GsαR201C and estrogen reveal different subsets of bone marrow adiponectin expressing osteogenic cells
Biagio Palmisano,Rossella Labella,Samantha Donsante,,Cristina Remoli,Emanuela Spica,Ilenia Coletta,Giorgia Farinacci,Michele Dello Spedale Venti,Isabella Saggio,Marta Serafini,Pamela Gehron Robey,Alessandro Corsi and Mara Riminucci✉
1Department of Molecular Medicine,Sapienza University of Rome,Rome 00161,Italy;2Tettamanti Research Center,Department of Pediatrics,University of Milano Bicocca/Fondazione MBBM,Monza 20900,Italy;3Department of Biology and Biotechnology,Sapienza University of Rome,Rome 00185,Italy;4Institute of Structural Biology and School of Biological Sciences Nanyang Technological University,639798 Singapore,Singapore;5CNR Institute of Molecular Biology and Pathology,Piazzale Aldo Moro 5,Rome 00185,Italy and6Skeletal Biology Section,National Institute of Dental and Craniofacial Research,National Institutes of Health,Department of Health and Human Services,Bethesda,MD 20892,USA
Correspondence:Mara Riminucci(mara.riminucci@uniroma1.it)These authors contributed equally:Biagio Palmisano,Rossella Labella
INTRODUCTION
The α subunit of the stimulatory G protein(Gsα)is a ubiquitously expressed protein encoded by the GNAS gene(GNAS complex locus;GNAS,OMIM*139320),that couples cell surface receptors to adenylyl cyclase to stimulate cAMP production after ligand/receptor binding.1–2The Gsα/cAMP signaling pathway participates in homeostasis of the post-natal skeleton by acting as a downstream effector of hormones and factors,such as parathyroid hormone,prostaglandin E2 and β-adrenergic agonists,which regulate the differentiation and function of different local cell types.3–4Indeed,mutations of GNAS that lead to a gain of function of the Gsα protein(GsαR201C/R201H)cause fibrous dysplasia of bone(Polyostotic FD/MAS,OMIM#174800)a disorder characterized by dysregulated bone resorption and abnormal bone formation that severely compromise the architecture and function of post-natal bone and bone marrow.5–6How Gsα modulates the activity of cells within the bone and marrow compartments is a complex and still largely unresolved issue.However,significant insights were provided by transgenic mice in which a gain of function mutation of Gsα was expressed in a ubiquitous and constitutive manner,7or at specific differentiation stages of maturation within the osteogenic lineage.8–9Overall,these models demonstrated that dysregulated activity of Gsα in differentiated osteoblasts stimulates bone formation but does not affect the bone marrow microenvironment.8In contrast,the expression of the mutation in early progenitor cells residing within the bone marrow stroma leads to a dramatic derangement of bone and marrow architecture and function.7,9These results are consistent with current knowledge of the biology and activity of bone marrow osteoprogenitor cells.However,the bone marrow stromal cell(BMSC)system is heterogeneous and comprises not only osteoprogenitor cells but also contains progenitors committed to the adipogenic lineage.10–11In particular,a marrow-specific adipogenic progenitor cell subset that expresses Adiponectin(Adipoq,Adq)(marrow adipogenic lineage precursors,MALPs)has been identified in mice.11This Adq-Cre-targeted(Adq-)marrow stromal cell was identified as a major player in bone homeostasis due to its role in generation of marrow adipocytes,regulation of bone resorption and support of the marrow vasculature.12
As another step towards the definition of the role of Gsα signaling within bone and bone marrow stroma,and of the cellular pathogenesis of FD,we investigated the effects ofexpression of GsαR201Cin marrow adipogenic cells by using the Adipoq promoter.We first demonstrate that the Adq-marrow stromal cell network was associated not only with an adipogenic function but also with an osteogenic activity that was manifested in the trabecular bone of most bone segments and increased during physiological mouse growth.We then show that the expression of GsαR201Cin bone marrow Adq-cells enhanced both bone resorption and bone formation and generated a complex combination of metaphyseal,diaphyseal and cortical phenotypes.In the metaphysis of lumbar vertebrae and long bones,GsαR201Ccaused an early phase of enhanced bone resorption followed by a phase of enhanced bone formation driven by Adq-bone-forming cells.In the diaphysis,GsαR201Cin combination with estrogen triggered the osteogenic activity of Adq-marrow perivascular/stromal cells leading to the deposition of intramedullary/perivascular bone.Finally,consistent with the existence of a previously unrecognized Adq-pericyte compartment associated with intraosseous blood vessels,we also observed that the expression of GsαR201Cled to the development of a FD-like lytic phenotype affecting both cortical(increased porosity)and trabecular(tunneling resorption)bone.
RESULTS
Adq-cells are found in the marrow cavity,trabecular bone and cortical blood vessel wall
We assessed the distribution of Adq-cells in the mouse skeleton by crossing Adq-Cre mice with the R26-mTmG reporter line(Fig.S1a),such that cells remain red in the absence of Adq-Cre expression,and green when it is expressed.Confocal analysis of multiple skeletal segments from Adq-Cre;R26-mTmG(Adq-mTmG)mice showed different age-and site-dependent patterns of GFP labeling.In long bones from 1-month-old mice,GFP expression was observed in a subset of stromal cells within the marrow cavity(Fig.1a)and among the trabeculae of the primary spongiosa(Fig.S1b).No labeling was detected in trabecular and cortical bone(Figs.1a,S1b).However,at 4 and 9 months of age,the same skeletal segments showed extensive GFP expression in the marrow stroma among hematopoietic cells(Fig.1a),where some of the GFP-positive cells were also stained by leptin receptor(LEPR)antibody(Fig.S1c).At 4 months,GFP-positive cells were also found in the primary spongiosa(Fig.S1b),around blood vessels(Figs.1a,S1d),and also in many osteoblasts and osteocytes in trabecular bone(Figs.1a,S1b).In contrast,only sparse osteocytes and some endosteal cells expressed GFP in the cortical bone(Fig.S1b).GFP expression in trabecular cells was observed in multiple skeletal segments except for the tail vertebrae and calvaria(Fig.S1e).In the tail vertebrae,the stromal compartment could not be visualized by confocal microscopy due to the massive presence of GFP-fluorescent marrow adipocytes,whereas in the calvaria only marrow stromal cells appeared to be labeled by GFP(Fig.S1e).Finally,as a previously unnoticed finding,we observed that throughout the skeleton and at all ages,some blood vessels running through the bony cortex also carried GFPpositive pericytes as observed in the marrow vasculature(Fig.1b).Outside of the skeleton,GFP labeling was found in all fat depots but not in other tissues(Fig.S1f).

Fig.1 Adipoq-Cre-targeted cell population in bone marrow and bone.a Representative confocal images of bone marrow and trabecular bone from 1-,4-and 9-month-old Adq-mTmG mice.GFP labeling was restricted to a small number of stromal cells at 1 month of age(arrow),while at 4 and 9 months it highlighted numerous marrow stromal(arrow)and perivascular(double arrow)cells,the majority of osteoblasts(arrowhead)and osteocytes(hollow arrowhead).b Representative fluorescent images of GFP-labelled intracortical perivascular cells(double arrow)in 4-and 9-month-old mice.bm Bone marrow,bt Bone trabecula,cb Cortical bone.Scale bars 25 μm
Generation of transgenic mice expressing GsαR201C in Adq-cells
To assess the effect of Gsα overactivity on the biology and function of Adq-cells in bone,we crossed Adq-Cre mice with the R26-lsl-GsαR201Ctransgenic line expressing the R201C gain-offunction mutation of Gsα in a Cre-regulated manner(Fig.S2a).Adq-Cre;R26-lsl-GsαR201C(Adq-GsαR201C)mice were viable and fertile.Consistent with the pattern of expression of GFP in AdqmTmG mice,Cre recombination occurred in skeletal segments and in adipose depots but not in other tissues(Fig.S2b).The amount of the GsαR201Ctranscript within the skeleton of 3-month-old mice was comparable in the tail vertebrae and in bones with few to virtually no marrow adipocytes such as in calvaria,thus confirming the expression of the mutated Gsα sequence in all Adq-cells,and was particularly abundant in the tibia(Fig.S2c).
The expression of GsαR201C in Adq-cells results in enhanced and unbalanced metaphyseal bone remodeling
The expression of GsαR201Cin Adq-cells caused an enhanced and unbalanced trabecular bone remodeling that resulted in an opposite metaphyseal phenotype in young and old mice.At 3 months of age,both male and female Adq-GsαR201Cmice showed osteopenic changes.In the lumbar vertebrae and distal femora,trabecular bone volume per tissue volume(BV/TV)was reduced(Fig.2a,b)and histomorphometry on TRAP stained sections showed increased values of osteoclast number per bone surface(N.Oc/BS)and osteoclast surface per bone surface(Oc.S/BS)compared with Rosa26 littermate controls(Fig.2c).These findings associated with high values of Rankl transcript and Rankl/Opg transcript ratio in homogenized tissue samples(Fig.2d).At the same time,histological and histomorphometric changes consistent with active osteoblast recruitment and function were also observed.Osteoblast number per bone surface(N.Ob/BS)and osteoblast surface per bone surface(Ob.S/BS)and dynamic parameters of bone formation were increased in Adq-GsαR201Cmice(Fig.2e,f).Moreover,the level of Alpl mRNA in homogenized bone tissue was higher compared with controls while no difference was found in the levels of Sp7 and Bglap transcripts(Fig.2g).Stimulation of osteogenic activity continued during mouse growth and in 9-month-old Adq-GsαR201Cmice,the trabecular bone mass of the same skeletal segments was higher than in controls,especially in femora(Fig.3a,b).At this age,bone surfaces were extensively rimmed by osteoblasts and the difference in the N.Ob/BS and Ob.S/BS values between Adq-GsαR201Cand control mice was greater than that observed at 3 months,especially in females(Fig.3c).Calcein labeling showed a significant increase in osteoblast activity parameters(Fig.3d)and Alpl,Sp7 and Bglap transcripts were also highly expressed in homogenized bone tissue(Fig.3e).The osteoclast parameters normalized on trabecular bone surfaces were lower than controls in female Adq-GsαR201Cmice(Fig.3f)although the total number of osteoclasts per tissue area(N.Oc/TA:15.22±1.924 vs.28.54±4.064,Mean±SEM,P<0.01)and the level of Rankl expression(Fig.3g)were still higher compared with controls.No significant difference was detected in the osteoclast parameters in male mice(Fig.3f).Interestingly,bone marrow adiposity in long bones of Adq-GsαR201Cmice was reduced at all ages,with marked differences observed in the number and area of bone marrow adipocytes in female mice(Fig.S3a,b).
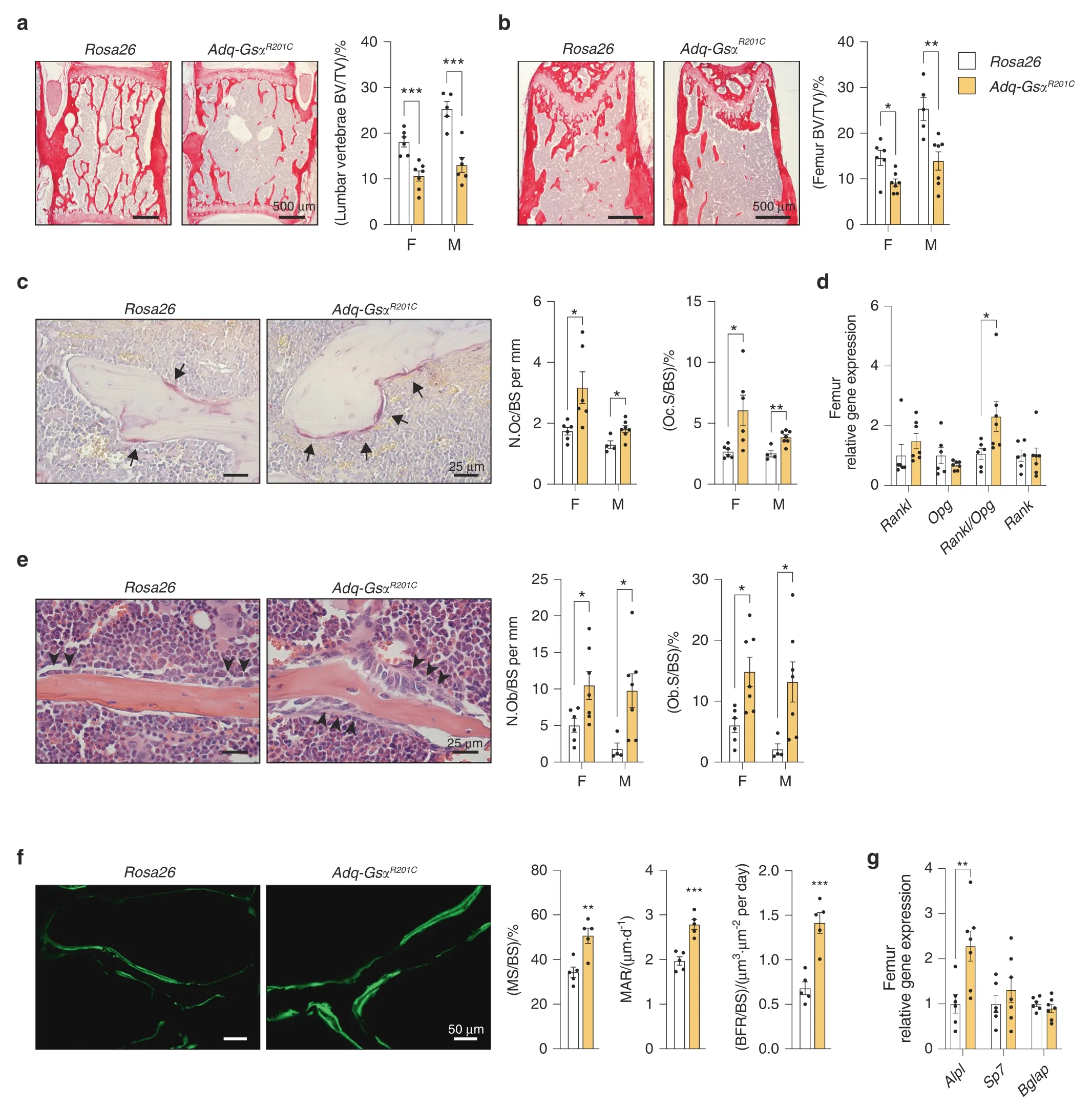
Fig.2 Enhanced trabecular bone resorption in 3-month-old Adq-GsαR201C mice.a,b Representative histological pictures from Sirius redstained sections and histomorphometric analysis of trabecular bone of lumbar vertebrae(a)and femora(b).BV/TV:bone volume per tissue volume.c TRAP histochemistry highlighting cells of the osteoclastic lineage(arrow)and histomorphometry of osteoclast parameters.N.Oc/BS:number of osteoclasts per bone surface.Oc.S/BS:osteoclast surface per bone surface.d Relative gene expression of Rankl,Opg,Rankl/Opg ratio and Rank in femora from female mice.e Representative images from H&E-stained sections showing osteoblasts(arrowhead)and histomorphometry of osteoblast parameters.N.Ob/BS:number of osteoblasts per bone surface.Ob.S/BS:osteoblast surface per bone surface.f Representative pictures of calcein labeling in trabecular bone of female mice and dynamic histomorphometry for bone formation parameters.MS/BS:mineralizing surface per bone surface.MAR:mineral apposition rate.BFR/BS:bone formation rate.g Relative gene expression of Alpl,Sp7 and Bglap in femora from female mice.F Females.M Males.Data are presented as mean±SEM.Statistical analysis was performed using Student t-test;*P<0.05,**P<0.01,***P<0.001

Fig.3 Enhanced metaphyseal bone formation in 9-month-old Adq-GsαR201C mice.a,b Representative histological pictures from Sirius redstained sections and histomorphometric analysis of trabecular bone of lumbar vertebrae(a)and femora(b).BV/TV:bone volume per tissue volume.c Representative images from H&E-stained sections showing osteoblasts(arrowhead)and quantitative histomorphometry of osteoblast parameters.N.Ob/BS:number of osteoblasts per bone surface.Ob.S/BS:osteoblast surface per bone surface.d Representative pictures of calcein labeling in trabecular bone of female mice and dynamic bone formation parameters.MS/BS:mineralizing surface per bone surface.MAR Mineral apposition rate.BFR/BS Bone formation rate.e Relative gene expression of Alpl,Sp7 and Bglap in femora from female mice.f TRAP histochemistry highlighting cells of the osteoclastic lineage(arrow)and quantitative histomorphometry of osteoclast parameters.N.Oc/BS:number of osteoclasts per bone surface.Oc.S/BS:osteoclast surface per bone surface.g Relative gene expression of Rankl,Opg,Rankl/Opg ratio and Rank in femora from female mice.F Females.M Males.Data are presented as the mean±SEM.Statistical analysis was performed using Student t-test;*P<0.05,**P<0.01,****P<0.000 1.The exact P-value was reported on male column bars in a and b
The bone Adq-cell network comprises metaphyseal osteoprogenitor cells that are regulated by Gsα
The trabecular osteopenic phenotype of 3-month-old Adq-GsαR201Cmice was consistent with the previously reported stimulatory activity of Adq-stromal cells on bone resorption12and revealed the regulatory role played by Gsα in this function.To assess the contribution of the same cells to the subsequent high metaphyseal trabecular bone mass phenotype we crossed Adq-GsαR201Cmice with the R26-mTmG reporter line(Fig.S2d).Ninemonth-old Adq-mTmG;GsαR201Cmice showed a less dramatic increase in metaphyseal bone compared with age-matched Adq-GsαR201Cmice,likely due to the presence of only one R26-lsl-GsαR201Callele.Nonetheless,confocal analysis of lumbar vertebrae and femora revealed the expression of GFP in the majority of osteoblasts and osteocytes in the metaphyseal bone trabeculae formed after the early resorption phase and an increased density of GFP positive stromal cells around their surface(Fig.4a).The fraction of GFP-positive osteocytes increased over time in both Adq-mTmG and Adq-mTmG;GsαR201Cmice,but at 9 months of ageit was significantly higher in Adq-mTmG;GsαR201Cmice compared with controls(Fig.4b).In the bone marrow of Adq-mTmG mice,this was associated with an expansion of Adq-GFP marrow stromal area(Figs.1a,4b)that was not appreciated in Adq-mTmG;GsαR201Cmice(Fig.4a,b).These results suggest an active recruitment of Adq-GFP stromal cells to osteogenesis(Fig.4c)and a role for Gsα in the stimulation of this process.To further support our findings,we performed heterotopic transplants with a mineralized osteoconductive carrier.BMSCs were isolated from long bones of 2-month-old Adq-mTmG;GsαR201Cand Adq-mTmG control mice,loaded onto ceramic particles and transplanted into the back of immunocompromised mice(Fig.4d).After 8 weeks,the control transplants showed abundant residual ceramic particles,the surfaces of which were extensively covered by lamellar bone,and marrow spaces occupied by hematopoietic cells and adipocytes(Figs.4e,S4a).In contrast,the Adq-mTmG;GsαR201Ctransplants showed very few residual ceramic particles and a remarkable amount of haphazardly distributed bone(Fig.4f,g).The newly formed bone had a mixed woven and lamellar structure(Fig.S4a),and its surfaces were covered by numerous TRAP positive osteoclasts(Fig.S4b).In these samples,the marrow spaces contained focal clusters of hematopoietic cells and adipocytes and were largely filled with ALP positive stromal cells(Fig.4f).Confocal analysis demonstrated GFP-expressing osteoblasts and osteocytes in control transplants(Fig.4h),thus supporting the existence of an Adq-stromal cell population in mouse long bone that is inherently osteogenic.In parallel with the higher amount of bone,GFP-labeled cells were numerous in AdqmTmG;GsαR201Ctransplants in which GFP expression was also found within the ALP positive marrow stroma(Fig.4i).
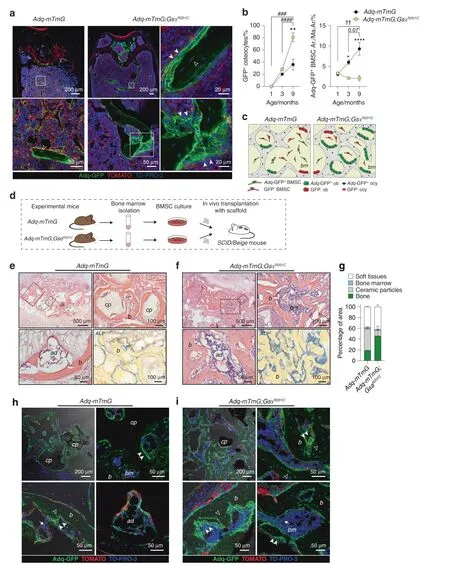
Fig.4 Osteogenic activity of Adq-marrow stromal cells in situ and in heterotopic transplants.a Representative images of trabecular bone from 9-month-old Adq-mTmG and Adq-mTmG;GsαR201C mice showing GFP-marked osteoblasts(arrowhead)and osteocytes(hollow arrowhead).b Quantification of the fraction of GFP-labeled osteocytes and the GFP-labeled stromal cells area in Adq-mTmG and Adq-mTmG;GsαR201C mice at different ages.Statistical analysis performed using Two-way ANOVA followed by Sidak’s multiple comparison test.*P<0.05,**P<0.01,****P<0.000 1 for comparison between Adq-mTmG and Adq-mTmG;GsαR201C mice.###P<0.001,####P<0.000 1 for comparison of different ages in Adq-mTmG;GsαR201C mice.††P<0.01,for comparison of different ages in Adq-mTmG mice.The exact P-value was reported for the comparison between 3 and 9 months in Adq-mTmG mice.c Scheme of changes in the trabecular bone mass and GFP-labeled osteoblasts,osteocytes and stromal cells in Adq-mTmG and Adq-mTmG;GsαR201C mice.d Experimental design for the heterotopic transplantation of BMSCs in SCID/beige mice.e,f H&E-stained section of transplants made with BMSCs derived from Adq-mTmG(e)and Adq-mTmG;GsαR201C(f)mice,showing newly generated bone(b),bone marrow(bm)and adipocytes(ad).Carrier particles(cp)were easily recognized only in Adq-mTmG samples.Numerous ALP positive stromal cells were detected in transplants generated with cells from Adq-mTmG;GsαR201C mice.g Quantification of fraction of transplant area occupied by soft tissue,bone marrow,ceramic particles and bone.h,i Representative confocal images of the same transplants showing GFP labeling in the majority of osteoblasts(arrowhead)and osteocytes(hollow arrowhead),in adipocytes(ad)and in stromal cells(arrow)
The marrow Adq-cell network comprises diaphyseal
osteoprogenitor cells that are regulated by Gsα and estrogen In addition to the metaphyseal bone changes,Adq-GsαR201Cmice also developed an unexpected skeletal phenotype characterized by bone formation within the diaphyseal marrow cavity.The medullary osteogenic process was restricted to females and to long bones,with the tibia being the first involved segment in allfemale mice(Fig.S5a).Bone deposition started within 3 months of age in the diaphyseal region at the tibia/fibula junction,and,as clearly shown by micro-CT scans,it involved the cortical endosteum only focally(Fig.5a),especially at sites in which large blood vessels entered the marrow cavity(Fig.5b).Bone formation continued along the blood vessel wall to extensively fill the marrow space in old animals(Figs.5a,b,S5a-c).The bone tissue had a mixed woven and lamellar structure(Fig.5b),showed a thin layer of osteoid,and was well mineralized(Fig.5c).In Adq-mTmG;GsαR201Cfemale reporter mice the expression of GFP was detected in the vastmajority of osteoblasts and osteocytes of the bone tissue wrapping the marrow vessels and in a few osteocytes in the inner side of the cortex in continuity with the medullary bone(Fig.5d).
The absence of medullary bone formation in Adq-GsαR201Cmale mice suggested that in the diaphysis of long bones,there is a subset of stromal/perivascular Adq-cells that are osteogenic and are modulated by Gsα but require estrogen stimulation to turn into bone-forming cells.To test this hypothesis,we treated 5-month-old Adq-GsαR201Cand control male mice with either 17β-estradiol(E2)or vehicle(Veh)in the drinking water for 6 weeks(Fig.6a).In mice expressing the Gsα mutation,E2 treatment led to deposition of bone in the diaphyseal marrowcavity(Fig.6b–d)similarly to what was observed in untreated female mice bearing the same genotype(Fig.5).The origin of the intramedullary bone was explored in the tibiae and femora of E2-treated Adq-mTmG;GsαR201Cmale reporter mice,in which it included only GFP positive osteoblasts and osteocytes(Fig.6e–g).After E2 treatment,an increase in the radiodensity of the metaphysis of long bones was detected in all male mice independent of their genotype(Fig.6b,c).Histomorphometric analyses of trabecular bone demonstrated that BV/TV(Fig S6a)and osteoblast parameters(Fig S6b)were significantly higher in Adq-GsαR201Cmice upon E2 treatment compared with Veh-treated Adq-GsαR201Cand E2-treated control mice,in the absence of significant changes in osteoclast parameters(Fig S6c).
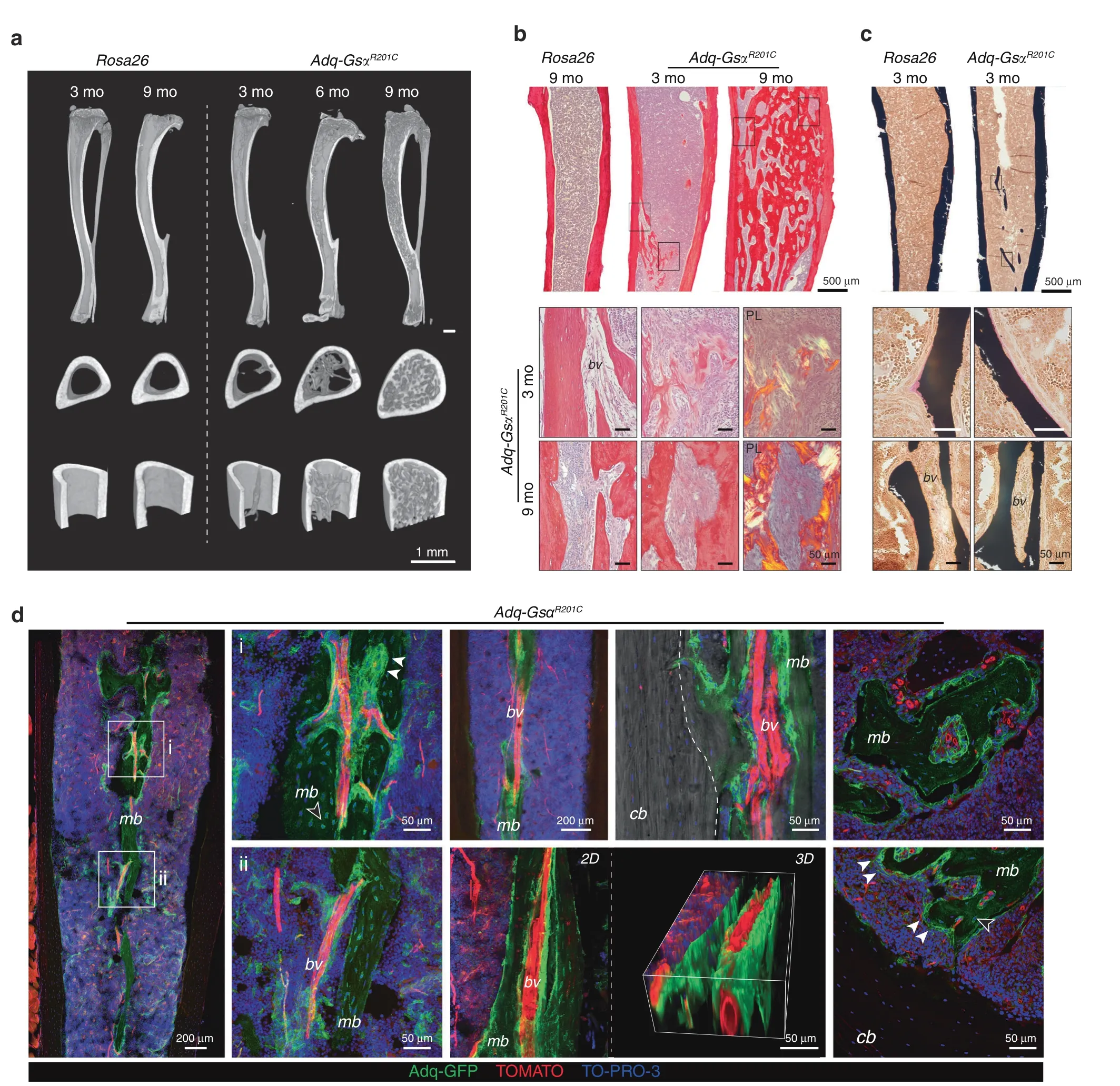
Fig.5 Diaphyseal intramedullary bone formation in female Adq-GsαR201C mice.a Representative micro-computed tomography images of mice at different ages,showing the appearance and progression of the diaphyseal intramedullary bone.Transverse images were taken 2 mm above the tibia-fibular junction.b Transmitted and polarized(PL)light microscopy views of Sirius red stained sections of the tibial midshafts showing the appearance of the intramedullary bone in Adq-GsαR201C mice at 3 months of age and its subsequent expansion with obliteration of the medullary canal at older ages.PL shows the mixed woven and lamellar bone structure.c Von Kossa/Van Gieson stained MMA sections of undecalcified tibial midshafts showing mineralized intramedullary bone with a thin layer of osteoid rimmed with osteoblasts.d Representative images from confocal microscopy,showing medullary bone(mb)distributed around marrow blood vessels(bv)and its focal connection with cortical bone(cb,dotted line).GFP expression was observed in osteoblasts(arrowhead)and osteocytes(hollow arrowhead).3D reconstruction was performed on a 50 μm-thick section using ImageJ software
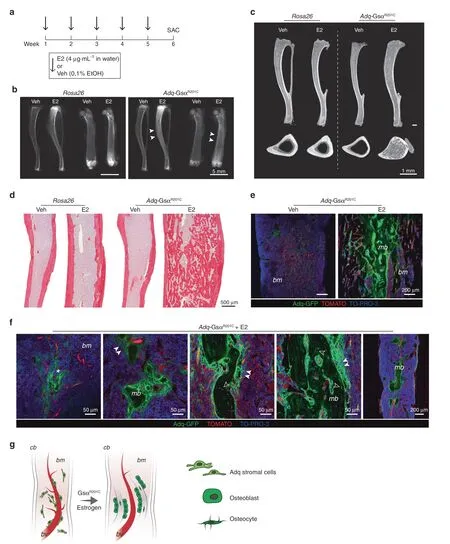
Fig.6 Diaphyseal intramedullary bone formation is reproduced in Adq-GsαR201C male mice by 17β-estradiol(E2)treatment.a Experimental scheme of E2 treatment started at 5 months of age.b Radiographs of dissected tibiae and femora at the end of E2 treatment.Arrowheads indicate the increased density in the diaphyseal region of E2-treated bone segments from Adq-GsαR201C mice.c Representative longitudinal and transverse micro-CT images of Veh-and E2-treated mice,showing the diaphyseal intramedullary bone in E2-treated Adq-GsαR201C male mice.Transversal images were taken 2 mm above the tibia-fibular junction.d Sirius red stained sections of the tibial midshafts showing intramedullary bone in Adq-GsαR201C male mice after 6 weeks of E2 treatment.e Representative confocal images showing GFP-labeled intramedullary bone in E2-treated Adq-GsαR201C male mice.No bone is observed in Veh-treated mice.f Cluster of perivascular GFP-labeled stromal cells(asterisk)preceding the appearance of bone with GFP-labeled osteoblasts(arrowhead)and osteocytes(hollow arrowhead)in the marrow cavity of E2-treated Adq-GsαR201C mice.g Schematic representation of GFP-labeled medullary bone formation by Adq-GsαR201C marrow perivascular/stromal cells.mb Medullary bone,bm Bone marrow,cb Cortical bone,bv Blood vessel
Osteogenic Adq-cells are not found in all skeletal segments
As observed in control Adq-mTmG mice,the expression of GFP in trabecular osteoblasts and osteocytes was never detected in the tail vertebrae of Adq-mTmG;GsαR201Cmice(Fig.S1e,Fig.7a).This indicated the lack of osteogenic potential of Adq-cells in the tail.To further confirm this finding,and to rule out potential inhibitory effects of the local microenvironment,we performed heterotopic transplantation of adherent cells grown after enzymatic digestion of tail vertebrae.The cells were harvested from 2-month-old Adq-mTmG;GsαR201Cand Adq-mTmG control mice,cultured in vitro(Fig.7b)and transplanted with ceramic particles into the back of immunocompromised mice(Fig.7c).After 8 weeks,bone tissue was detected in all samples(Fig.7d,e).However,despite the presence of GFP-labeled adherent cells in the cell cultures(Fig.7b)only Tomato positive osteocytes were detected by confocal analysis in all transplants(Fig.7f,g).GFP labeling was restricted to marrow adipocytes(Fig.7f,g)and,in Adq-mTmG;GsαR201Csamples,to a small number of stromal cells distributed within the marrow spaces(Fig.7g).
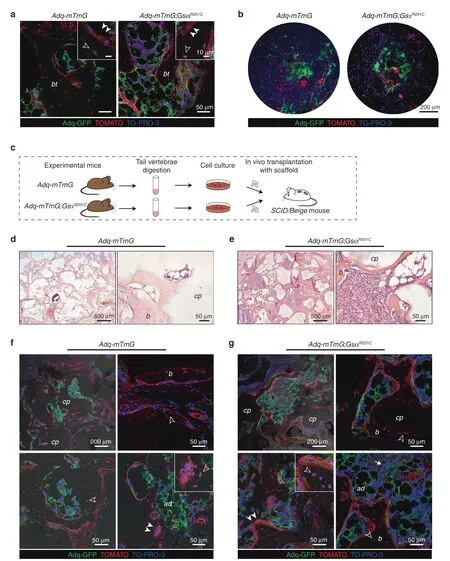
Fig.7 Adq-marrow stromal cells from tail vertebrae are not osteogenic.a,Representative confocal microscopy images of bone trabeculae(bt)in the tail vertebrae of Adq-mTmG and Adq-mTmG;GsαR201C mice showing only Tomato positive osteoblasts(arrowhead)and osteocytes(hollow arrowhead).b GFP-labeled adherent cells in BMSC cultures isolated from tail vertebrae of 2-month-old mice.c Experimental design for the heterotopic transplantation of bone marrow stromal cells isolated from tail vertebrae.d,e Representative transmitted light microscopy images of Adq-mTmG and Adq-mTmG;GsαR201C transplants showing newly formed bone on the surfaces of carrier particles and inter-particle spaces occupied by bone marrow and adipocytes.f,g Representative confocal microscopy images of the same transplants showing GFP labeling in adipocytes(ad)and stromal cells(arrow)within the inter-particle spaces.Only Tomato positive osteoblasts(arrowhead)and osteocytes(hollow arrowhead)were found in these transplants.b Bone,cp Carrier particles
The expression of GsαR201C in Adq-cells is associated with cortical lysis and trabecular tunneling(dissecting)resorption
Stimulation of bone resorption was a persistent feature of Adq-GsαR201Cmice at all ages.While in young mice it involved predominantly the bone surface in the trabecular bone(Fig.2a,b),during mouse growth,abnormal bone resorption was also observed around some blood vessels running within the cortical bone and in the larger,predominantly sub-cortical,bone trabeculae.Consequently,areas of lysis(increased porosity)and tunneling(dissecting)resorption developed in cortical and trabecular bone respectively.This phenotype was markedly expressed in the tail vertebrae(Fig.8a–e)in which osteoclastenriched lytic lesions(Fig.8c,d)tended to expand over time and were always filled by a stromal tissue that was ALP-positive/OSXnegative and expressed RANKL,as assessed by immunohistochemistry(Fig.8e).In Adq-mTmG;GsαR201Cmice,GFP labeling was observed in perivascular cells and in some stromal cells within lytic regions,but not in the surrounding bone cells(Fig.8f,g).Areas of cortical and trabecular bone resorption were also observed in other skeletal segments such as long bones and lumbar vertebrae(Fig.S7a).However,at these sites,the pattern of evolution was different compared with the tail since the resorption spaces were progressively replaced by hematopoietic marrow(Fig.S7b).In Adq-mTmG;GsαR201Cmice,GFP labeling was localized to perivascular cells at sites of lesion development and in osteoblasts bordering the lytic area(Fig.S7c,d).
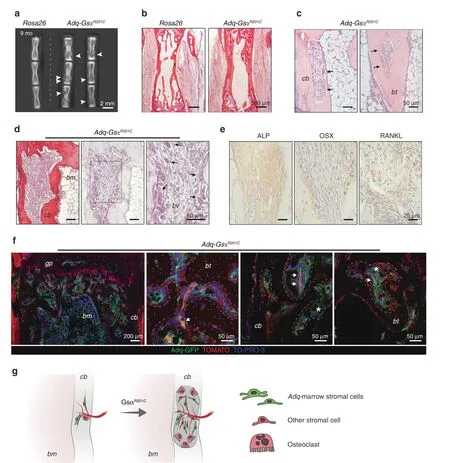
Fig.8 Adq-intraosseous pericytes associate with lytic lesions in cortical and trabecular bone in Adq-GsαR201C mice.a Radiographic analysis of dissected tail vertebrae from 9-month-old Rosa26 and Adq-GsαR201C mice showing several osteolytic lesions(arrowhead).b Representative Sirius red stained sections of tail vertebrae from 9-month-old mice.c H&E-stained sections showing dissecting and tunneling resorption in tail vertebrae.Note the massive presence of osteoclasts(black arrow).d Sirius red and TRAP-stained sections showing intracortical bone resorption in tail vertebrae.Resorption areas progressed over time and were enriched in osteoclasts(black arrow)in close association with blood vessels(bv).e Immunolocalization of ALP,OSX and RANKL in a tail vertebra lytic lesion of Adq-GsαR201C mice.f Representative confocal images from Adq-mTmG;GsαR201C mice tail vertebrae showing areas of osteolysis(dashed line)filled with GFP-positive perivascular cells(arrow)and stromal cells(asterisk).g Schematic representation of lytic lesions associated with intracortical Gsα-mutated Adq-pericytes.cb:cortical bone,bt:bone trabecula,bm:bone marrow,gp:growth plate
DISCUSSION
Adipoq-Cre recombination in the mouse skeleton occurs in marrow adipocytes,in a network of stromal cells residing among hematopoietic cells and around blood vessels,and in cells lining the trabecular bone surfaces.11,13–15Adq-marrow stromal cells were reported to coincide with a fraction of Lepr-Cre-targeted stromal cells13and to largely overlap with the PDGFRβ+/VCAM-1+/CXCL12+(CAR)stromal cell subsets.14In addition,it was shown that the majority of Adq-marrow stromal/perivascular cells are post-natal adipocyte progenitors,named MALPs,that support marrow vasculature and are involved in the regulation of bone resorption.11–12Here we show that Adq-Cre in bone marrow targets a population that includes stromal cells,some of which express LEPR,and perivascular cells.This Adq-Cre-targeted cell population is associated with an osteogenic activity that is expressed in the metaphysis of different skeletal segments in physiological conditions and in the diaphysis of long bones under specific stimulatory settings.
Adq-Cre targeting of osteoblasts was previously noted by Zhou et al.as a rare event in vivo13and by Mukohira et al.as an agedependent phenomenon14but neither further investigated its significance.In agreement with Mukohira’s report,we observed that in Adq-mTmG mice,GFP-labeled osteoblasts were virtually absent at a very young age,but their number progressively increased in the metaphyseal region of the long bones and lumbar vertebrae during mouse growth.This suggested the existence of Adq-marrow stromal osteoprogenitor cells that undergo progressive,age-dependent recruitment to trabecular bone formation and remodeling.This hypothesis was supported by the presence of GFP-positive osteoblasts and osteocytes in heterotopic ossicles made by BMSCs.Previously,other groups performed in vivo transplantation studies that failed to reveal the osteogenic potency of the Adq-marrow stromal compartment.11Although we cannot exclude Adipoq promoter activation during the transplantation process,the expression of osteogenic activity in our transplants,could be ascribed to the different experimental conditions used in the in vivo assays compared with previous studies.For example,while in previous work,gelatin sponges(GelfoamTM)were used as a carrier and the constructs were analyzed after 4 weeks,11we transplanted the cells with a ceramic carrier and harvested the ossicles after 8 weeks.Thus,it is possible that the type of carrier and the longer time before harvesting in our experiments generated the optimal conditions for Adqmarrow stromal progenitors to express their osteogenic potency in vivo.In addition,we did not perform cell sorting before transplantation and we cannot exclude the possibility that Adqcell survival and differentiation was favored in the presence of different populations of marrow stromal cells.Based on the evidence that Adq-marrow stromal cells represent an adipogenically committed cell population11–12and that GFP-labeled adipocytes are also observed in our heterotopic ossicles,our data point to the existence of a subset of Adq-progenitors that are able to generate both adipocytes and osteoblasts.
The involvement of Adq-marrow stromal cells in both bone resorption and bone formation and the regulatory role played the Gsα/cAMP pathway in the two processes may explain the skeletal phenotype of Adq-GsαR201Cmice.The Gsα/cAMP pathway is known to enhance the osteoclastogenic activity of marrow osteoprogenitor cells by initially stimulating the secretion of RANKL,16–19which is physiologically produced by Adq-marrow stromal cells.12On the other hand,Gsα is also known to stimulate cell commitment and subsequent bone formation within the osteogenic lineage.8,20In Adq-GsαR201Cmice at 3 months of age,increased Rankl expression by mutated stromal cells leads to the prevalence of trabecular bone resorption over bone formation,in spite of the increased osteoblastogenesis.During growth of the mouse,when the Adq-stromal compartment expands,as demonstrated in control Adq-mTmG mice,the Gsα mutation massively stimulates its osteogenic differentiation.Thus,in 9-month-old mice,bone formation prevails over bone resorption,leading to a high trabecular bone mass phenotype,despite the persistent increase in Rankl levels.
Of note,previous work demonstrated that in the bone marrow stromal compartment,Gsα enhances osteogenic differentiation at the expense of adipogenesis;21–22accordingly,in Adq-GsαR201Cmice,bone formation is associated with an overall reduction in bone marrow adipose tissue.We did not investigate the phenotypic effects and functional mechanisms affected by GsαR201Cin Adq-marrow stromal cells.However,we showed that the events observed in Adq-GsαR201Cmice were reproduced in heterotopic transplants in which the presence of Adq-GsαR201Cmarrow stromal cells associated with an unusual extensive“resorption”/disintegration of the ceramic particles23and with the deposition of a high amount of bone that was largely made by GFP-positive bone cells.
Interestingly,our Adq-GsαR201Ctransgenic model also demonstrated that a synergy exists between GsαR201Cand estrogen in stimulating bone formation by Adq-stromal cells within the diaphyseal marrow of long bones.The role of estrogen in this ectopic and non-structural osteogenic process was suggested by its spontaneous appearance in Adq-GsαR201Cfemale mice and was confirmed by its reproduction in male mice treated withexogenous hormone.Estrogen-dependent bone deposition in the marrow diaphysis is a long-known phenomenon in birds,as a physiological calcium storage mechanism during the egg-laying cycle,24and in female mice,as a non-physiological phenomenon induced by treatment with high doses of exogenous hormone.25–26However,estrogen-related medullary osteogenic processes previously reported in birds and mice relied on the activation of cells lining the endosteal surface and resulted in a centripetal process of“endosteal trabecularization”.25In our Adq-GsαR201Cfemale mice and estrogen-treated male mice,the intra-medullary bone resulted from the activation of Adq-stromal cells on the blood vessel abluminal side and involved the cortical endosteum only focally,consistent with the discontinuous distribution of Adq-cells at this site.Thus,the topographic pattern of estrogen-dependent bone formation in the diaphyseal cavity of GsαR201Cmice was rather reminiscent of that observed in some estrogen-independent experimental models of medullary osteogenesis,as for example,in colchicine treated rats.27–28The reason why Adq-diaphyseal perivascular marrow stromal cells require both GsαR201Cand estrogen to express their osteogenic potential remains to be
addressed.However,it is interesting to note that recently,Sivaraj et al.demonstrated that diaphyseal stromal progenitors have different biological properties compared with their metaphyseal counterparts including a higher baseline commitment to adipogenesis.29This finding may explain the different response of metaphyseal and diaphyseal Adq-marrow stromal cells to GsαR201Cin our Adq-GsαR201Ctransgenic model.In metaphyseal Adq-marrow stromal cells,GsαR201Calone was sufficient to stimulate the osteogenic function that was further enhanced by estrogen.In contrast,in diaphyseal Adq-marrow stromal cells the synergic action of both stimuli was required to trigger and complete the osteogenic program.
Regardless of the mechanism of synergy of GsαR201Cand estrogen to enhance osteogenesis,the marrow osteogenic process in GsαR201Cmice provides further in situ evidence of the ability of Adq-marrow stromal cells to form bone and has some interesting implications.First,it reveals that R201-mutated Gsα and estrogen together may induce an osteogenic fate choice in a subset of Adqstromal cells that do not form bone in physiological conditions.Further investigation of this phenomenon may help to better understand the process of osteogenesis and to therapeutically approach low bone mass diseases.Second,consistent with Sivaraj’s work,29it shows for the first time and in the intact bone,there is a major difference in the regulation of diaphyseal Adq-Cre-targeted osteoprogenitor cells compared with their metaphyseal counterparts and it seems to confirm the existence of an“internal plasticity”29in the marrow stromal cell system.Finally,our data show that Adq-GsαR201Cmice provide a unique model to study bone formation by marrow diaphyseal stromal cells in mammals in the absence of any type of mechanical or pharmacological manipulation of the local microenvironment and/or of hematopoiesis.
Interestingly,the metaphyseal and diaphyseal osteogenic activities of Adq-cells were not homogeneously distributed throughout the mouse skeleton since neither calvaria nor the tail vertebrae ever showed Adq-Cre-targeted osteoblasts and osteocytes.The reason for the lack of osteogenic activity of Adqcells at these skeletal sites remains to be clarified.Meanwhile,we ruled out potential inhibitory effects of the local microenvironment by demonstrating that marrow stromal cells isolated from the tail of Adq-mTmG;GsαR201Cmice did not form GFP-labeled bone cells in heterotopic transplants.
In this work,we also identified a compartment of Adqperivascular cells within the cortical bone that was unnoticed in previous studies.Consistent with this finding and with the evidence that GsαR201Cenhances the bone resorption stimulatory activity of Adq-marrow stromal cells,as shown in this study,Adq-GsαR201Cmice developed a cortical lytic(increased porosity)and trabecular tunneling(dissecting)resorption phenotype that was particularly evident in the tail vertebrae.This phenotype may indicate a role for Adq-perivascular cells in the physiological remodeling of the transcortical vascular channels.30In addition,it may shed some light on the cell type involved in the pathogenesis of Gsα-related skeletal diseases with abnormal bone resorption such as hyperparathyroidism and certain phases of lesion development in FD of bone.
FD is a severely crippling skeletal disorder associated with Gsα activating mutations.5In FD lesions,normal bone is resorbed and replaced by fibrous marrow and newly formed,woven bone.We previously reported a FD phenotype in transgenic mice with ubiquitous expression of the GsαR201Cmutation.7Our current study shows that specific aspects of the FD phenotype are also reproduced in Adq-GsαR201Cmice,thus revealing the involvement of the Adq-marrow stromal cell subset in the pathogenesis of the disease.Specifically,it suggests that Gsα-mutated Adqmarrow stromal cells might act as the trigger of the bone resorption processes(cortical lysis and dissecting resorption)that precedes the growth of the fibro-osseous tissue,and that they might also contribute to the deposition of FD bone.Interestingly,the post-natal appearance and progressive expansion of the Adq-marrow stromal cell network perfectly fit with the natural history of this skeletal dysplasia that,despite the early post-zygotic occurrence of the Gsα mutation,does not affect embryonic and fetal development of the skeleton,appears after birth,and worsens during skeletal growth.31Thus,although Adq-GsαR201Cmice fail to develop the full-blown FD skeletal phenotype observed in mice with ubiquitous and constitutive expression of GsαR201C7,they could represent a useful tool to study the early stages of FD lesion development.
CONCLUSION
In conclusion,in this work we demonstrated that Adq-cells in the marrow stromal cell network act not only as stimulators of bone resorption,but also as osteoprogenitor cells.We also showed that Gsα mediates their regulatory and progenitor functions and that the activity of the Gsα/cAMP signaling pathway must be tightly controlled in the entire Adq-marrow cell network in order to preserve the homeostasis of the post-natal bone/bone marrow organ.
MATERIALS AND METHODS
Mice generation
All studies were performed in compliance with relevant Italian laws and Institutional guidelines and all procedures were IACUC approved.
To generate the Rosa26-lsl-GsαR201Cvector,the SalI/HindIII cassette was excised from the R201C rat Gsα cDNA,which included a hemagglutinin(HA)epitope as a flag(ATCC 63317,GenBank M12673),32and inserted it into the pBigT vector(Addgene #21270),which contained a splicing acceptor site of adenovirus(SA),a stop region,including a neo cassette downstream to the PGK promoter and,downstream,a triple SV40 polyadenylation sequence.The stop cassette was flanked by two loxP sites and followed by the bovine growth hormone polyadenylation signal(BGHpA).The PacI and AscI restriction sites,placed 5’to the SA and 3’to the BGHpA,respectively,were used to subclone the functional sequence into the pRosa26PA plasmid(Addgene#21271),which included the Rosa26 sequences required for targeted recombination into the murine Rosa26 locus.R26-lsl-GsαR201Cmice were generated by electroporating the R26-lsl-GsαR201Cvector into murine ES CK35(129/Sv Pas strain)cells.Upon electroporation,mouse ES cells were selected by neo screening,and 216 neo resistant clones were analyzed by multiple PCRs and sequencing.Two positive ES clones were implanted in C57Bl/6 N blastocysts,which,in turn were transferred into surrogate B6CBAF1 female mice.Mouse chimeras were backcrossed with C57Bl/6 N and F1 animals were genotyped by PCR.Heterozygous F1 mice were obtained from both founder clones.Two lines were serially backcrossed and showed regular Mendelian inheritance of the transgenic cassette.
Homozygous(homo)R26-lsl-GsαR201Cmice(Rosa26)were crossed with heterozygous(het)Adipoq-Cre mice(#028020,The Jackson Laboratory);the resulting progeny were crossed again with Rosa26 mice to generate Adiponectin-Cre(het);R26-lsl-GsαR201C(homo)(Adq-GsαR201C)mice,which expressed the mutant form of Gsα in adipogenic cells.
To generate Adq-mTmG and Adq-mTmG;GsαR201Clineage reporter mice,we crossed heterozygous Adq-Cre and double heterozygous Adq-GsαR201Cmice with homozygous loxP-mT-pAloxP-mG-pA(mTmG)mice(#007676 The Jackson Laboratory).The triple heterozygous Adq-mTmG;GsαR201Cmice harbor one R26 allele with GsαR201Ctransgene while the other R26 allele contains the mTmG transgene.
All mice were maintained in cabin-type isolators at standard environmental conditions(temperature 22–25°C,humidity 40%–70%)with 12:12 h dark/light photoperiod.Food and waterwere provided ad libitum.Mice were genotyped by using the oligonucleotides listed in Table 1.

Table 1.Sequence of primers used for genotyping and qPCR
X-ray analysis and micro-CT scanning
Radiographic analyses were performed on femora and tibiae using Faxitron MX-20 Specimen Radiography System(Faxitron X-ray Corp.,Wheeling,IL,USA)set at 24–25 kV for 6–8 s with Kodak MINR2000 18×24 films.
For micro-CT scanning,tibiae were placed within a plastic tube,mounted onto the instrument rotational stage and scanned at 8 μm voxel size using 80 kV,50 μA X-ray settings and a 1 mm aluminum filter with exposure time of 100 ms per frame,with a Bruker SkyScan 1275 micro-CT(Micro Photonic,Allentown,PA,USA).Three-dimensional reconstruction was performed with Bruker’s NRecon software and visualization occurred using Bruker’s DataViewer and CTscan software programs.
Histology
Mice were euthanized by carbon dioxide inhalation and skeletal segments were dissected and processed for either paraffin embedding,methylmethacrylate(MMA)or gelatin embedding.
For paraffin embedding,samples were fixed with 4%formaldehyde in PBS pH 7.4 for 48 h at 4°C and decalcified in 10% EDTA for 14–21 days at 4°C with gentle shaking.Threemicron-thick sections were used for standard histology after staining with Hematoxylin-Eosin(H&E)or with Sirius red to visualize collagen fibers,for Tartrate-Resistant-Acid-Phosphatase(TRAP)histochemistry to highlight cells of the osteoclastic lineage and for histomorphometry.
MMA embedding was performed on undecalcified bone segments.After dissection,bone samples were fixed in 4%formaldehyde for 24 h and dehydrated through a series of increasing ethanol concentrations.Bones were then infiltrated for 3 days with the plastic embedding mixture containing 60 mL of MMA,35 mL butylmethacrylate,5 mL methylbenzoate,1,2 mL polyethylene glycol 400 and 0.8 g of dry benzoyl peroxide.The polymerization mixture was prepared by adding 400 μL of N,Ndimethyl-p-toluidine to the infiltrating solution.Sections of 4–7 μm in thickness were cut from MMA blocks,deplasticized with 2-methoxyethylacetate(all reagents were purchased from Sigma Aldrich,Saint Louis,MO,USA),stained with Von Kossa and counterstained with Van Gieson.
For gelatin embedding,freshly dissected femora,tibiae and tail vertebrae were fixed in cold 4% formaldehyde solution for 4 h,washed in 1X PBS and decalcified in 0.5 M EDTA at 4°C.Soft tissues were fixed in 4%formaldehyde for 4 h.Samples were then placed in 20%sucrose and 2%Polyvinylpyrrolidone(PVP)solution in PBS for a further 48 h.Samples were embedded in an 8% porcine gelatin solution containing 20%sucrose and 2%PVP as previously reported.33Twenty to 50 μm-thick sections were cut,air-dried for 30 min,hydrated with 1X PBS,stained with TO-PRO-3(#T3605,Thermo Fisher Scientific,Waltham,Massachusetts,USA)for nuclei visualization and imaged with Leica Confocal Microscope(Wetzlar,Germany).For heterotopic transplants,gelatin embedded samples were also used to perform TRAP and Alkaline Phosphatase(ALP)histochemistry.
For measurements of GFP-positive BMSC area(Adq-GFP+BMSC Ar/Ma.Ar),pictures at 40X magnification were taken with Leica Confocal Microscope,color channel split by ImageJ software and green channel used for quantification of the area of signal that was then normalized on the marrow area.The fraction of GFPlabeled osteocytes(GFP+osteocytes)was calculated by counting them on bone trabeculae of femora and tibiae.
Histochemistry
TRAP and ALP histochemistry were performed using Sigma Aldrich reagents(Sigma Aldrich).Briefly,for TRAP histochemistry working solution,50 mg of Naphtol AS-BI phosphate were dissolved in 4 mL N,N-dimethylformamide added to 4 mL acetate buffer and 92 mL of distilled water;then,150 mg of Tartaric Acid and 30 mg of Fast Garnet were added.The slides were incubated with the working solution at 37°C.For histochemical detection of ALP on gelatin sections,30 mg of Naphtol AS Phosphate were dissolved in 0,5 mL N,N-dimethylformamide and added to a 100 mL borate buffer with 100 mg of AS blue BB salt.The solution was added to the slide and incubated for 5–10 min at 37°C.
Immunohistochemistry
Immunolocalization of OSX,RANKL and ALP was performed using rabbit anti-mouse antibodies(anti-OSX #ab22552,anti-RANKL#ab37415,Abcam,Cambridge,UK;anti-ALP # 11187–1-AP,Proteintech,Rosemont,Illinois,USA)applied at a dilution of 1:200 in PBS+1% turn BSA,overnight at 4°C.After repeated washing with PBS,sections were incubated for 30 min with biotinconjugated swine anti-rabbit IgG(#P0217,Agilent,Santa Clara,CA,USA)1:200 in PBS+1% BSA and then exposed for 30 min to peroxidase-conjugated ExtrAvidin(#P0217,Agilent)(1:50 in PBS+1% BSA).The peroxidase reaction was developed using DAB substrate kit(SK-4100,Vector Laboratories,Burlingame,CA,USA).
Immunolocalization of LEPR,endomucin(EMCN)and alphasmooth muscle actin(α-SMA)was performed using goat anti-LEPR(#AF497,R&D Systems,Minneapolis,MN,USA),rat anti-EMCN(#ab106100,Abcam)and rabbit anti-α-SMA(#ab5694,Abcam).Twenty-five μm-thick sections from gelatin-embedded samples were rehydrated with PBS and then immunostained overnight.After primary antibody incubation,sections were repeatedly washed with PBS and incubated with appropriate Alexa Fluor 647-conjugated(rabbit anti-goat IgG #A-21446,goat anti-rat IgG#A-21247,goat anti-rabbit IgG #A27040 Thermo Fisher Scientific)secondary antibodies for 1 h at room temperature.Nuclei were counterstained with TO-PRO3.
Histomorphometry
Quantitative bone histomorphometry was conducted on lumbar vertebrae(3rdand 4th)and on distal femora.Experiments were performed in a blinded fashion.Different bone parameters,using standard nomenclature and abbreviations,34were measured in a region of interest(ROI)in the secondary spongiosa of distal femora,starting 300 μm below the growth plate and for a length of 1 mm,and between the two growth plates in lumbar vertebrae.H&E and Sirius red-stained sections were used to measure trabecular bone volume per tissue volume(BV/TV),osteoblast number per bone surface(N.Ob/BS)and osteoblast surface per bone surface(Ob.S/BS).TRAP-stained sections were used to measure osteoclast number per bone surface(N.Oc/BS)and osteoclast surface per bone surface(Oc.S/BS).
Dynamic bone histomorphometry was performed on lumbar vertebrae dissected from mice that were treated with 30 mg/kg of calcein(Sigma Aldrich),5 and 2 days before euthanasia.Calcein fluorescent labeling was used to quantify mineralizing surface(MS/BS),mineral apposition rate(MAR)and bone formation rate(BFR/BS).
Bone marrow adiposity was analyzed by manual counting of the number of adipocytes per marrow area(N.Ad/Ma.Ar)and by measuring their area(Ad.Ar/Ma.Ar)in H&E-stained sections of distal femora,according to standard procedures and nomenclature.35–36
Pictures were acquired with an optical microscope(Zeiss Axiophot,Jena,Germany)through a digital camera(Jenoptik ProgrRes C5,Jena,Germany)and all histomorphometric analyses were performed using ImageJ.37
Transplantation assay
BMSCs were isolated from 3-month-old female Adq-mTmG and Adq-mTmG;GsαR201Cmice by flushing long bones(femora,tibiae,humeri)and crushing sacral and lumbar vertebrae.Cell suspensions were collected after vigorous pipetting,filtered through a 70 μm nylon mesh cell strainer,and grown at 37°C 5% CO2as multiclonal cell strains38in αMEM Sigma Aldrich)supplemented with 20% Fetal bovine serum(Thermo Fisher Scientific),1%L-glutamine,1% penicillin/streptomycin Sigma Aldrich).To isolate BMSCs from the tail vertebrae,the tails were skinned,cleaned of muscle and tendons and incubated in 5 mL of 2 mg·mL−1Collagenase I solution in HBSS for 15 min and then spun at 200 g(relative centrifugal force)to remove the periosteum.The vertebrae were then minced and incubated in 10 mL of 2 mg·mL−1Collagenase solution in HBSS for 1 h and spun at 100 g.After digestion,the supernatant was collected,filtered through 70 μm nylon mesh and centrifuged at 1 300 g for 6 min.The resulting pellet was washed with αMEM and cells were grown in the supplemented culture medium reported above.
After 1 week,all cell populations were transplanted.Constructs were made by loading 7×106cells onto 40 mg of ceramic particles(a component of AttraX,NuVasive,San Diego,CA,USA)and transplanted into 2-month-old female CB17.Cg-PrkdscidLystbg-j/Crl(SCID/beige)mice(Charles River,Wilmington,Massachusetts USA)as previously described.39After 8 weeks,samples were harvested,fixed in 4% formaldehyde in PBS pH 7.4 for 12 h and decalcified in 0.5 mol·L−1EDTA for 1 week.Samples were then processed for porcine gel embedding as described above.Tenmicron-thick sections were stained with H&E for morphology evaluation and histomorphometric quantification of the different tissue area,and for TRAP and ALP histochemistry.Twenty μm-thick sections were cut and analyzed by confocal microscopy for visualization of GFP and tdTomato fluorescence.
Estradiol treatment
For 17β-estradiol(E2,Sigma Aldrich)treatment,two experimental groups were established:a vehicle group including Rosa26,Adq-GsαR201Cand Adq-mTmG;GsαR201Cmice(n≥4 for each genotype,5 months of age)and a E2 group including Rosa26,Adq-GsaR201Cmice,Adq-mTmG;GsαR201Cmice(n≥4 for each genotype,5 months of age).E2 was dissolved in ethanol at a concentration of 5 mg·mL−1and added to 300 mL of drinking water to a final concentration of 4 μg·mL−1.The vehicle group received ethanol in drinking water at a final concentration of 0.1%.Water bottles were changed every week.The dose of E2 ingested was calculated as previously reported.40After 6 weeks of treatment,mice were sacrificed by CO2inhalation.Radiographic analyses were performed before the treatment and at sacrifice.
Gene expression analysis by quantitative PCR
Femora and tibiae from 3-and 9-month-old female mice were dissected and snap frozen in liquid nitrogen and kept at−80°until use.Bone samples were homogenized by Mikro-Dismembrator U(Gottingen,Germany)and total RNA was isolated using the TRI Reagent®(Thermo Fisher Scientific)protocol.Reverse transcription was performed by using QuantiTect® Reverse Transcription Kit(Qiagen,Hilden,Germany).cDNA samples were used as templates for quantitative PCR(qPCR)analysis on a 7500 Fast Real-Time PCR System(Applied Biosystem,Waltham,Massachusetts,USA),performed using PowerUP Sybr Green(Thermo Fisher Scientific)and specific primers(Table 1).Gene expression levels of each gene were normalized to GAPDH expression.
Statistical analysis
The comparisons between two groups were performed using the unpaired t-test.Changes in the GFP+osteocyte fraction and Adq-GFP+BMSC Area between Adq-mTmG and Adq-mTmG;GsαR201Cmice at different ages were analyzed with the two-way ANOVA followed by a Sidak’s multiple comparison test.The comparison of histomorphometrical parameters during estrogen treatment was performed using one-way ANOVA followed by a Tukey’s multiple comparison test.In all experiments a P-value less than 0.05 was considered statistically significant.All graphs and statistical analyses were performed using GraphPad Prism version 8(GraphPad Software,La Jolla,CA,USA).
DATA AVAILABILITY
The authors declare that all data supporting the findings of this study are available within the paper and its supplementary information files.
ACKNOWLEDGEMENTS
This study was supported by grants from Telethon GGP15198,University of Pennsylvania Orphan Disease Center in partnership with the Fibrous Dysplasia Foundation MDBR16-114-FD,MDBR17-114-FD,MDBR18-114-FD/MAS and Sapienza University RM11916B839074A8 to M.R.;Fibrous Dysplasia Foundation MDBR-22-101-FDMAS to B.P.and M.R.;Sapienza University RM118164289636F0 to A.C.;ZIA DE000380 to PGR;AIRC IG-24614 to I.S.
AUTHOR CONTRIBUTIONS
B.P.and M.R.designed the research study and drafted the manuscript.B.P.conducted all the experiments,acquired,analyzed the data,and oversaw all aspects of the study.R.L.is listed as co-first author as she contributed to the design of the study,conducted the experiments and acquired the data.S.D.,C.R.,E.S.,I.C.,G.F.,and M.D.S.V.conducted the experiments and acquired histological and molecular data.I.S.contributed to the vector generation.MS contributed to the micro-CT analysis.P.G.R.and A.C.provided expertise related to the experiments and contributed to theinterpretation of the results.B.P.,P.G.R.,A.C.and M.R.edited and revised the manuscript.All the authors read and approved the manuscript.
ADDITIONAL INFORMATION
Supplementary informationThe online version contains supplementary material available at https://doi.org/10.1038/s41413-022-00220-1.
Competing interests:The authors declare no competing interests.
- Bone Research的其它文章
- Runx1 is a key regulator of articular cartilage homeostasis by orchestrating YAP,TGFβ,and Wnt signaling in articular cartilage formation and osteoarthritis
- Fetuin-A is an immunomodulator and a potential therapeutic option in BMP4-dependent heterotopic ossification and associated bone mass loss
- Endothelial PDGF-BB/PDGFR-β signaling promotes osteoarthritis by enhancing angiogenesis-dependent abnormal subchondral bone formation
- Ammonia promotes the proliferation of bone marrow-derived mesenchymal stem cells by regulating the Akt/mTOR/S6k pathway
- Systemic IL-27 administration prevents abscess formation and osteolysis via local neutrophil recruitment and activation
- Strontium-incorporated bioceramic scaffolds for enhanced osteoporosis bone regeneration

