一例四核Gd(Ⅲ)簇的结构、磁性、抑菌活性及大的磁热效应
侯银玲 季 甲 左 瑶 陆胜桃 王欣超 胡晓蒙 黄晓强
(1凯里学院理学院,凯里 556011)
(2太原师范学院化学系,晋中 030619)
The design and construction of polynuclear Ln(Ⅲ)-based clusters with fascinating structures and interesting magnetic properties,such as single-molecule magnets(SMMs)and magnetocaloric effects(MCEs),have always attracted great attention from coordination chemists and materials scientists[1-3].Up to now,lots of polynuclear lanthanide clusters with various geometric topologies have been constructed[4-8],and some of them display outstanding MCEs or remarkable SMMs behaviors[9-13].It is well known that MCE is related to the magnetic entropy change and the adiabatic temperature change with a varying applied magnetic field[14].For obtaining large MCE,weak superexchange interactions and large magnetic density are of great importance[15].Based on this,Gd(Ⅲ)ion is an excellent and best choice to construct refrigerant materials due to its negligible magnetic anisotropy[16],and small ligands can also enhance the magnetic density of Ln(Ⅲ)-based clusters,which is beneficial to get a large MCE[17].It is worth mentioning that various small ligands combined with Gd(Ⅲ)ion are employed to construct high polynuclear clusters[18-22].In 2013,a Gd24nanocapsule with the formula[Gd24(DMC)36(μ4-CO3)18(μ3-H2O)2]·6H2O displaying a larger- ΔSmof 46.12 J·kg-1·K-1(T=2.0 K,and ΔH=7.0 T)was reported by Zhao's group[23];whereafter,Long's group reported an incomparable Gd104cluster which displays well MCE with- ΔSm=46.9 J·kg-1·K-1at T=2.0 K and ΔH=7.0 T[24].In 2015,a[Gd60]nanocage with the formula{[Ln3(μ6-CO3)(μ3-OH)6]OH}nshowing the largest MCE with- ΔSm=66.5 J·kg-1·K-1has been reported by Zhao's group[25].
On the other hand,Ln(Ⅲ)-based compounds have been found to own special medicinal properties due to their less toxic side effects[26].Hence,how to effectively use Ln(Ⅲ)-based compounds,as far as possible to avoid or reduce its toxic side effects,has become the research target of medical scientists[27].The research emphasis of Ln(Ⅲ)-based compounds focuses on how to combine Ln(Ⅲ)ions with ligands and display specific physiological activities[28].Currently,lots of works revolving around the biological activity of Ln(Ⅲ)-based compounds have been reported[29].
Based on these research backgrounds,as part of our research interests,we have long been tracing some literature about polynuclear Ln(Ⅲ)-based clusters[30-33],which employ Schiff-base ligands to construct Ln(Ⅲ)-based clusters with outstanding magnetic properties.Combined with biological activity studies of Ln(Ⅲ)-based compounds,in this paper,we report the design and synthesis of a new Gd4cluster[Gd4(CH3COO)6(H3L)2]·2CH3OH(1)based on a polydentate ligand 1,3-bis(tris(hydroxymethyl)methylamino)propane(H6L).Meanwhile,we thoroughly characterized the Gd4cluster by magnetic property and antibacterial activity.What is worth mentioning that cluster 1 displayed well MCE(-ΔSm=40.6 J·kg-1·K-1at T=2.0 K and ΔH=7.0 T).It is larger than the-ΔSmof the mostly reported Gd4cluster.In addition,antibacterial activity study shows that cluster 1 possesses a prominent antibacterial effect on common bacteria.
1 Experimental
1.1 Materials and methods
Gadolinium (Ⅲ) nitrate hydrate(Gd(NO3)3·6H2O),acetic acid,the solvents(CH3OH,CH3CN,CH2Cl2,and N,N-dimethylformamide(DMF)),and H6L were purchased from Energy Chemical Co.,Ltd.The Luria-Bertani(LB)medium and five bacteria,namely Escherichia coli(E.coli),Staphylococcus aureus(S.aureus),Staphylococcus albus(S.albus),Bacillus subtilis(B.subtilis),and Micrococcus luteus(M.luteus),were purchased from Shanghai Macklin Biochemical Co.,Ltd.Elemental analyses(C,H,and N)for cluster 1 were performed on a PerkinElmer 2400 elemental analyzer.The thermogravimetric analysis(TGA)for cluster 1 was determined on a Netzsch TG 209 TG-DTA analyzer from room temperature to 800℃under a nitrogen atmosphere with a heating rate of 10℃·min-1.The directcurrent(dc)magnetic susceptibility data of cluster 1 was collected for polycrystalline samples using a Quantum Design MPMS(SQUID)-XL magnetometer and PPMS-9T system.
1.2 Synthesis of cluster 1
The synthetic route of cluster 1 is shown in Scheme 1.Gd(NO3)3·6H2O(0.15 mmol,0.067 7 g),H6L(0.15 mmol,0.042 4 g),and acetic acid(45µL)were dissolved in a mixture solvent of CH3OH(5.0 mL),CH3CN(5.0 mL),and CH2Cl2(2.0 mL).The mixture was sealed in a 20 mL glass sample vase and heated at 80℃for 18 h under autogenous pressure to yield yellow block-shaped crystals suitable for X-ray diffraction.Yield:35%(based on Gd(NO3)3·6H2O).Elemen-tal analysis Calcd.for C36H72Gd4N4O26(%):C,26.90;H,4.48;N,3.49.Found(%):C,26.93;H,4.44;N,3.45.
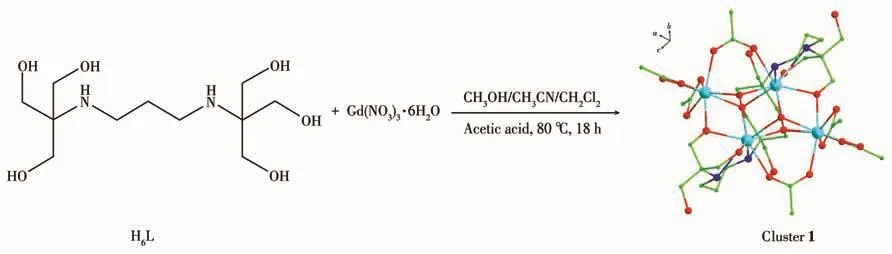
Scheme 1 Synthetic route of cluster 1
1.3 Bacteriostatic activity measurement
1.3.1 Preparation of bacteriostatic agent
The solutions of Gd(NO3)3·6H2O,H6L,and cluster 1 were prepared by DMF with concentrations of 10-1,10-2,10-3,10-4,and 10-5mol·L-1,respectively.Highpressure steam sterilization was used.
1.3.2 Preparation of bacteria suspension
LB medium(1.0 mL)was slowly added into centrifuge tubes containing various bacteria(1.5 mL),whereafter,they were placed into an oscillator under shaking for about 6.0 h.Then,the above strains were transferred into a conical flask containing LB liquid medium(100 mL),and they were placed into the oscillator under shaking for another 12 h.The bacteria suspensions have been obtained.
1.4 Single-crystal X-ray diffraction measurement
The crystallographic diffraction data for 1 was measured by a Bruker APEX-Ⅱ CCD diffractometer equipped with a graphite-monochromatic Mo Kα radiation(λ=0.071 073 nm),solved through direct methods then refined to convergence by least-squares method on F2using the SHELXTL(Olex2)program.All the atoms except the hydrogen atoms were refined with anisotropic parameters.Due to the existence of disordered solvent molecules in the crystal of cluster 1,some attempts to locate and refine were unsuccessful.Hence,the disordered solvent molecules were removed using PLATON/SQUEEZE program.Detailed crystal data and structure refinements for cluster 1 are listed in Table 1.The important bond lengths and angles for cluster 1 are listed in Table S1(Supporting information).

Table 1 Crystal data and structure refinement parameters for cluster 1
CCDC:2114735.
2 Results and discussion
2.1 Structure description of cluster 1
The crystals of cluster 1 are obtained by using the multidentate chelating ligand H6L(0.15 mmol)reacting with Gd(NO3)3·6H2O(0.15 mmol)of a mixed solvents CH3OH(5.0 mL)/CH3CN(5.0 mL)/CH2Cl2(2.0 mL)at 80℃under solvothermal conditions.Single-crystal X-ray diffraction analysis reveals that cluster 1 crystallizes in orthorhombic space group Pbca with Z=4,and the formula of cluster 1 is[Gd4(CH3COO)6(H3L)2]·2CH3OH.As shown in Fig.1,cluster 1 mainly includes four Gd(Ⅲ)ions,two deprotonated H3L3-ions,and six CH3COO-ions.There are two distinct coordination environments around the center Gd(Ⅲ)ions in 1,the central Gd1(Ⅲ)ion is eight-coordinate and connected by eight oxygen atoms(O1,O5,O6,O7,O8,O9,O10,and O11),while the Gd2(Ⅲ)ion is nine-coordinate and connected by two nitrogen atoms(N1 and N2)and seven oxygen atoms(O1,O3,O5,O5a,O6,O6a,and O12)(Fig.2).As shown in Fig.3,there are two distinct coordination geometries for the central Gd(Ⅲ)ions in cluster 1:a distorted bi-augmented trigonal prism(C2v,Gd1 ion)and muffin(CS,Gd2 ion),which are also determined by the software SHAPE 2.0(Table S2).The four central Gd(Ⅲ)ions are bridged by four μ3-O atoms and two μ2-O atoms from two H3L3-ions and four μ-O atoms from two CH3COO-ions(Fig.S1).The four central Gd(Ⅲ)ions display a butterfly Gd4core.The H3L3-adopts a polydentate mastiff coordination mode to connect four central Gd(Ⅲ)ions(Fig.S2).In the Gd4core,the adjacent distances of Gd1…Gd2 and Gd1…Gd1a are 0.392 02(5)and 0.357 66(5)nm,respectively.The diagonal distances of Gd…Gd are 0.667 05(5)and 0.343 86(6)nm.The Gd—O distances fall in a range of 0.229 5(4)-0.257 3(4)nm and the Gd—N bond lengths are 0.266 2(5)and 0.254 5(5)nm,respectively.The bond angles of O—Gd—O are in a range of 52.74(15)°-149.11(15)°.These Gd—O/N distances and O—Gd—O bond angles are comparable with the corresponding bond lengths and bond angles of reported Ln4compounds[34-36].
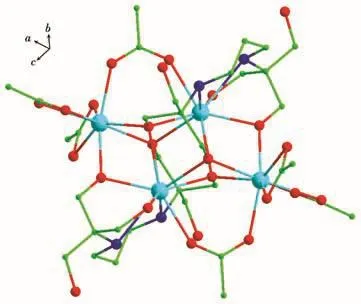
Fig.1 Molecular structure of cluster 1

Fig.2 Coordination atoms of central Gd1(Ⅲ) and Gd2(Ⅲ)ions in cluster 1

Fig.3 Two different polyhedral geometrical configurations around the Gd(Ⅲ)ions in cluster 1
2.2 TGA of cluster 1
The TGA of cluster 1 was performed under an N2atmosphere by the heating rate of 10 ℃·min-1,as shown in Fig.S3.From room temperature to about 200℃,the sample gradually lost weight,and the weight loss ratio was 4.17%,corresponding to the theoretical value of losing two free CH3OH molecules(3.99%).After about 200℃,the weight loss was rapid,indicating the decomposition of organic ligands and the collapse of the crystal structure.
2.3 Magnetic property of cluster 1
In the temperature range of 300-2 K,and under a magnetic field of 0.1 T,variable-temperature dc magnetic susceptibility measurement of cluster 1 was performed.The χMT vs T plot is shown in Fig.4.The observed χMT value of cluster 1 at room temperature was 31.51 cm3·mol-1·K,which is consistent with the theoretical value of 31.52 cm3·mol-1·K for four free Gd (Ⅲ) ions(8S7/2,g=2).Upon cooling,the χMT value remained almost unchanged between 300 and 25 K.As the temperature further decreased,the χMT value of 1 quickly decreased and reached a minimum value of 23.79 cm3·mol-1·K.The decreasing curve trend of 1 indicates the presence of an antiferromagnetic interaction between the adjacent Gd(Ⅲ)ions in 1[37].As shown in Fig.S4,the magnetic susceptibility of 1 could be fitted by the Curie-Weiss law: χMT=(T- θ)/C(θ is the Weiss constant,and C is the Curie constant),and the two parameters were obtained:C=31.54 cm3·mol-1·K,θ=-0.88 K(R2=0.999 67).The θ parameter of 1 was negative and small,which further suggests the occurrence of weak antiferromagnetic coupling between the adjacent Gd(Ⅲ)ions in 1[38].
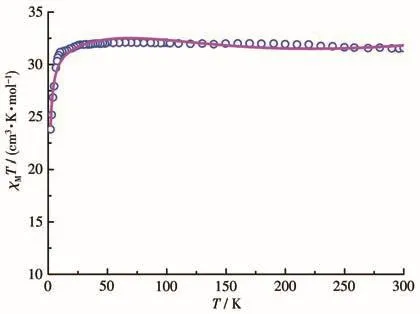
Fig.4 Temperature dependence of χMT at 1.0 kOe for cluster 1
To further explore the magnetic interaction between the neighboring Gd(Ⅲ)ions of cluster 1,the experimental χMT vs T plot was fitted using the Hamiltonian(Eq.1)based on the model of Fig.5[39].The data fitted gave three significant parameters:g=2.03,J1=-0.07 cm-1and J2=-0.04 cm-1.A low and negative J value implies weak antiferromagnetic exchange between the adjacent Gd(Ⅲ)ions in 1[40].
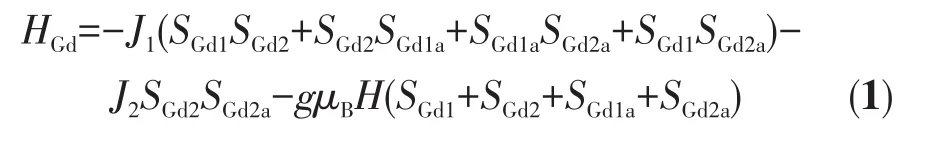
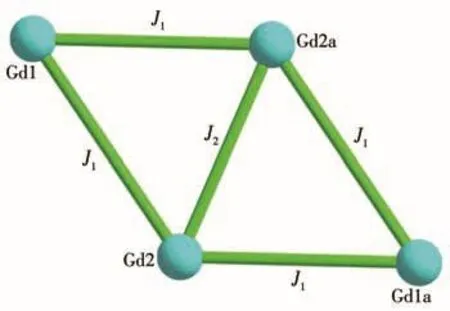
Fig.5 Magnetic coupling model of Gd(Ⅲ)ions in cluster 1
The field dependence of the magnetization for cluster 1 was studied in the temperature range of 2.0-10.0 K and magnetic field range of 0-70 kOe(Fig.6a).The M of cluster 1 was 28.1Nβ at 2.0 K which was consistent with the saturation value of 28Nβ.Due to the large isotropy of Gd(Ⅲ)ions and small Mr/NGdratio[41],the magnetocaloric effect of cluster 1 was investigated.The magnetic entropy change-ΔSmof cluster 1 can be calculated using the Maxwell equation:ΔSm(T)=∫[∂M(T,H)/∂T]HdH(2)[42].The-ΔSmvs T plots are shown in Fig.6b.The experimental- ΔSmof 40.6 J·kg-1·K-1(T=2.0 K,and ΔH=7.0 T)was smaller than the theoretical value of 43.1 J·kg-1·K-1(based on the equation:4Rln(2S+1))for four free Gd(Ⅲ)ions,which may be due to the antiferromagnetic interactions between Gd(Ⅲ)ions in cluster 1[43-47].Compared with the recently reported Gd4compounds,it is worth mentioning that the-ΔSmof 1 was much larger than those of mostly reported Gd4compounds(Table S3).Therefore,cluster 1 is a possible magnetic refrigerant to be used in practice.
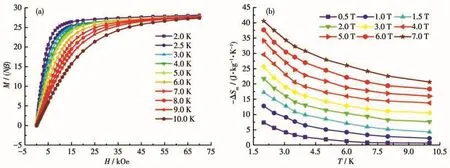
Fig.6 (a)M vs H plots for 1 at T=2.0-10.0 K and H=0-70 kOe;(b)Plots of-ΔSmvs T for cluster 1
2.4 Bacteriostatic activity of cluster 1
To explore the biological activity of cluster 1,the bacteriostatic activity of circles of H6L,Gd(NO3)3·6H2O,and cluster 1 were determined.With a concentration of 10-2mol·L-1for H6L,Gd(NO3)3·6H2O,and cluster 1,and a concentration of 1 mmol·L-1for bacterial suspension,the bacteriostatic activity results are shown in Fig.7.For H6L and Gd(NO3)3·6H2O,there were almost no bacteriostatic effects,however,the diameters of bacteriostatic circles of cluster 1 for E.coli,S.aureus,S.albus,B.subtilis,and M.luteus were 18.25,17.89,21.66,19.12,and 22.46 mm,respectively.It proves that cluster 1 possesses a remarkable bacteriostatic effect on common bacteria.By comparison,cluster 1 had the best bacteriostatic effect on M.luteus.
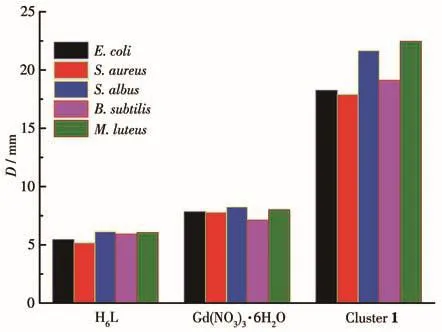
Fig.7 Diameters of bacteriostatic circles of H6L,Gd(NO3)3·6H2O,and cluster 1
To further study the bacteriostatic effect of cluster 1,the bacteriostatic activity of cluster 1 at various concentrations has been measured(Fig.8).Cluster 1 showed similar bacteriostatic effects on the five bacteria,and with the increase of the concentration,the bacteriostatic activity increased gradually.According to the above results,the bacteriostatic effect of cluster 1 is neither from the ligand nor Gadolinium(Ⅲ)ion,but it is probably from the synergistic effect of the ligand with Gd(Ⅲ)ion.After the Gd(Ⅲ)ion reacts with the ligand to form the Gd(Ⅲ)-based complex,the bacteriostatic effect on bacteria is greatly enhanced.
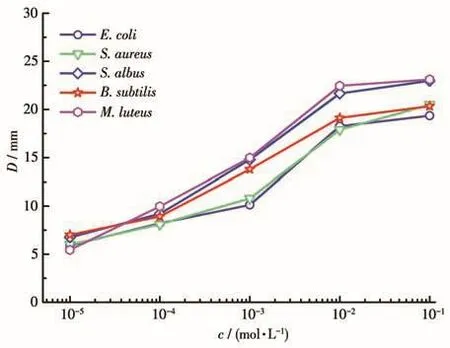
Fig.8 Diameters of bacteriostatic circles of cluster 1 for the five common bacteria
3 Conclusions
In summary,we designed and successfully synthesized a novel Gd4cluster[Gd4(CH3COO)6(H3L)2]·2CH3OH(1)via the solvothermal method by using a polydentate ligand H6L.Structural analysis shows that cluster 1 mainly contains a butterfly Gd4core and includes four Gd(Ⅲ)ions,six CH3COO-ions,and two deprotonated H3L3-ions.Magnetic study reveals that cluster 1 displayed significant cryogenic magnetic refrigeration property with a large-ΔSm:-ΔSm=40.6 J·kg-1·K-1at T=2.0 K and ΔH=7.0 T.Meanwhile,cluster 1 possesses remarkable antibacterial activity.The results of this work afford a way to design and construct polynuclear Gd(Ⅲ)-based clusters as multifunctional materials.Other property studies of polynuclear Ln(Ⅲ)-based clusters are currently under investigation in our group.
Supporting information is available at http://www.wjhxxb.cn
———理学院

