijkstra RRM, et al., 2011. Microscopic structure influencing macroscopic splash at high weber number. Soft Matter, 7(24):11325-11333. https://doi.org/10.1039/c1sm05801k
Tse C, Whiteley R, Yu T, et al., 2016. Inkjet printing Schwann cells and neuronal analogue NG108-15 cells. Biofabrication, 8:015017. https://doi.org/10.1088/1758-5090/8/1/015017
Unagolla JM, Jayasuriya AC, 2020. Hydrogel-based 3D bioprinting: a comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Applied Materials Today, 18:100479. https://doi.org/10.1016/j.apmt.2019.100479
Wijshoff H, 2010. The dynamics of the piezo inkjet printhead operation. Physics Reports, 491(4-5):77-177. https://doi.org/10.1016/j.physrep.2010.03.003
Workman VL, Tezera LB, Elkington PT, et al., 2014. Controlled generation of microspheres incorporating extracellular matrix fibrils for three‐dimensional cell culture. Advanced Functional Materials, 24(18):2648-2657. https://doi.org/10.1002/adfm.201303891
Wu DZ, Xu CX, 2018. Predictive modeling of droplet formation processes in inkjet-based bioprinting. Journal of Manufacturing Science and Engineering, 140(10):101007. https://doi.org/10.1115/1.4040619
Xie MJ, Gao Q, Fu JZ, et al., 2020. Bioprinting of novel 3D tumor array chip for drug screening. Bio-Design and Manufacturing, 3(3):175-188. https://doi.org/10.1007/s42242-020-00078-4
Xu CX, Chai WX, Huang Y, et al., 2012. Scaffold‐free inkjet printing of three‐dimensional zigzag cellular tubes. Biotechnology and Bioengineering, 109(12):3152-3160. https://doi.org/10.1002/bit.24591
Xu CX, Zhang ZY, Christensen K, et al., 2014. Freeform vertical and horizontal fabrication of alginate-based vascular-like tubular constructs using inkjetting. Journal of Manufacturing Science and Engineering, 136(6):061020. https://doi.org/10.1115/1.4028578
Xu HQ, Liu JC, Zhang ZY, et al., 2022a. Cell sedimentation during 3D bioprinting: a mini review. Bio-Design and Manufacturing, 5(3):617-626. https://doi.org/10.1007/s42242-022-00183-6
Xu HQ, Martinez Salazar DM, Xu CX, 2022b. Investigation of cell concentration change and cell aggregation due to cell sedimentation during inkjet-based bioprinting of cell-laden bioink. Machines, 10(5):315. https://doi.org/10.3390/machines10050315
Xu T, Jin J, Gregory C, et al., 2005. Inkjet printing of viable mammalian cells. Biomaterials, 26(1):93-99. https://doi.org/10.1016/j.biomaterials.2004.04.011
Xu T, Rohozinski J, Zhao WX, et al., 2009. Inkjet-mediated gene transfection into living cells combined with targeted delivery. Tissue Engineering Part A, 15(1):95-101. https://doi.org/10.1089/ten.tea.2008.0095
Xu T, Binder KW, Albanna MZ, et al., 2012. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication, 5(1):015001. https://doi.org/10.1088/1758-5082/5/1/015001
Xu T, Zhao WX, Zhu JM, et al., 2013. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials, 34(1):130-139. https://doi.org/10.1016/j.biomaterials.2012.09.035
Yang JX, Zheng F, Derby B, 2021. Stability of lines with zero receding contact angle produced by inkjet printing at small drop volume. Langmuir, 37(1):26-34. https://doi.org/10.1021/acs.langmuir.0c01928
Yarin AL, 2006. Drop impact dynamics: splashing, spreading, receding, bouncing…. Annual Review of Fluid Mechanics, 38:159-192. https://doi.org/10.1146/annurev.fluid.38.050304.092144
Yin J, Zhao DK, Liu JY, 2019. Trends on physical understanding of bioink printability. Bio-Design and Manufacturing, 2(1):50-54. https://doi.org/10.1007/s42242-019-00033-y
Yumoto M, Hemmi N, Sato N, et al., 2020. Evaluation of the effects of cell-dispensing using an inkjet-based bioprinter on cell integrity by RNA-seq analysis. Scientific Reports, 10(1):7158. https://doi.org/10.1038/s41598-020-64193-z
Zhang B, He JK, Li X, et al., 2016. Micro/nanoscale electrohydrodynamic printing: from 2D to 3D. Nanoscale, 8(34):15376-15388. https://doi.org/10.1039/C6NR04106J
Zhang B, Gao L, Ma L, et al., 2019. 3D bioprinting: a novel avenue for manufacturing tissues and organs. Engineering, 5(4):777-794. https://doi.org/10.1016/j.eng.2019.03.009
Zhang YS, Khademhosseini A, 2020. Engineering in vitro human tissue models through bio-design and manufacturing. Bio-Design and Manufacturing, 3:155-159. https://doi.org/10.1007/s42242-020-00080-w
Zhao DK, Zhou HZ, Wang YF, et al., 2021. Drop-on-demand (DOD) inkjet dynamics of printing viscoelastic conductive ink. Additive Manufacturing, 48:102451. https://doi.org/10.1016/j.addma.2021.102451
Zhou HZ, Liu P, Gao ZQ, et al., 2022. Simultaneous multimaterial multimethod bioprinting. Bio-Design and Manufacturing, 5:433-436. https://doi.org/10.1007/s42242-022-00203-5
Zhu W, Ma XY, Gou ML, et al., 2016. 3D printing of functional biomaterials for tissue engineering. Current Opinion in Biotechnology, 40:103-112. https://doi.org/10.1016/j.copbio.2016.03.014
https://doi.org/10.1631/jzus.A2200569
Jun YIN, https://orcid.org/0000-0002-1937-6812
Nov. 28, 2022; Revision accepted Dec. 13, 2022;
Crosschecked Dec. 25, 2022
© Zhejiang University Press 2022
 Journal of Zhejiang University-Science A(Applied Physics & Engineering)2022年12期
Journal of Zhejiang University-Science A(Applied Physics & Engineering)2022年12期
- Journal of Zhejiang University-Science A(Applied Physics & Engineering)的其它文章
- Optimum insulation thickness of external walls by integrating indoor moisture buffering effect: a case study in the hot-summer-cold-winter zone of China
- Investigations on lubrication characteristics of high-speed electric multiple unit gearbox by oil volume adjusting device
- Hole-growth phenomenon during pyrolysis of a cation-exchange resin particle
- A novel stacking-based ensemble learning model for drilling efficiency prediction in earth-rock excavation
- Enhanced photocatalytic performance of S-doped covalent triazine framework for organic pollutant degradation
- Total contents
