苦毒烷型萜类化合物及其生物活性的研究进展*
杨田融,杨雅琳,许 敏
(昆明理工大学生命科学与技术学院制药工程中心,云南 昆明 650500)
Picrotoxanes are a group of alkaloids,sesquiterpenes,and nor-diterpenes featuring 6/5 dicyclic ring skeleton.A variety of oxidation and skeletal rearrangement occurring in the basic 6/5 dicyclic ring led to various picrotoxane derivatives,e.g.,picrotoxane sesquiterpenes with the fused or the bridged γ-lactone and picrotoxane nor-diterpenes with spiro-γ-lactone.They are bearing with highly complex tracyclic,or pentacyclic structures.And some structures have up to 12 stereogenic centers.In addition,lactone functionalities are opened formally to alcohols and in some case glycosylated.
Picrotoxanes are mainly reported to exist in the Picrodendraceae,Orchidaceae,Menispermaceae,Phyllanthaceae and Coriariaceae families.Among them,picrotoxane terpenoids are mainly distributed in a variety of Traditional Chinese Medicines(TCMs),e.g.,Dendrobium findlayanum[1],D.nobile[2],D.amoenum[3],D.aduncum[4]and D.williamsonii[5].A few picrotoxane terpenoids are also found in ethnomedicines,e.g.,Bccaurea ramiflora[6],Coriaria nepalensis[7],Picrodendron baccatum[8],Hyaenanche globosa[9]and Celaenodendron mexicana[10],Maesobotrya floribunda[11],Austrobuxus swanii[12],A.carunculatus[13]and Anamirta cocculus[14].It is noted that a small number of picrotoxane sesquiterpenes from honey were reported occasionally[15-16].Picrotoxanes are mostly crystals or white powders,and a small amount of them are oily substance[17-19].For example,the coriaria oil extracted from seed of C.nepalensis contained 0.24% picrotoxane sesquiterpenes[19].The picrotoxanes are the indispensable neuropharmacological tool molecules as the antagonist of aminobutyric acid(GABA)receptors and ligand gated chloride channels[20-21].They also showed potent biological activities such as anti-tumor[22],insecticidal[21,23],immunoregulation[24]and antibacterial[25]activities,which have been used in the treatment of neurological disorder[20],cancer[22]and immune system diseases[24].
In recent years,a number of novel picrotoxanes with significant biological activities have been continuously reported such as picrotoxanes from Dendrobium spp.Until now,picrotoxane alkaloids[26-30]and the synthesis of picrotoxane terpenoids[31-32]had been summarized.Herein,this review mainly surveyed recently advances in chemical structures and biological activities of picrotoxane terpenoids.
1 Picrotoxane terpenoid derivatives
Picrotoxane terpenoids are a group of sesquiterpenes and nor-diterpenes bearing with high complex tetracyclic,or pentacyclic structures and up to 12 stereogenic centers.A variety of oxidation,skeletal rearrangement and and heterozygosis occurring in the basic 6/5 dicyclic ring led to various derivatives,e.g.,picrotoxane sesquiterpenes with the fused or the bridged γ-lactone and picrotoxane nor-diterpenes with spiro-γ-lactone.In addition,lactone functionalities are reduced formally to alcohols and in some case glycosylated.Plaus biostnthetic pathways of picrotoxane terpenoids are shown in Figure 1.
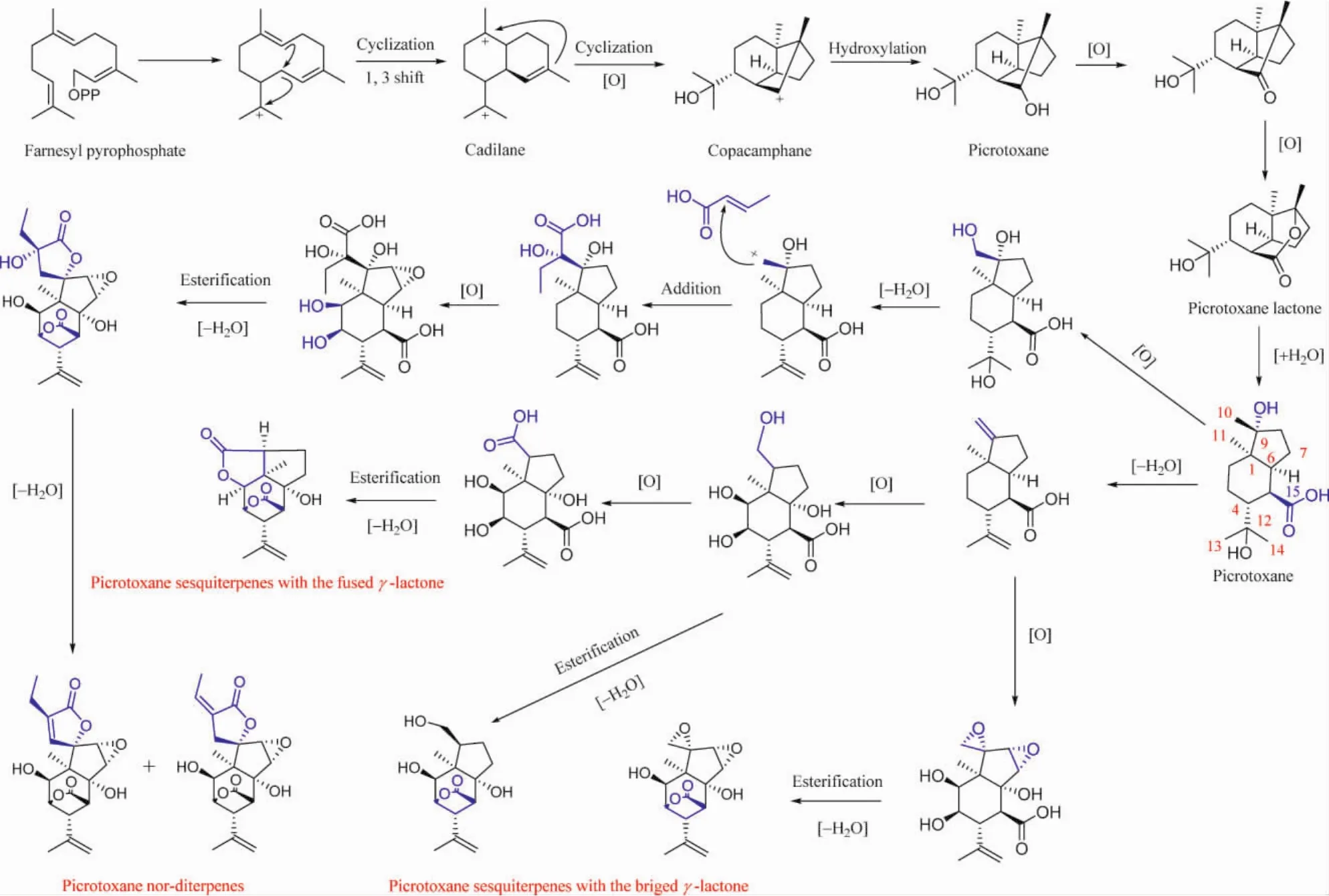
Fig.1 Biosynthesis of picrotoxane terpenoids
1.1 Picrotoxane sesquiterpenes with the γ-lactone and the bridged γ-lactone These are a group of sesquiterpeneswith 6/5 dicyclic skeleton with a bridged γ-lactone.Theyaredistributed in C.nepalensis,C.japonica,D.nobile,D.amoenum,D.findlayanum,P.baccatum,and a few in B.ramiflora and A.carunculatus.Their structures were shown in Fig.2,and their names and the related plant sources were organized in Table 1.
Tab.1 Picrotoxane sesquiterpenes with the bridged γ-lactone and their plant sources

Continuation Tab.1
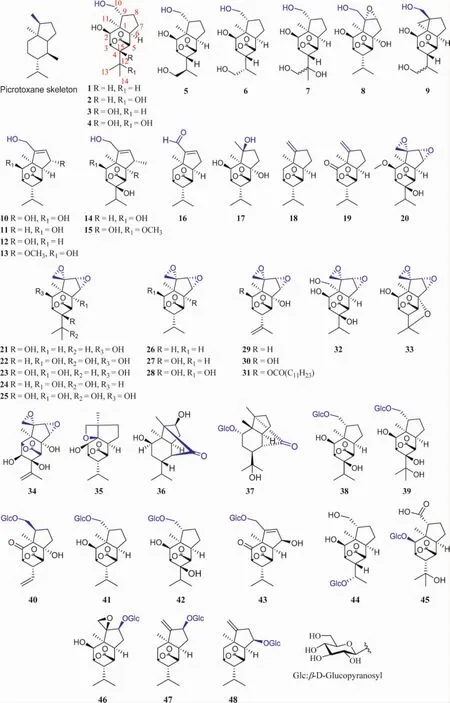
Fig.2 Structures of picrotoxane sesquiterpenes with the bridged γ-lactone
In general,a variety of oxidation occurring at C2,3,7,8,9,10,12,13 or/and 14 leads to the hydroxyl group positioned in the 6/5 dicyclic skeleton.In some case,the hydroxyl group at C2,7,8,10 or 13 is glycosylated,e.g.compounds 37-48.It is noted that the oxidation occurring at C10 led to the formation of aldehyde or carboxyl group,e.g.corialactone C(16)and dendromoniliside B(45)[7].The hydroxyl functionalities are reduced formally t o terminal olefin positioned between C9 and C10,e.g.compounds 18[7],19[33],47 and 48[34].In addition,most epoxides of picrotoxane sesquiterpenes have been reported.They feature in the fused oxirane at C9(10)and C7(8),e.g.compounds 20-34.They mainly exist in the stem and bark of P.baccatum,as well as the root and aerial parts of C.nepalensis.
1.2 Picrotoxane sesquiterpenes with the fused γlactone These are a group of sesquiterpenes with 6/5 dicyclic skeleton with a fused γ-lactone.Most of the sesquiterpenes feature in the fused γ-lactone at C1(2,9)except for a bridged γ-lactone at C3(5).Their structures were shown in Fig.3,their names and therelated plantsourceswere organized in Table 2.
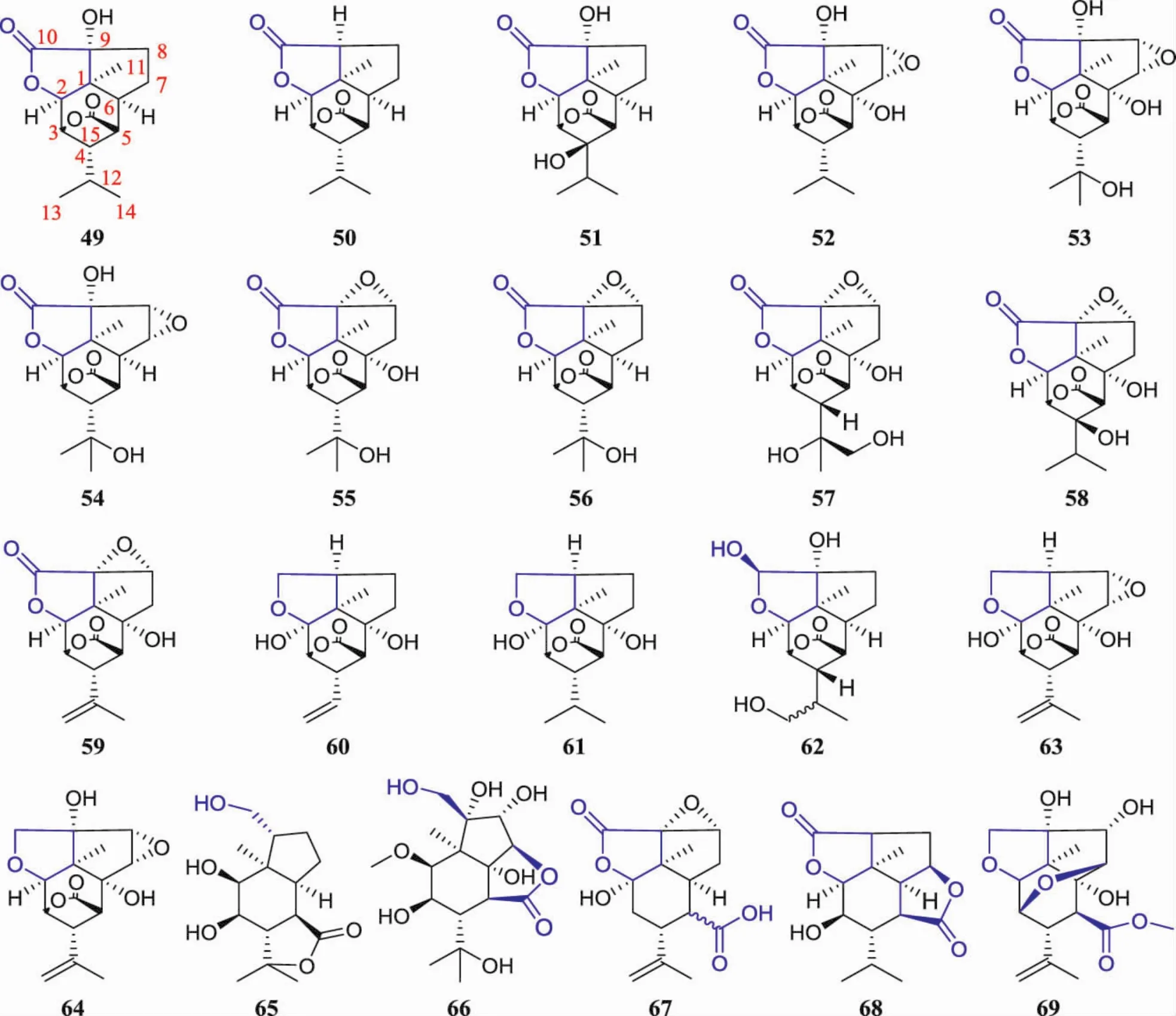
Fig.3 Structures of picrotoxane sesquiterpenes with the fused γ-lactone

Tab.2 Picrotoxane sesquiterpenes with the fused γ-lactone and their plant sources
Similar to the picrotoxane sesquiterpenes with the bridged γ-lactone,these compounds are hydroxy lated at C9 and most of them are fused with oxirane at C7(8)or C8(9).It is noted that some of them are bearing with an additional fused γ-lactone at C5(6,7)without the bridged γ-lactone at C3(5).For example,compounds 66[41]and 68[45]fused a γ-lactone at C5(6,7),and compound 65 fused at C4(5,12)[1].Sometimes,the bridged γ-lactone at C3(5)is opened,and in the same case further methyl esterification,e.g.compounds 67[14]and 69[7].Compared with the picrotoxane sesquiterpenes with only bridged γ-lactone,there are few reports of sesquiterpenes with the fused γlactone.They were mainly distributed in stem of D.nobile,whole portion of D.amoenum,D.williamsonii and D.findlayanum,and are the characteristic compounds of Dendrobium spp.A few of them were distributed in the berry of B.ramiflora,the root of C.nepalensis,the leaf of P.baccatum,the berry of M.floribunda and M.coccus.
1.3 Picrotoxane sesquiterpenes without the γ-lactone Lactone functionalities of these picrotoxane sesquiterpenes are reduced formally to carbonyl and in some case glycosylated.For example,the bridged-lactone at C3(5)of compounds 70 and 71 is opened,and they distributed in C.nepalensis.It is noted that corianlactone(72)derived from coriatone(71)is the only one picrotoxane sesquiterpene with 5/7 dicyclic skeleton[18].In addition,dendronobilosides A(73)and B(74)without γ-lactone were isolated from D.nobile,but there were glycosylated at C5 and C10.Compound 73 is the only picrotoxine sesquiterpene with two glycosyl units[47].Their structures were shown in Figure 4,and their names and the related plant sources were organized in Table 3.

Fig.4 Structures of picrotoxane sesquiterpenes without the γ-lactone
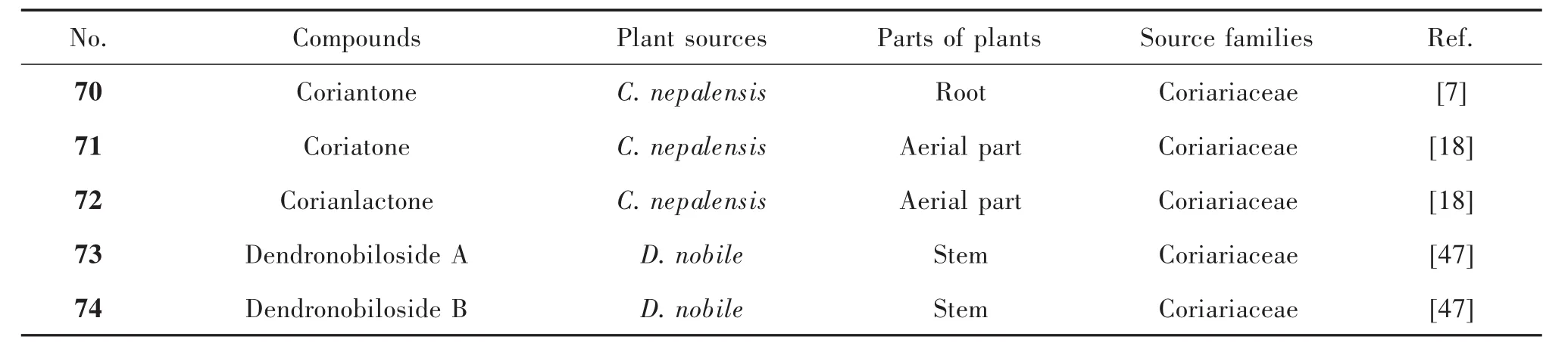
Tab.3 Picrotoxane sesquiterpenes without the γ-lactone and their plant sources
1.4 Picrotoxane nor-diterpenes These compounds sometimes possess a unique carbon skeleton containing a spiro-γ-lactone positioned at C13 except for a bridged-lactone at C3(5,15)[13].The physical state of these compounds are mostly prismatic crystal and white powder[24,35],and they are one of the characteristic chemical constituents of P.baccatum and A.swanii.Their structures were shown in Figure 5,and their names and the related plant sources were organized in Table 4.
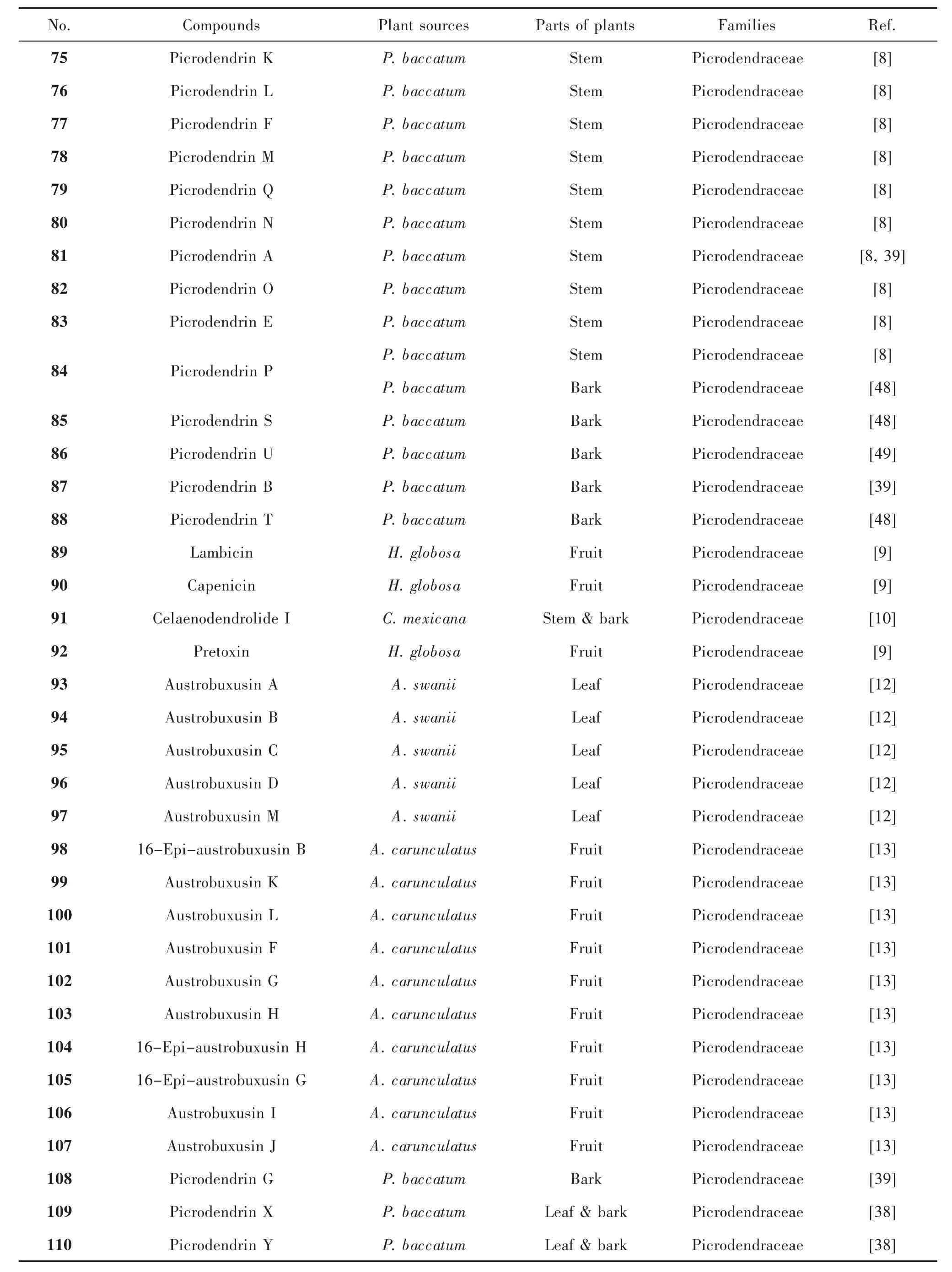
Tab.4 Picrotoxane nor-diterpenes with spiro γ-lactone and their plant sources
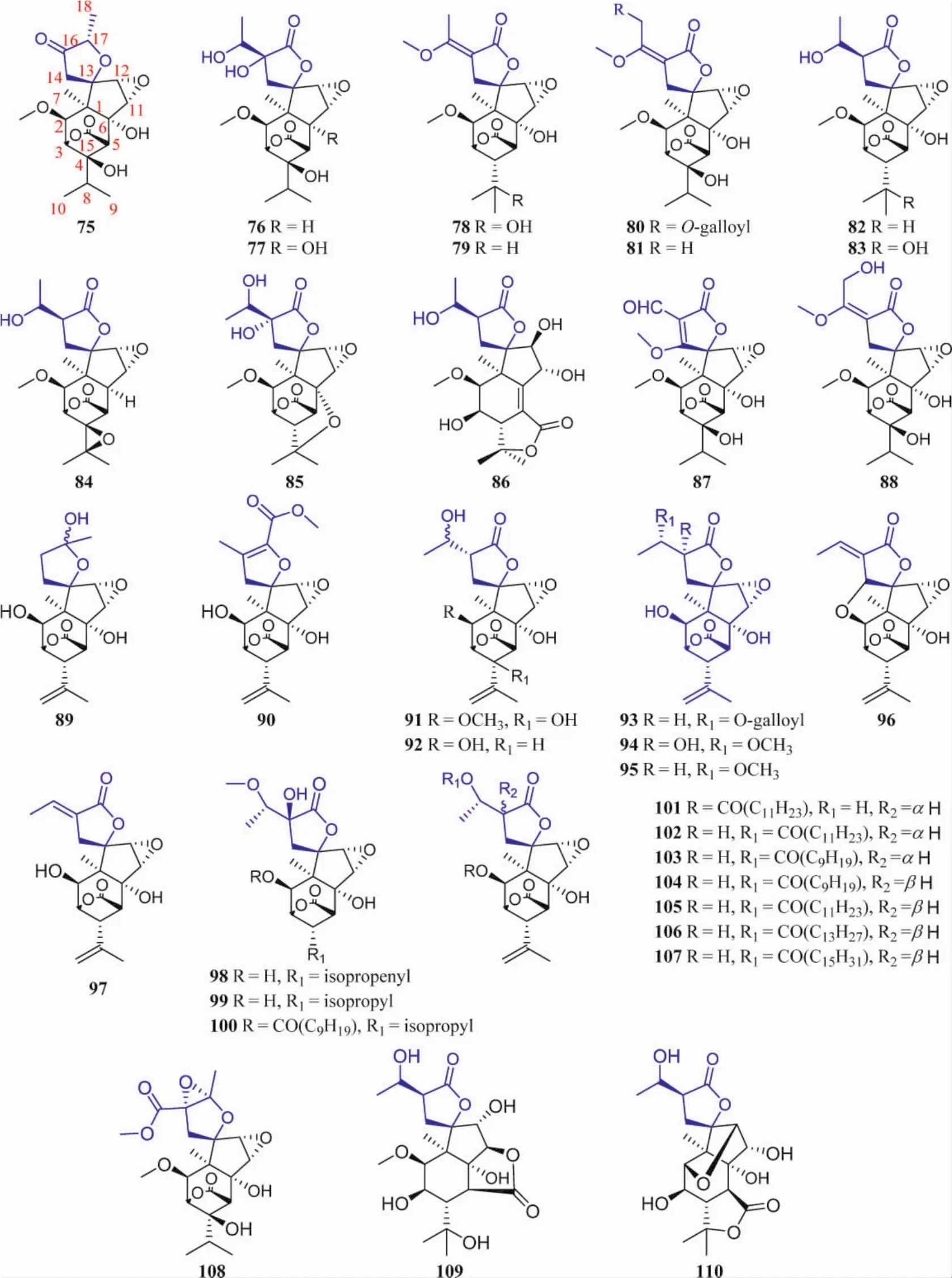
Fig.5 Structures of picrotoxane nor-diterpenes with spiro γ-lactone
In same case,an isopropyl moiety or isopropenyl functionality was linked with C16.Similar to picrotoxane sesquiterpenes,a variety of oxidation occurring at C2,4,6,11,12,or/and 13 led to the various hydroxyl groups and in some case methylated.In addition,most epoxides of picrotoxane nor-diterpenes have been reported.They feature in the fused oxirane at C11(12).Few of them bear an oxygen bridge at C2 (14),e.g.compound 96[12].It is noted that the rearrange occurs at isopropyl moiety at C16 letting to a methyl linked with C16 and or carboxyl group positioned at C17,e.g.compound 90[9].Some picrotoxane nor-diterpenes formed an ester at C18 with long alkane chain,e.g.compounds 101-107[13].Picrodendrin X(109)is fused a γ-lactone at C5(6,11),but picrodendrin Y(110)fused at C4(5,8)[38].
2 Bioactivity of picrotoxane terpenoids
2.1 Neuroprotective effects Picrotoxane terpenoids were ligand gated chloride channels and ionic GABA receptor antagonists[20-21].The tutin(30)from the parasitism of mulberry has been used to treat schizophrenia.Tutin(30)with an isopropyl moiety at C4 had more antagonistic effect on GABA than that of with hydroxyisopropyl group[50].In addition,the compounds 1a-1e(Figure 6)were the synthetic derivatives of nepalactone A(47),which showed better neurotrophic activity than that of compound 47[45,50-51].Compared with 21.11%±1.62% for 10 ng/mL NGF(Nerve growth factor)alone,compound 1c at a concentration of 10 μM increased the numbers of neurite-bearing cells by 27.81%±1.48%.The neurotrophic activities of the compound 1a decreased due to the glycolation at C8.
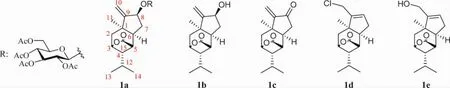
Fig.6 Structures of synthetic picrotoxane sesquiterpenes 1a-1e
2.2 Cytotoxic effects Dendronobilin B(3)promoted the proliferation of intersegmental vessel(ISV)and endothelial cells with a dose-dependent manner on the recovery of ISV deletion induced by sunitinib.Dendronobilin B(3)repaired ISV injury in Tg zebrafish embryos induced by sunitinib by promoting the proliferation of ISV endothelial cells[22].Austrobuxusin E(31),austrobuxusin L(100)and austrobuxusin F(101)showed different antiproliferative effects against U87-MG glioblastoma,HCT116 colon,and A549 cells.Importantly,austrobuxusin L(100)and austrobuxusin F(101)showed IC50values of 5.8 ± 0.5 μM for the A549 cells and 0.7 ± 0.1 μM for the U87-MG cell line,respectively.Among them,picrotoxane nor-diterpenes bearing an acyl chain at C2 exhibited potential antitumor effects[13].
2.3 Insecticidal effects Coriamyrtin(29)had obvious antifeedant behavior,which could delay the pupation time of Plutella xylostella and inhibit the development of P.xylostella[52].The structure activity of relationships(SARs)suggested that the double bond of C12/13 and hydroxyl group at C2 may be a key active group.Picrodendrin Q(79)showed a potent inhibition in the development of Musca domestica L.with an IC50value of 22 nM.The presence or absence of the C4,C8-dihydroxyl groups and electronegativity of the 16-carbon atom were important determinants of the potency of nor-diterpenes in inhibiting M.domestica[21].
2.4 Immunomodulatory effects Dendromoniliside C(46)stimulated the proliferation of B cells and inhibited the proliferation of T cells[24],and dendroside F(41)significantly stimulated the proliferation of mouse T or B lymphocytes.
2.5 Antifungal effects Picrotoximaesin (61),ramifloside(40)and sapidolide A(60)exhibited weak antibacterial activities against Colletotrichum gloeosporioides,and showed strong activity against C.gloeosporioides with minimum inhibitory concentrations(MICs)of 12.5,12.5 and 50 μg/mL[6].It was speculated that the double bond between C-12 and C-13 is the key unit for the strong antibacterial ability of compounds 40 and 60.
2.6 Anti-α-glucosidase effects Using acarbose as positive control(IC50=0.72 mM),dendroterpene C(50)manifested inhibitory activity on α-glucosidase with IC50value of 0.97 mM.However,dendroterpene D(49)had no effect on α-glucosidase(IC50> 1 mM),indicating that the activity decreased due to the hydroxyl group at C-9[46].
3 Conclusion
Picrotoxin,the first picrotoxine terpenoid,was isolated in 1811 shortly after isolation of morphine in 1806,which signified the start of plant secondary products research.Due to complex structures and potential activities,a number of novel picrotoxanes with significant biological activities have been reported continuously.Picrotoxanes bearing with unique skeleton are one of the main active components in TCMs and herbs.Interestingly,some picrotoxanes has certain therapeutic effects on neurologicaldisorder,e.g.schizophrenia.These active natural products will be potent tool molecules for investigating on target identification and mechanism of action for complex brain diseases.Chemical proteomics is one of new approaches for identifying targets by active natural product probes,especially affinity-based protein profiling(ABPP).Thus,it will be an interesting research work to use active picrotoxine terpenoid as probe to reveal new drug target and its mechanism,especially druggable target of neurological disorder.

