Cross-protective effect of acid adaptation on ethanol tolerance in Salmonella Enteritidis
Shoukui He, Beining Ye, Zengfeng Zhng, Yn Cui, Siyun Wng, Xinming Shi,*
a MOST-USDA Joint Research Center for Food Safety, School of Agriculture and Biology, State Key Lab of Microbial Metabolism,Shanghai Jiao Tong University, Shanghai 200240, China
b Food, Nutrition and Health, Faculty of Land and Food Systems, The University of British Columbia, Vancouver BC V6T 1Z4, Canada
Keywords:Salmonella Enteritidis Acid adaptation Ethanol tolerance Growth phase Fatty acid
A B S T R A C T Cross protection can undermine the effectiveness of control measures on foodborne pathogens, and therefore brings major implications for food safety. In this work, the capacity of Salmonella Enteritidis to mount ethanol tolerance following acid adaptation was characterized by analysis of cell viability and cell membrane property. It was observed that preadaptation to pH 4.5 significantly (P < 0.05) increased the tolerance of log-phase cells to ethanol; in contrast, stationary-phase cells displayed reduced ethanol tolerance after acid adaptation. However, acid adaptation did not cause cell leakage and morphological change in both log-phase and stationary-phase S. Enteritidis. Fatty acid analysis further revealed that the amount of C14:0, C17:0 cyclo and C19:0 cyclo fatty acids was increased, while that of C16:1ω7c and C18:1ω7c fatty acids was decreased, respectively,in response to acid adaptation, regardless of bacterial growth phase. Notably, acid adaptation signif icantly(P < 0.05) increased the proportion of C16:0 fatty acid in log-phase cells, but this effect did not occur in stationary-phase cells. Moreover, exogenous addition of C16:0 fatty acid to stationary-phase acid-adapted cultures was able to enhance bacterial ethanol tolerance. Taken together, C16:0 fatty acid is involved in the growth-phase-dependent protective effect of acid adaptation on ethanol tolerance in S. Enteritidis.
1. Introduction
Non-typhoidalSalmonellaposes a great threat to public health in both developing and industrialized countries [1,2]. Annually, a total of 80.3 million foodborne salmonellosis is reported worldwide,resulting in 155 000 deaths [3]. There are currently more than 2 610Salmonellaserovars, of whichSalmonellaEnteritidis stands out as the world-leading cause of salmonellosis, accounting for 40%-60%human cases [4,5]. To reduce foodborne diseases associated with this pathogen, food industries have long employed low pH to controlS.Enteritidis in foods [6,7]. Moreover, acidic disinfectants have also been utilized for cleaning food contact surfaces [8]. Hence, acid adaptation conditions can be encountered byS.Enteritidis during food processing and preservation.
Acid adaptation inS.Enteritidis has received much attention in the f ield of food safety in recent years [9-14]. This adaptive response contributes to the development of bacterial tolerance to homologous(direct protection) or heterologous (cross protection) stressing agents [13]. Acid direct protection is also known as acid tolerance response (ATR), which is generally comprised of log-phase ATR and stationary-phase ATR based on bacterial growth phases employed for adaptation. Furthermore, acid adaptation is able to confer cross protection against heat and salt stresses inS.Enteritidis [13]. To date,however, the number of studies regarding acid cross protection is much smaller compared with acid direct protection inS.Enteritidis.
Concerning ethanol tolerance resulting from acid adaptation, it was previously demonstrated that sublethal acid treatment renderedListeria monocytogenesandVibrio parahaemolyticusmore resistant to ethanol stress [15,16]. Both acid and ethanol have been utilized for antimicrobial purposes in food industries such as meat processing plants [17,18]. The emergence of cross protection between acid adaptation and ethanol stress may diminish the efficacy of food control measures, thus posing critical implications for food safety.However, it is currently unknown whether acid adaptation could protect other important foodborne pathogens (e.g.,S.Enteritidis)against ethanol stress.Furthermore, the role of bacterial growth phase in this cross protective response has not yet been elucidated.
Despite the knowledge on cross protection response resulting from acid adaptation in several foodborne pathogens, few studies have focused on elucidation of the underlying mechanisms.Practically,regulation of cell membrane characteristics (e.g., fatty acid composition, membrane integrity) is a strategy by which foodborne pathogens respond to both acid adaptation and ethanol stress [19,20].It is therefore expected that physiological analysis will be helpful to understand the effect of acid adaptation on bacterial ethanol tolerance.Hence, this work was carried out to assess the protective effect of acid adaptation on ethanol tolerance in log-phase and stationary-phaseS.Enteritidis by stress tolerance phenotype and cell membrane property analyses, which might be useful in designing effective control strategies over this pathogen.
2. Materials and methods
2.1 Bacterium
A well-defined standard strain,S.Enteritidis ATCC 13076, was served as the target organism. This bacterium was preserved at -80 °C in Luria-Bertani (LB) broth supplemented with 25% glycerol. The frozen culture was streaked onto LB agar and incubated overnight at 37 °C prior to each experiment. A single colony was transferred into 5 mL LB broth and incubated at 37 °C for 12 h with shaking. A 1-mL aliquot of bacterial suspension was then inoculated into 100 mL fresh LB broth, followed by incubation at 37 °C and 200 r/min for 5 and 9 h to reach the log-phase and stationary-phase, respectively (Fig. 1).
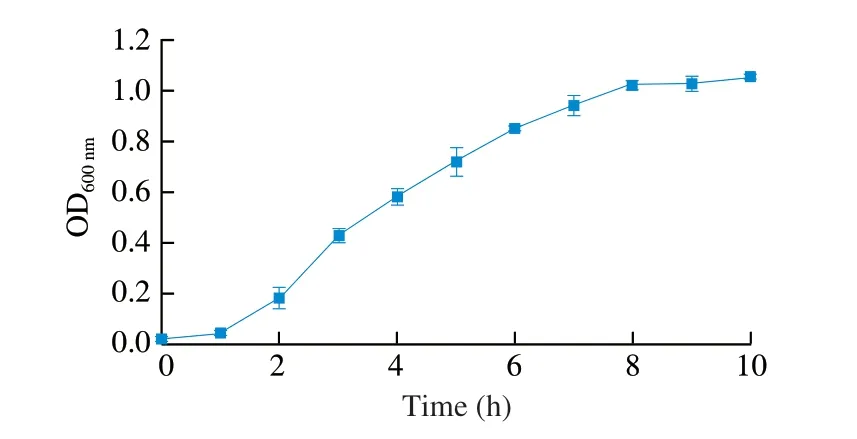
Fig. 1 Growth curve of S. Enteritidis ATCC 13076 in LB broth. Vertical bars show standard deviations. Symbols without visible bars indicate that standard deviations are smaller than the symbol size.
2.2 Acid adaptation treatment
Acid-adapted cells ofS.Enteritidis were prepared as previously described [13]. Briefly, log-phase and stationary-phase cultures(1 mL) were centrifuged at 3 000 r/min for 5 min and resuspended in 10 mL LB broth acidified to pH 4.5 with hydrochloric acid,followed by incubation at 37 °C for 3 and 1 h, respectively, to prepared acid-adapted cells.Log-phase and stationary-phase cultures that were not exposed to mild acid treatments were utilized as non-adapted controls.
2.3 Ethanol tolerance test
To assess the protective effect of acid adaptation on ethanol tolerance inS.Enteritidis, an aliquot (100 µL) of acid-adapted and non-adapted cells were transferred into 10 mL LB broth containing 12% ethanol to reach an initial bacterial concentration of approximately 106CFU/mL. The cell number was measured after 0,10, 20, 30 and 40 min by plate counting method.
2.4 Cell membrane permeability test
The influence of acid adaptation on cell membrane permeability ofS.Enteritidis was assessed as previously described [21]. Briefly, an aliquot (1 mL) of bacterial cultures in log-phase and stationary-phase were centrifuged, resuspended in PBS and submitted to acid adaptation treatment as mentioned above. In the positive control group, disodium EDTA (a known membrane-permeabilizing agent)was added to bacterial suspensions to reach a final concentration of 0.2 mol/L. Samples were taken before and after acid adaptation or disodium EDTA treatment and centrifugated at 12 000 r/min for 2 min. The supernatant’s absorbance at 260 and 280 nm was determined by a Nanodrop 2000c spectrophotometer.
2.5 Scanning electron microscopy (SEM) analysis
The cell morphology of acid-adapted and non-adapted cells ofS.Enteritidis was observed by SEM as detailed previously [21,22].Briefly, bacterial suspensions (1 mL of approximately 108CFU/mL)were centrifuged at 4 000 r/min for 10 min and cell pellets were fixed with 2.5% glutaraldehyde at 4 °C for 12 h. The resulting cells were washed with PBS and dehydrated with 25%, 50%, 70%, 80%, 90% and 100% ethanol. Afterwards, bacterial samples were critical point-dried and gold-coated, followed by imaging on a Sirion 200 scanning electron microscope.
2.6 Membrane fatty acid composition analysis
Acid-adapted and non-adapted cells ofS.Enteritidis were centrifugated at 12 000 r/min for 2 min and washed twice with PBS.Pelleted cells (about 40-60 mg) were subjected to fatty acid methyl esters extraction according to Sasser (2001) with slight modifications [23]. In the last extraction step, saturated NaCl (500 µL)was added into each tube after base wash, followed by tumbling for 3 min. Afterwards, 2/3 of organic phases were pipetted into a GC vial.Fatty acid methyl esters in acid-adapted and non-adaptedS.Enteritidis were determined using a 7890A gas chromatography instrument coupled to a flame ionizing detector (Agilent Technologies Inc., New York, USA) as described in our previous study [24]. The relative content of individual fatty acid was expressed as the molar ratio of the fatty acid to total measured fatty acids.
2.7 Fatty acid supplementary assay
Both ethanol tolerance and C16:0fatty acid content in acid-adaptedS.Enteritidis were dependent on bacterial growth phase in the current work. Therefore, fatty acid supplementary assay was conducted to further explore the role of C16:0fatty acid in the development of acid-induced ethanol tolerance inS.Enteritidis.Briefly, log-phase and stationary-phase acid-adaptedS.Enteritidis cells were transferred into 10 mL LB broth containing 12% ethanol with or without 0.5 mmol/L C16:0fatty acid. The cell number was determined after 0, 20, and 40 min by plate counting method.
2.8 Statistical analysis
Three independent experiments were carried out with three replicates in each experiment. Comparison of means was performed by one-way ANOVA and Duncan’s test in SAS software. Statistical
significance was determined at the level ofP< 0.05.
3. Results and discussion
3.1 Ethanol tolerance was influenced by acid adaptation
Cross-protection between different stresses is considered as a major food safety concern since it can compromise currently employed food control measures [18]. Therefore, the influence of acid adaptation on ethanol tolerance inS.Enteritidis was assessed in the current study. As shown in Fig. 2,S.Enteritidis cells in log-phase demonstrated significantly (P< 0.05) higher ethanol tolerance after acid adaptation. For example, log-phase acid-adapted cells exhibited a survival rate of 103.39% after exposure to 12% ethanol for 20 min,which was increased for 17.73-fold compared to non-adapted cells.On the contrary,S.Enteritidis cells in stationary-phase became more susceptible to ethanol stress after acid adaptation; the survival rate of acid-adapted cells (0.19%) was significantly (P< 0.05) lower than that of non-adapted cells (73.86%) after exposure to 12% ethanol for 20 min. These results suggested that the protective effect of acid adaptation on ethanol tolerance inS.Enteritidis was dependent on bacterial growth phase.

Fig. 2 Tolerance of log-phase (A) and stationary-phase (B) S. Enteritidis to 12% ethanol after acid adaptation at pH 4.5. Vertical bars show standard deviations. Symbols without visible bars indicate that standard deviations are smaller than the symbol size. Different letters above bars at each time point signify significant difference (P < 0.05).
The growth phase-dependent cross protection has also been observed by Chiang et al. [25] inV. parahaemolyticus.For instance,log-phase and stationary-phase cells of this pathogen exhibited enhanced and unaltered ethanol tolerance after heat shock, while reduced and unaltered acid tolerance after ethanol adaptation,respectively. The authors attributed this growth phase-specific bacterial stress response to the level of synthesized shock proteins(e.g., heat shock proteins). Therefore, growth phase was a crucial factor affecting cross protective response in bacterial pathogens.
Acid adaptation was also able to provide bacterial pathogens cross-protection against other stress factors (e.g., heat, cold and salt)commonly applied in food industries, thus representing a potential risk to food safety [18]. In terms of mechanisms on cross protection induced by acid adaptation, it has been revealed that alteration of cell membrane is a strategy by which acid-adapted cells ofSalmonellamount cross tolerance to heat and cold stresses [26,27]. For example,Yang et al. [26] found that adaptation ofS.Enteritidis to lactic acid conferred cross-protection against heat stress presumably due to alterations in the cytoplasmic membrane such as decreased membrane fluidity under acidic conditions.Interestingly, regulation of membrane integrity and fatty acid composition has been reported to be involved in the response ofSalmonellato both acid and ethanol stresses [19-21].Hence, these cell membrane characteristics will be analyzed to provide an insight into the physiological bases of the effect of acid adaptation on ethanol tolerance inS.Enteritidis.
3.2 Cell membrane integrity was maintained in response to acid adaptation
Acid-adaptedS.Enteritidis was subjected to cell membrane permeability and cell morphology analyses in the current study. It was found that there existed no significant difference (P> 0.05)in absorbance at 260 nm (intracellular nucleic acids) and 280 nm(intracellular proteins) between acid-adapted and non-adaptedS.Enteritidis in both log-phase and stationary-phase (Fig. 3). This finding indicated that no obvious leakage of intracellular genetic materials was observed after acid adaptation inS.Enteritidis,in regardless of bacterial growth phase. SEM analysis further demonstrated that both acid-adapted and non-adapted cells had a regular and smooth morphology with an intact cell membrane(Fig. 4). Taken together, cell membrane integrity inS.Enteritidis was maintained in response to acid adaptation.
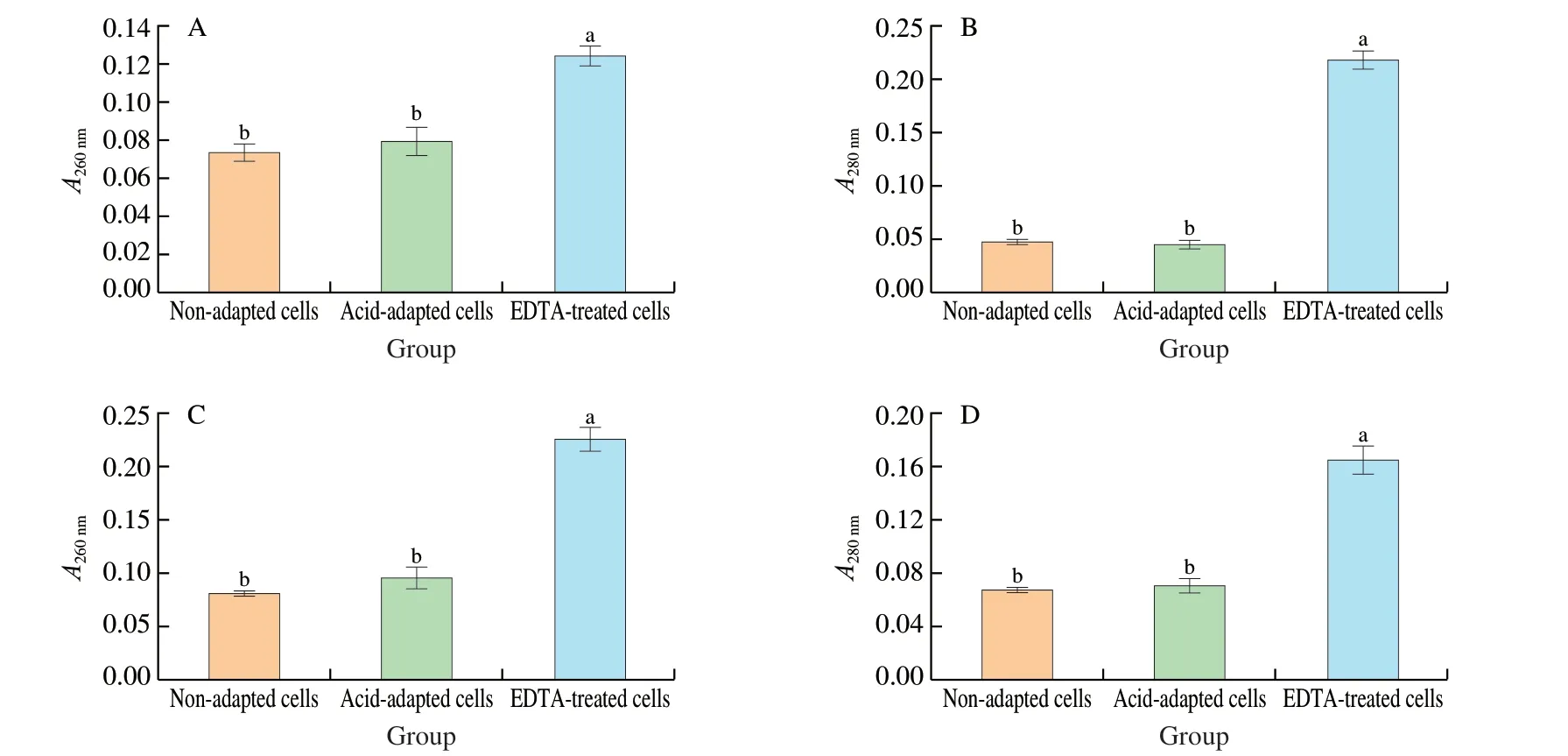
Fig. 3 Effect of acid adaptation on the leakage of intracellular 260 nm (A, C) and 280 nm (B, D) absorbing materials in log-phase (A, B) and stationary-phase (C, D)S. Enteritidis. Vertical bars show standard deviations. Different letters above bars signify significant difference (P < 0.05). EDTA-treated cells with leakage of 260 and 280 nm absorbing materials were served as a positive control.
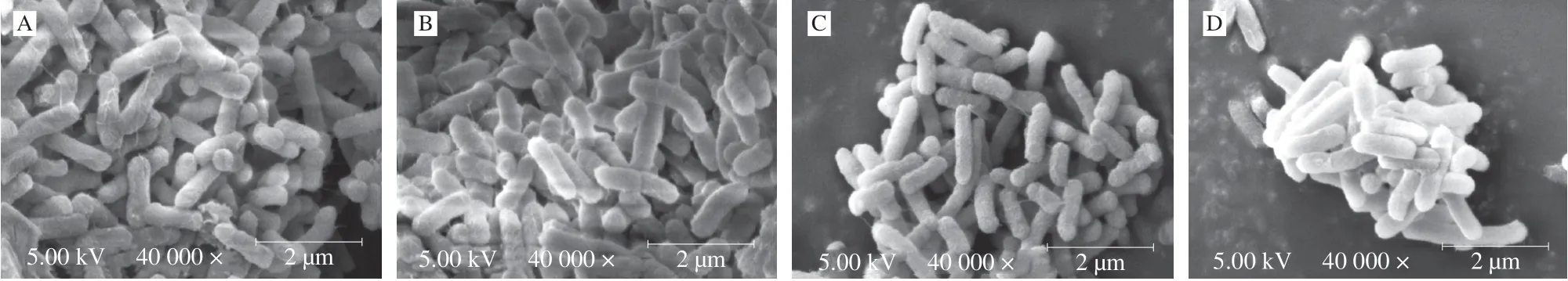
Fig. 4 Effect of acid adaptation on cell morphology of log-phase (A, B) and stationary-phase (C, D) S. Enteritidis. Magnification: 40 000×, bar marker: 2 μm.(A) log-phase non-adapted cells; (B) log-phase acid-adapted cells; (C) stationary-phase non-adapted cells; (D) stationary-phase acid-adapted cells.
As the initial line of defense, it is crucial for bacterial pathogens to regulate cell membrane integrity in response to environmental stresses.In the current study, both log-phase and stationary-phase acid-adaptedS.Enteritidis had an intact cell membrane, but displayed enhanced and reduced ethanol tolerance, respectively. It thus appeared that membrane integrity was not responsible for the growth-phase-dependent protective effect of acid adaptation on ethanol tolerance inS.Enteritidis.Similarly, it was previously suggested that maintenance of cell membrane integrity might not be essential for bacterial pathogens to mount ethanol tolerance response [21,28]. Therefore,some other factors (e.g., membrane fatty acid composition) were probably involved in ethanol tolerance ofS.Enteritidis in log-phase,which were induced by acid adaptation.
3.3 Fatty acid composition was altered in response to acid adaptation
Regulation of membrane fatty acid composition is an important adaptation mechanism for the survival of bacterial pathogens under environmental stresses such as acid adaptation, ethanol shock and osmotic stress [19,20,29]. Therefore, fatty acid composition was measured to determine whether acid adaptation affects the cell membrane composition ofS.Enteritidis in the current study. As shown in Fig. 5, the major identified fatty acids were lauric acid(C12:0), myristic acid (C14:0), palmitic acid (C16:0), palmitoleic acid(C16:1ω7c), cyclo-heptadecanoic acid (C17:0cyclo), vaccenic acid (C18:1ω7c),and cyclo-nonadecanoic (C19:0cyclo). The amount of C12:0in both log-phase and stationary-phaseS.Enteritidis was not significantly(P> 0.05) influenced by acid adaptation. However, acid adaptation increased the amount of C14:0, C17:0cycloand C19:0cyclofatty acids,while it decreased the amount of C16:1ω7cand C18:1ω7cfatty acids inS.Enteritidis, regardless of bacterial growth phase. It was also noted that variations in C16:0fatty acid content were dependent on bacterial growth phase; the proportion of this fatty acid in log-phase and stationary-phaseS.Enteritidis was enhanced and unaltered after acid adaptation, respectively.acid adaptation on cell membrane fluidity. It was observed that the relative UFA/SFA ratio in both log-phase and stationary-phaseS.Enteritidis was significantly (P< 0.05) reduced by acid adaptation(Fig. 6). A decrease in UFA/SFA ratio can lead to lower membrane fluidity, which usually correlates with higher bacterial tolerance to environmental stresses [24]. Nevertheless, log-phase and stationary-phase acid-adaptedS.Enteritidis with reduced membrane fluidity became more tolerant and susceptible to ethanol stress in the current study, respectively. Hence, the role of particular fatty acids such as C16:0should be considered to understand why bacterial growth phase influences the induction of ethanol tolerance by acid adaptation inS.Enteritidis.
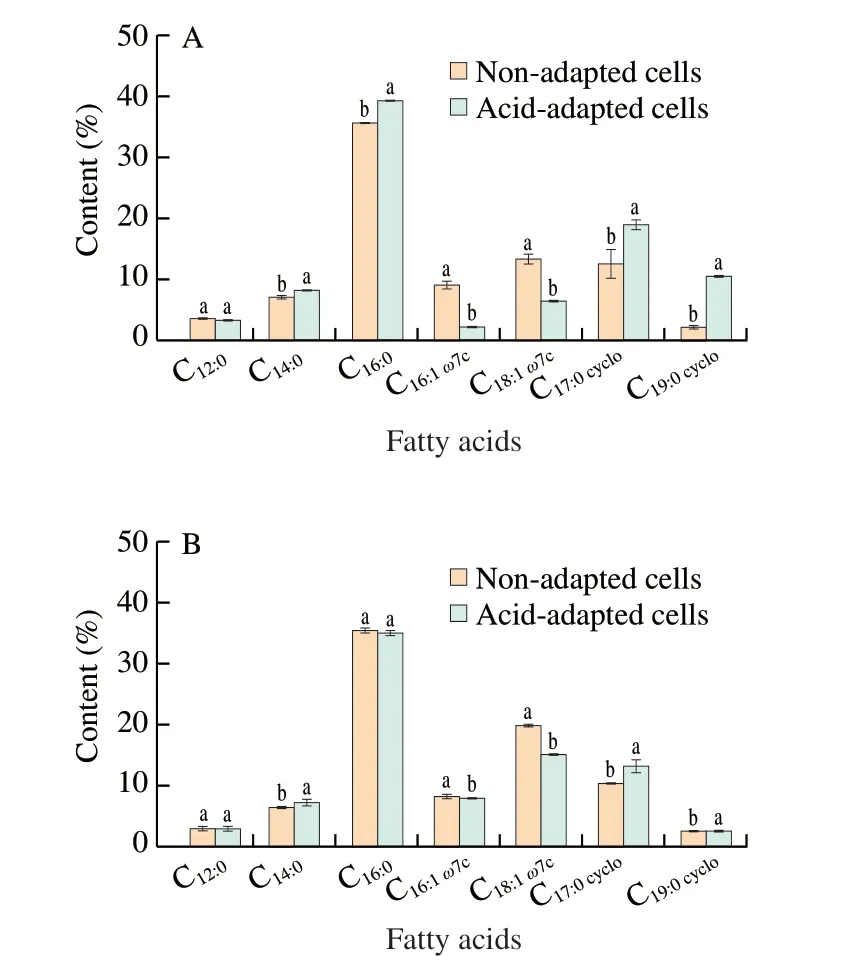
Fig. 5 Effect of acid adaptation on fatty acid composition of log-phase (A)and stationary-phase (B) S. Enteritidis. Vertical bars show standard deviations.Symbols without visible bars indicate that standard deviations are smaller than the symbol size. Different letters above bars within a column signify significant difference (P < 0.05).
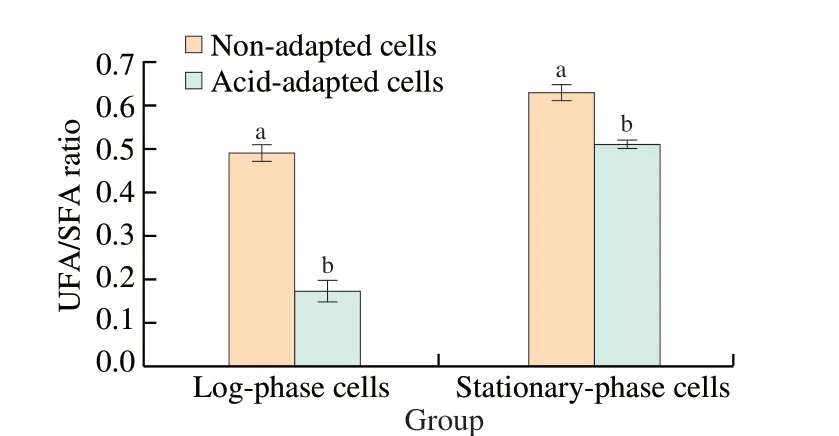
Fig. 6 Effect of acid adaptation on the ratio of unsaturated to saturated fatty acids (UFA/SFA) in log-phase and stationary-phase S. Enteritidis. Vertical bars show standard deviations. Different letters above bars within a column signify significant difference (P < 0.05).
3.4 C16:0 fatty acid was involved in acid-induced ethanol tolerance
In the current study, log-phase acid-adapted cells with evaluated amounts of C16:0fatty acid exhibited increased ethanol tolerance, while stationary-phase acid-adapted cultures with unaltered proportions of C16:0fatty acid displayed reduced ethanol tolerance. Thus, it can be inferred that C16:0fatty acid might be involved in acid-induced ethanol tolerance inS.Enteritidis.To verify this speculation, fatty acid supplementary assay was carried out and it was revealed that exogenous addition of C16:0fatty acid to stationary-phase acid-adapted cultures was able to enhance bacterial ethanol tolerance, although this effect was not observed in log-phase cells (Fig. 7). Therefore, C16:0fatty acid was indeed a factor responsible for the different survival behavior of log-phase and stationary-phaseS.Enteritidis under ethanol stress after acid adaptation.
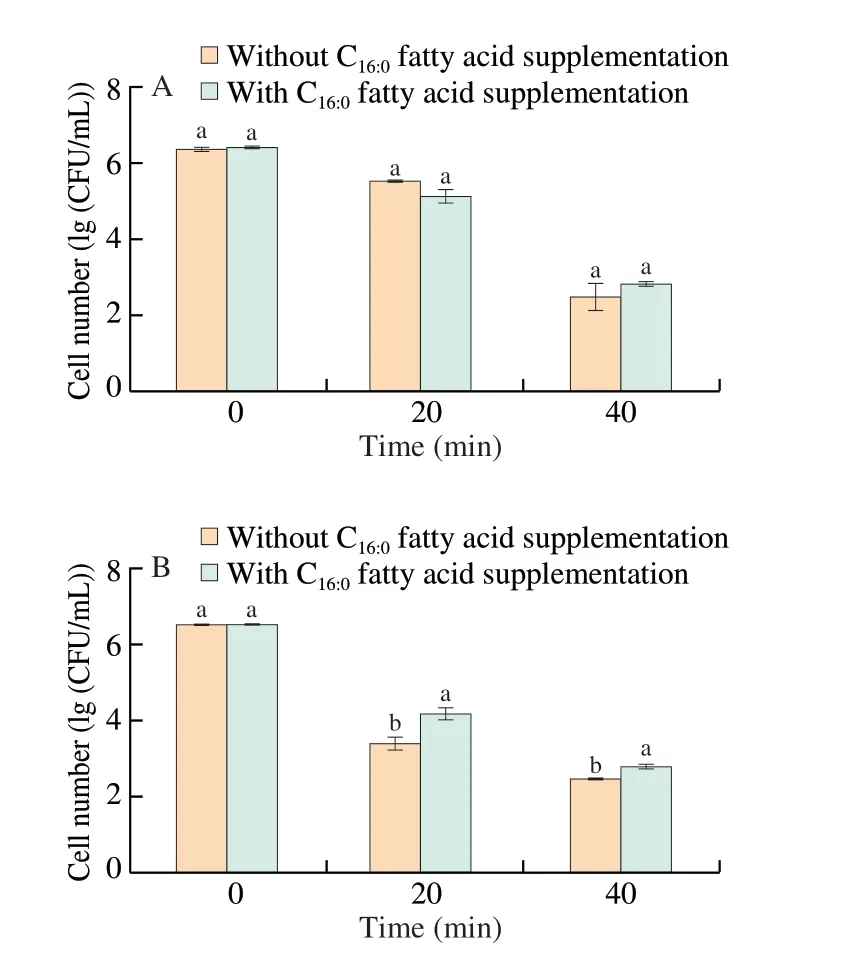
Fig. 7 Effect of exogenous supplementation of C16:0 fatty acid on ethanol tolerance of acid-adapted S. Enteritidis in log-phase (A) and stationary-phase (B).Vertical bars show standard deviations. Symbols without visible bars indicate that standard deviations are smaller than the symbol size. Different letters above bars within a column signify significant difference (P < 0.05).
The C16:0fatty acid content has been previously reported to be associated with microbial direct tolerance to ethanol stress [30-34].For example,Saccharomyces cerevisiaecells grown with exogenously supplemented C16:0fatty acid displayed pronounced ethanol tolerance [30].Interestingly, our current study revealed that C16:0fatty acid was involved in cross tolerance to ethanol conferred by acid adaptation inS.Enteritidis, which provided a new insight into bacterial ethanol tolerance mechanisms from the viewpoint of cross protection.
Notably, a standard strain ofS.Enteritidis (ATCC 13076) was utilized as a model bacterium to characterize acid-induced ethanol tolerance in the current work. It would be interesting to explore if this cross protective effect is representative for otherS.Enteritidis strains in future studies.This kind of population-wide survey has already been conducted to determine the response ofSalmonellato high pressure, acid stress and heat shock [35-37], which will contribute to a better understanding of bacterial stress response.
4. Conclusions
The protective effect of acid adaptation on ethanol tolerance inS.Enteritidis was dependent on bacterial growth phase.Log-phase and stationary-phase cells exhibited enhanced and reduced ethanol tolerance after acid adaptation, respectively,accompanied by maintained cell membrane integrity and reduced membrane fluidity. Moreover, C16:0fatty acid was involved in this growth-phase-dependent cross protective effect as revealed by fatty acid composition analysis and supplementary assay. Overall, these results provide an initial insight into mechanisms of acid-induced cross-protection against ethanol stress in log-phaseS.Enteritidis.Furthermore, this cross-protection phenomenon should be taken into consideration when developing control measures forS.Enteritidis.
Declarations of interest
None.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2019YFE0119700), the National Natural Science Foundation of China (32001797), the Science and Technology Innovation Agricultural Project of Shanghai Science and Technology Commission (19391902100), and the Natural Science Foundation of Shanghai (22ZR1429900).
- 食品科学与人类健康(英文)的其它文章
- Call for Papers from Journal of Future Foods
- GUIDE FOR AUTHORS
- Metabolomics and gene expression levels reveal the positive effects of teaseed oil on lifespan and aging process in Caenorhabditis elegans
- Ameliorative effect of Lacticaseibacillus rhamnosus Fmb14 from Chinese yogurt on hyperuricemia
- Study on the interaction between β-carotene and gut microf lora using an in vitro fermentation model
- Transcriptomics reveals substance biosynthesis and transport on membranes of Listeria monocytogenes affected by antimicrobial lipopeptide brevilaterin B

