Relationship Between Micromorphological Structure of Leaf Epidermis and Drought Resistance in Callisia repens
Haofeng OUYANG, Silin CHEN, Jinxiang LIU, Jie WU, Yudan SUN
College of Life Science and Technology, Lingnan Normal University, Zhanjiang 524048, China
Abstract [Objectives] To discuss the relationship between left epidermis structures and drought resistance. [Methods] The leaf epidermis of Callisia repens was studied by optical microscope. [Results] The upper and lower epidermal cells of the leaves of Callisia repens arranged closely, and no cell gap was arranged. The morphology of the epidermal cells was hexagonal, few pentagon or heptagon, the equivalent elliptical aspect ratio was 1.20, the vertical wall was straight and there was no stomatal distribution. Compared with the epidermal cells, the morphology of the lower epidermal cells was irregular. The equivalent elliptic aspect ratio was 1.35, and the vertical wall was smooth and curved. The mean oval aspect ratio of the stomatal guard cells was 1.42, the average stomatal density was 11.79/mm2, and the average stomatal index was 17.21. [Conclusions] These characteristics provide the theoretical basis for the drought resistance of Callisia repens and the ornamental plants as roof greening.
Key words Callisia repens, Leaf epidermis, Micromorphology
1 Introduction
Callisiarepensis a perennial herb of the genusCallisiain the family Commelinaceae, native to tropical America and distributed in Guangdong, China. The stem ofC.repensis trailing and can crawl or hang along the ground[1-2]. The leaves are heart-shaped or long-ovate, thin and less fleshy, with waxy surface and bright luster, and occasionally purple spots are distributed along the middle axis of the leaves. Leaf margin and leaf sheath, as well as stem vine, are purplish. It likes high temperature, humidity and shows shade tolerance, and the latest research has found thatC.repensalso has the characteristics of sun tolerance. The suitable temperature for growth is about 20-28 ℃, the plant height is about 10 cm, the crown diameter is uncertain, and the tolerable lowest temperature is 15 ℃. It is not only suitable for fertile and well-drained soil, but also suitable for low-nutrient and shallow soil[3]. The stems and leaves are oily green and soft, suitable for indoor viewing of hanging pots, and it can be ground cover in the sun, and suitable for cultivation on the roof[4-5].C.repenshas high ecological benefit and ornamental value. The plant has good creeping property, strong reproductive ability, fast growth rate, strong expansion ability, low cost of building and maintenance[6-7], and there are few diseases and pests. In urban landscaping, it can be promoted as an important ornamental ground cover plant[8].
In the process of plant evolution, due to the role of natural selection, plants that can adapt to the environment can survive, so different selection forms different types of adaptation. Plants feel the changes in the environment through their own organs, in which leaves are the organs most sensitive to environmental changes. When the environment changes, the leaves are the first to receive information, so the structure of leaves is compatible with the environment for plants. The change in plant structure will inevitably affect the change in physiological function, so understanding the structure of plant leaves plays an important role in exploring why this plant can survive in a certain environment. The study shows that the stomata, epidermis, stratum corneum and other structures of plant leaves are closely related to the drought tolerance of plants[9-11]. In roof greening plants, it is necessary to have the properties of drought resistance, heat resistance and water resistance in order to achieve the effect of greening. The drought and high temperature resistance ofC.repensmakes it one of the roof greening plants, and this stress resistance is compatible with the structural characteristics of leaf epidermis. At present, there are many studies on the roof greening plants, including Crassulaceae, sedum,etc., but the study on the leaf epidermis structure ofC.repenshas not been found. In this paper, the micromorphological structure of leaf epidermis ofC.repenswas observed by optical photographic microscope, and the morphological characteristics of leaf epidermis cells were discussed in order to provide morphological basis for the popularization ofC.repensinto roof greening plants.
2 Materials and methods
2.1 MaterialsC.repens(provided by the grass experimental base of Lingnan Normal University); 2.5% glutaraldehyde fixed solution (25% glutaraldehyde 1 mL, double distilled water 4 mL, 0.2 mol/L phosphate buffer 5 mL); sodium dihydrogen phosphate; sodium hydrogen phosphate; alcohol (30%, 50%, 70%, 90%, 95%); anhydrous ethanol; OLYMPUS BX43 photographic microscope.
2.2 Methods
2.2.1Procedure. Fresh leaves were rinsed with distilled water for a while, then distilled water was poured away, and then put into a petri dish, and 2.5% glutaraldehyde was added as stationary liquid for 10 h, making the leaves go under the surface of stationary liquid.
The fixed leaves were clamped, the surface stationary solution was washed with distilled water, and put into an empty petri dish. The upper epidermis was torn off with tweezers, and the excess mesophyll cells were scraped off by scraping the lower epidermis, and then dehydrated by 30%, 50%, 70%, 90% and 95% alcohol for 1 min, then spread on the slide and covered with the slide.
The typical characteristics of leaf epidermis were observed under OLYMPUS BX43 photographic microscope and microphotographed. The upper and lower epidermis were observed in 10 different visual fields, and the morphology of epidermal cells, the number of epidermal cells and stomata were observed. 40 stomatal apparatuses in different visual fields in the lower epidermis were randomly selected, and the long axis length and diameter axis length of the stomatal apparatus were measured by Image Pro Plus.
2.2.2Calculation. The number of epidermal cells: 10 visual fields were randomly selected and the cells were counted (objective lens 20×eye lens 10). The size of epidermal cells: the equivalent ellipse length and width and equivalent ellipse length-width ratio of 40 epidermal cells were randomly measured, and the average value was taken (objective lens 20×eye lens 10).
Stomatal size: the long axis (L) and diameter axis (W) of 40 stomatal apparatuses were measured randomly, and the average value was taken (objective lens 40×eye lens 10).
Stomatal density (SD): the number of stomata per unit area was observed (objective lens 20×eye lens 10). The calculation formula is: SD=S/M.
Stomatal index (SI): 10 visual fields were observed, and the number of stomata per unit visual field (S) and the number of common epidermal cells per unit visual field (E) were calculated as follows:
SI= (S/E+S)×100 (objective lens 20×eye lens 10).
whereSrepresents the number of stomata,Erepresents the number of epidermal cells in the same visual field, andMrepresents the area of each visual field (0.95 mm2).
3 Results and analysis
3.1 Characteristics of upper epidermal cellsThe upper epidermal cells ofC.repensare shown in Fig.1. There were 19-25 cells per unit area of visual field, with an average of 22 cells. The cells were of different sizes and arranged neatly, the cells were close to each other, and the anticlinal walls were straight and thick, which could be seen clearly. Most of the cells were regular hexagonal (Fig.1A1), similar to honeycomb shape, some cells were irregular hexagonal (Fig.1A2), a few cells were pentagonal (Fig.1A3) and heptagonal (Fig.1A4). The equivalent ellipse length of cells was 86.64-120.99 μm, with an average of 104 μm; the equivalent ellipse width of cells was 75.38-101.27 μm, with an average of 88.09 μm. The ratio of equivalent ellipse length to width ranged from 1.02 to 1.45, with an average of 1.20.
3.2 Characteristics of epidermal cellsThe lower epidermal cells ofC.repensare shown in Fig.1B. There were 57-72 cells per unit area of visual field, with an average of 65 cells. The shape of the cells was different, most of the cells were irregular, the size was different, the arrangement was tight, and the anticlinal wall was smooth and curved with the change of the cell shape. The equivalent ellipse length of cells was 49.88-82.67 μm, with an average of 65.92 μm. The equivalent ellipse width of cells was 36.78-71.12 μm, with an average of 50.56 μm. The ratio of equivalent ellipse length to width was 1.03-2.03, with an average of 1.35.
3.3 Characteristics of stomatal apparatusThe stomatal apparatus ofC.repenswas only distributed in the lower epidermis. Under the 100-fold magnification of the optical microscope, as shown in Fig.2A, the long axis of most stomatal apparatuses was parallel to the long axis of the leaf, distributed in a row in the lower epidermis, and the stomatal apparatuses were not connected, showing single distribution. Under the 400-fold magnification of the optical microscope, as shown in Fig.2B, the stomatal apparatus consisted of two guard cells and four subsidiary cells, the two guard cells were kidney-shaped, and the concave surface formed stomata. The four subsidiary cells were in a parallel quadruple cell type, and the two smaller subsidiary cells near the guard cells were perpendicular to the long axis of the guard cells, and the outermost two guard cells were parallel to the long axis of the guard cells. There were 12-20 green granules in the guard cells, which were clusters of granules, that is, chloroplasts.
The long axis length of guard cells was 22.36-31.43 μm, with an average of 26.99 μm, and the diameter axis length was 16.83-23.18 μm, with an average of 19.38 μm. The ratio of equivalent ellipse length to width of guard cells was 1.15-1.75, with an average of 1.42. There were 9-15 stomatal apparatuses per unit area of visual field, the stomatal density was 9.47-15.79/mm2, with an average of 11.79/mm2, and the stomatal index was 12.50-20.83, with an average of 17.21.
4 Discussion
4.1 The relationship between the characteristics of upper epidermal cells and drought resistance inC.repensThe study of Sanford G. and You Fengli showed that the thickness of anticlinal wall, the degree of bending and the degree of compactness between cells were obviously related to the protective effect. The thicker the anticlinal wall is, the higher the degree of bending is, the tighter the arrangement of cells is, and the more obvious the protective effect is. This is the characteristic of stronger drought resistance of plants[12-13]. It was found that the anticlinal wall of the upper epidermal cells ofC.repenswas straight and thickened, and the thicker anticlinal wall made the epidermal
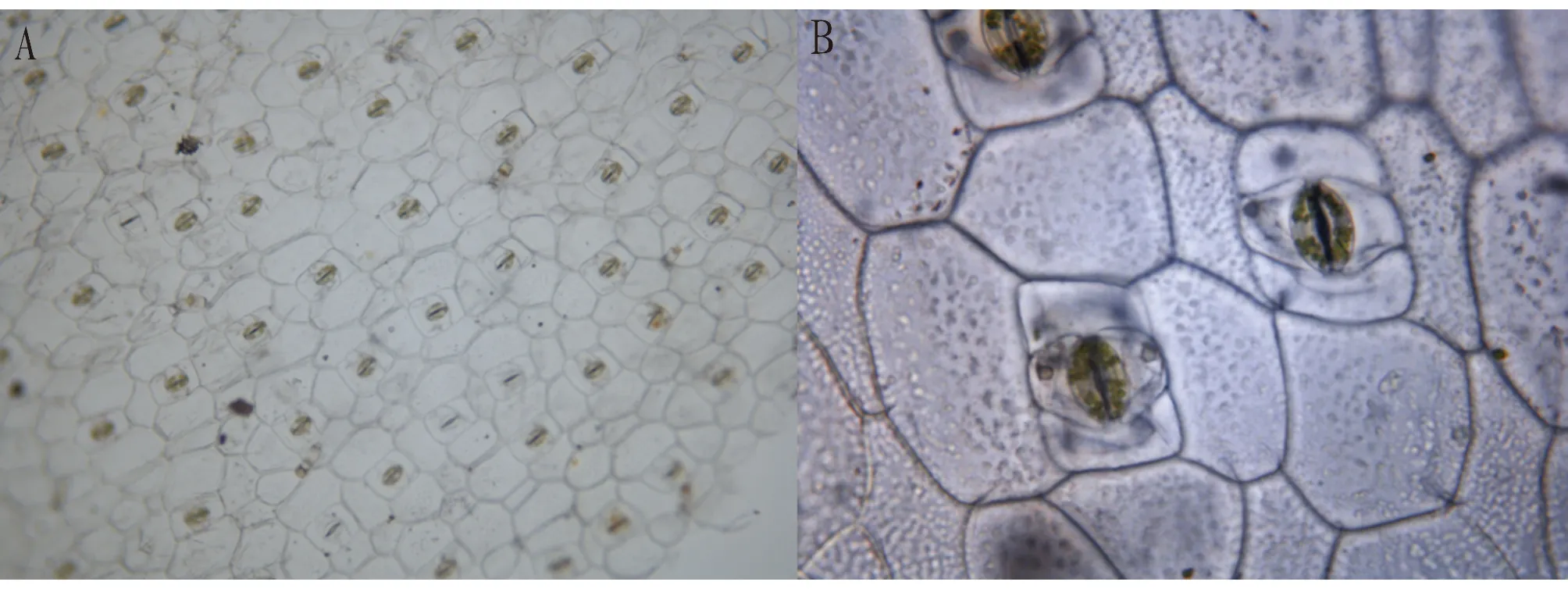
Note: A. 1∶100; B. 1∶400.
cells combine more closely, which enhanced the mechanical support ability and stress resistance of the leaves. The close combination of cells could reduce the loss of water, which was beneficial to enhancing the drought resistance of plants. In addition, the upper epidermal cells showed a certain regular polygonal shape. The average length of equivalent ellipse was 104 μm, the width of equivalent ellipse was 88.09 μm, and the ratio of length to width of equivalent ellipse was 1.20. The average number of cells per unit area of visual field was 22. Compared with drought-tolerant plants such as tomato and eggplant[14], the upper epidermis cells were obviously larger and the number was smaller, which improved the light capture ability of leaf cells, made it easier for light radiation to penetrate the leaf epidermis and reach the mesophyll tissue, and then improved the photosynthetic capacity and promoted cells to produce drought-resistant chemicals in the adverse environment.
4.2 The relationship between the characteristics of lower epidermal cells and drought resistance inC.repensThe anticlinal wall of the lower epidermis ofC.repenswas wavy and thickened, and the cells combined closely, which was consistent with the characteristics of the upper epidermis, so the lower epidermis also played an important role in drought resistance. Yang Huiminetal.found that the lower epidermis cells of leaves of wheat growing in arid environment tended to become smaller[15]. Li Fanglanetal.found that the leaf epidermis of plants growing in arid environment tended to become smaller, the anticlinal wall thickened and had endodermis, which reflected the adaptation and response of plants to environmental stress[16]. The average length of equivalent ellipse, width of equivalent ellipse, length-width ratio of equivalent ellipse, and the average number of cells per unit area of visual field ofC.repenswere 65.92 μm, 50.56 μm, 1.35 and 65 respectively. Compared with drought-tolerant plants such as tomato and eggplant[14], the lower epidermal cells ofC.repenswere obviously smaller, and the number was also relatively small, and the smaller cells could reduce the loss of water and had strong water retention capacity.
4.3 The relationship between the stomatal apparatus characteristics and drought resistance inC.repensStomatal apparatus has the function of controlling water and gas exchange. It is an important channel for plants to regulate water and directly affect plant transpiration. Plants resist drought through stomatal apparatus regulation under water stress, which is one of the mechanisms for plants to adapt to the environment.
The number and distribution of stomatal apparatus on plant leaves vary with plant species. There are stomata in the upper and lower epidermis of most plants, and the number of stomatal apparatus in the lower epidermis is larger than that in the upper epidermis, but in a few plants, the number of stomatal apparatus in the upper epidermis is larger than that in the lower epidermis; stomatal apparatus exists only in the lower epidermis of a few plants[17].C.repensis relatively special, there is no stomatal apparatus in the upper epidermis, and the stomatal apparatus is only distributed in the lower epidermis. The stomatal apparatus shows single distribution, and the lower epidermal cells are separated between the adjacent stomatal apparatuses. In hot weather, irradiated by the sun, the temperature of the upper epidermis is often relatively higher than that of the lower epidermis. Stomata are a channel for plants to control transpiration, and the water in the leaves is easily lost from the stomata of the upper epidermis. The stomatal distribution characteristics ofC.repensare compatible with the living environment, which ensures not only the normal operation of photosynthesis, but also the normal operation of respiration, so that the plant can resist the adverse environment.
There is a certain relationship between the size of plant stomatal apparatus and plant drought resistance. Li Zhonghuaetal.found that the plant stomatal apparatus which can adapt to arid environment has a decreasing trend[18]. The average long axis length of stomatal apparatus ofC.repensis 26.99 μm, the average diameter axis length is 19.38 μm, and the average ratio of equivalent ellipse length to width is 1.42. This is similar to the plants of Cruciferae and Crassulaceae which have strong drought resistance. The average long axis length of stomatal apparatus is 30.21 μm, the average diameter axis length is 19.74 μm, and the average ratio of equivalent ellipse length to width is 1.52[19]. The average long axis length of stomatal apparatus in Crassulaceae is 31.25 μm, the average diameter axis length is 18.40 μm, and the average ratio of equivalent ellipse length to width is 1.53[20]. The smaller stomatal apparatus plays an important role in adapting to the high temperature and dry environment. When the environment becomes dry, the leaf moisture keeps at a certain level. Stomatal conductivity decreases, transpiration weakens, and stomata close faster, preventing water loss in the body, and improving the drought resistance of the plant.
Plant stomatal density and stomatal index can reflect the ability of plant to resist drought to some extent. Stomatal density measures the number of stomatal apparatus per unit area, and stomatal index measures the percentage of stomatal apparatus in the total number of cells, both of which reflect the number of stomatal apparatus. Li Zhonghuaetal.found that the stomatal density and stomatal index of plants that could adapt to arid environment were smaller than those that could not adapt to arid environment[18]. The average stomatal density and stomatal index ofC.repenswere 11.79/mm2and 17.21, while those of tomato and pepper were 226.85/mm2, 212.31/mm2and 22.74, 23.48[14], respectively. In comparison, the stomatal density and stomatal index ofC.repenswere lower than those of tomato and pepper. In the environment of high temperature and drought, few stomatal apparatuses can not only reduce transpiration, prevent water loss, but also maintain water in plants and ensure effective respiration, which is the performance of plants to adapt to arid environment.
There is also a certain relationship between chloroplasts in stomatal guard cells and plant drought resistance. Most plant leaf epidermis cells do not have chloroplasts, but stomatal guard cells contain chloroplasts. Compared with chloroplasts in mesophyll cells, the volume and number of chloroplasts in guard cells are relatively reduced, and the lamellar structure of chloroplasts in guard cells is underdeveloped. However, the physiological and biochemical functions of guard cell chloroplast and mesophyll cell chloroplast are similar, with photosystem I (PS I) and photosystem II (PS II). According to Thomson and De. Fournett’s study, the total amount of photosynthesis in the chloroplasts of guard cells was sufficient to maintain the function of these guard cells. The chloroplasts in stomatal guard cells ofC.repenswere observed (round and granular, with a quantity of 12-20). When the environment was friendly, water and sunlight were sufficient, the chloroplast in the guard cell produced ATP and transported it to the cytoplasm. After it was utilized by the plasma membrane H+-ATPase, H+was pumped out of the guard cell, resulting in a significant decrease in intracellular water potential, and leading to water influx, guard cell expansion, and stomatal opening. When the environment became dry, chloroplasts in guard cells produced energy to promote the entry of H+into guard cells, resulting in an increase in intracellular water potential, and leading to water outflow, reduction of guard cells and closure of stomata[21].
5 Conclusions
In the urban planning and development space, the roof space has been ignored, and even regarded as a "sundries room", which is a common fault in urban construction and management. With the economic development, the overall urban construction area far exceeds the urban green area, and the building roof area is large, but few roofs are fully utilized, and most of them are abandoned. In a city with high cost of land, how to make full use of this last space has become the primary consideration of architectural design experts, ecological environment experts and urban planning experts. Therefore, roof greening is gradually respected, which can not only take into account the architectural landscape, create a unique urban style, but also improve the urban ecological environment and increase the charm of the city.
But the natural environment of the roof is very different from that of the ground and interior. First, the building has a certain load limit, so the thickness of the soil layer for planting on the roof is also limited. There is no rising effect of underground capillary water, and the effective water content in the soil is low. Affected by the weather, the soil temperature changes greatly. The soil is easy to dry or flooded, and the water needed by plants on the roof depends entirely on natural precipitation and artificial irrigation. Second, the soil environment for planting on the roof is not completely consistent with the soil environment on the ground, so the roots of plants are vulnerable to burns in summer and frost damage in winter. In addition, in urban buildings, the roof is generally in a higher position, the surrounding space is relatively empty, the wind speed is higher, and water evaporation is faster, so the plants on the roof are more vulnerable to wind damage. The higher the roof is from the ground, the stronger the wind on the roof is, the worse the greening conditions are, and the greater the restrictions on the plants that can be cultivated. Third, the soil moisture in the planting layer of roof is easy to lose, and irrigation is relatively frequent, which can easily lead to the loss of nutrients in the soil, so fertilizers are often needed, and the cost of roof greening is rising. On the roof, good drainage is not easy to cause wet damage, and the large temperature difference between day and night is beneficial to the accumulation of plant nutrition. However, in general, the planting environment on the roof is not very good[22].
As an ideal roof greening plant, the specific leaf epidermis structure ofC.repensenables it to actively adapt to the harsh environment of the roof and make a positive response to reduce environmental stress. For example, the large but few upper epidermis cells enhance the ability of leaf cells to capture light, which promotes light radiation to penetrate the leaf epidermis and reach the mesophyll tissue, so as to improve the photosynthetic capacity, promote the cells to produce drought-resistant chemicals and withstand the adverse environment. The small and many lower epidermal cells reduce the loss of water and maintain moisture in the plant. The specific structure of stomatal apparatus regulates water transport and photosynthesis to achieve a balance, so that it can maintain a certain capacity of photosynthesis without losing too much water. However, the response ofC.repensto drought environment is a very complex process, which involves not only the structural characteristics of leaf epidermal cells, but also the internal mechanisms of cell differentiation, material movement, physio-logical and biochemical reactions and other biological reactions. With the continuous improvement in experimental methods and the development of genetic engineering, molecular markers and electronic technology in recent years, the physiological process of drought resistance inC.repenswill be gradually discovered. This study can provide a solid theoretical basis for promotingC.repensas a roof greening plant to promote the development of roof greening industry.
 Asian Agricultural Research2022年11期
Asian Agricultural Research2022年11期
- Asian Agricultural Research的其它文章
- Monitoring and Evaluation of Benefits of Project of Returning Farmland to Forests in Henan Province
- Impact and Policy Implications of Swine Epidemic on Price Fluctuation of Livestock Products in China
- Present Situation of Dictyophora Industry in China and Cultivation Technique of Dictyophora rubrovolvata
- Impacts of ENSO on the Welfare of Rural Residents in China: A Stochastic CGE Model Assessment
- Impacts of Sand and Dust Storms on Regional Economy Based on Stochastic CGE Model: A Case Study in Inner Mongolia, China
- Current Situation, Problems and Countermeasures of Marine Ranching Development in Guangdong Province, China
