一系列基于混合配体策略构筑的金属有机骨架的合成、结构及性质
曹阳政 潘 伟 周川江 张俊勇*, 徐 昊宫春华 徐慧婷 沈润溥 刘遂军 谢景力*,,,
(1嘉兴学院生物与化学工程学院,嘉兴 314001)
(2嘉兴南湖学院新材料工程学院,嘉兴 314001)
(3绍兴文理学院脂溶性维生素浙江省工程研究中心,绍兴 312000)
(4江西理工大学化学化工学院,赣州 341000)
0 Introduction
Metal-organic frameworks(MOFs)are ordered porous solids in which inorganic building units are joined together by organic linkers[1-2].This type of materials could exhibit permanent porosity and they have attracted considerable interest due to their structural diversity and intrinsic variety of topologies[3].Moreover,these materials have diverse applications in catalysis[4-6],gas storage and capture[7-8],chemical sensors[9-10],magnetism[11-13],and luminescence[14-18].
Recently,by exploiting the mixed-ligand synthetic strategy,various types of MOFs with fascinating structures and interesting properties have been constructed,especially in the acid-base system.Intriguingly,those acid and base ligands could compensate for charge balance,coordination deficiency,repulsive vacuum,and weak interaction simultaneously[19-21].For example,Hu et al.synthesized two novel Mn-MOFs by a mixed-ligand strategy in solvothermal conditions and explored the relationships between the magnetic properties and magnetic structures of MOFs[22].Du et al.have constructed an N-rich metal-organic framework with four kinds of cages through the mixed-ligand strategy,and the MOF could selectively absorb cationic dyes in which the faded solutions can be further used for the detection of methanol and other small alcohol molecules[23].
In this contribution,based on the mixed-ligand strategy,we designed a rational organic base ligand,2,6-bis(4-pyridyl methylidene)cyclohexanone(BPCH),and selected three auxiliary acid ligands:terephthalic acid(H2TP),isophthalic acid(H2IP),and 1,3,5-benzenetricarboxylic acid(H3TMA)(Scheme 1)to react with transition metal ions.MOFs 1-7,namely{[Zn2(TP)2(BPCH)]·2DMF·4H2O}n(1),{[Cd2(TP)2(BPCH)2]·DMF·H2O}n(2),{[Cd(IP)(BPCH)]·DMF·2H2O}n(3),{[Co(HTMA)(BPCH)]·DMF·H2O}n(4),{[Cd(HTMA)(BPCH)]·DMF·H2O}n(5),{[Cu(HTMA)(BPCH)]·DMF·H2O}n(6)and{[Mn(HTMA)(BPCH)]·DMF·H2O}n(7),have been synthesized successfully.Notably,MOFs 1-3 exhibit different 3D structures,while MOFs 4-7 are isomorphic crystalline complexes with 2D structures.All complexes have been characterized by single-crystal X-ray diffraction,IR,powder X-ray diffraction(PXRD),and thermogravimetric analysis(TGA).Investigation of the fluorescence properties of MOFs materials has indicated those complexes had selectivity towards Fe3+ion.In addition,several complexes could adsorb dye molecules.
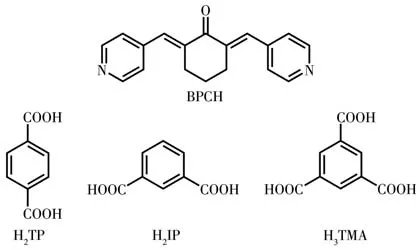
Scheme 1 BPCH(base)ligand and different auxiliary ligands(acid)used in this work
1 Experimental
1.1 Materials and instrumentations
All the chemicals were reagent grade and used as received.The IR spectra were obtained on a Varian 640 FT/IR spectrometer as a KBr pellet in a 4 000-400 cm-1region.PXRD was performed on an XRD-7000 diffractometer equipped with a Cu-sealed tube(Cu Kα,λ=0.154 178 nm)with 40 kV and 30 mA at room temperature(2θ=5°-50°).Thermalgravimetric analyses(TGA)were carried out in flowing N2on an SDT 2960 differential thermal analyzer.The fluorescence emission spectra of the solid and liquid were recorded on an F-7000 fluorescence spectrophotometer.
1.2 Synthesis of BPCH
A mixture of cyclohexanone(2.45 g,25 mmol)and 4-pyridinecarboxaldehyde(5.36 g,50 mmol)was dissolved in 100 mL of water,and 15 mL of 6 mol·L-1KOH solution was added dropwise.The mixture was then stirred at room temperature for ten hours and neutralized to a pH value of 7 by using dilute hydrochloric acid.The resulting yellow solid was then filtered out and recrystallized in ethanol to give purified BPCH(Fig.S1,Supporting information).IR(KBr powder,cm-1):3 444(w),3 021(w),2 945(w),2 838(w),1 668(m),1 593(s),1 578(s),1 414(s),1 276(s),1 172(s),995(m),818(s)(Fig.S2a).
1.3 Synthesis of MOFs 1-7
A mixture of BPCH(0.013 8 g,0.05 mmol),Zn(NO3)2·6H2O(0.014 9 g,0.05 mmol),H2TP(0.008 3 g,0.05 mmol),DMF(4 mL),H2O(2 mL),C2H5OH(1 mL)was placed in a 10 mL glass vial and under ultrasonic treatment for 30 min.The bottle was then placed in an oven and heated at 80℃for 48 h.Light yellow transparent crystals of MOF 1 were formed and isolated.Anal.Calcd.for C34H24N2O9Zn2·2DMF·4H2O(%):C,50.38;H,4.86;N,5.88.Found(%):C,49.52;H,4.08;N,5.71.IR(KBr powder,cm-1):3 420(m),2 931(w),1 638(s),1 610(s),1 530(s),1 389(vs),1 273(m),1 222(m),1 165(m),825(s),748(s)(Fig.S2b).
MOFs 2-7 were obtained by using a similar procedure as for 1 except for metal salt or acid ligand(Table 1).The transparent crystals of 2-7 were isolated from the mixture respectively.Anal.Calcd.for 2(C55H49Cd2N5O12,%):C,55.20;H,4.13;N,5.85.Found(%):C,54.84;H,4.16;N,6.68;Anal.Calcd.for 3(C29H25CdN3O8,%):C,53.10;H,3.84;N,6.41.Found(%):C,53.32;H,4.10;N,6.45;Anal.Calcd.for 4(C30H29CoN3O9,%):C,56.79;H,4.61;N,6.62.Found(%):C,56.43;H,4.50;N,6.76;Anal.Calcd.for 5(C30H29CdN3O9,%):C,52.37;H,4.25;N,6.11.Found(%):C,51.80;H,3.82;N,5.61;Anal.Calcd.for 6(C30H29CuN3O9,%):C,56.38;H,4.57;N,6.57.Found(%):C,54.45;H,4.24;N,6.28;Anal.Calcd.for 7(C30H29MnN3O9,%):C,57.15;H,4.64;N,6.66.Found(%):C,56.71;H,4.52;N,6.79.IR(KBr powder,cm-1):2,3 416(m),2 922(w),1 676(m),1 576(vs),1 500(m),1 369(s),1 275(m),1 146(m),745(s)(Fig.S2c);3,3 408(s),2 950(w),1 670(m),1 601(s),1 560(s),1 424(s),1 383(s),1 275(m),1 246(m),1 170(m),1 145(m),820(m),734(m)(Fig.S2d);4,3 406(m),3 075(w),2 948(m),1 714(s),1 673(s),1 620(s),1 608(s),1 583(s),1 436(s),1 383(vs),1 266(s),1 165(s),758(s),723(s)(Fig.S2e);5,3 424(w),3 081(w),2 923(w),1 674(m),1 615(s),1 576(s),1 479(m),1 425(s),1 377(s),1 276(m),1 166(s),1 141(s),815(m),779(s),697(m)(Fig.S2f);6,3 416(m),3 047(w),2922(w),1710(s),1669(s),1623(s),1611(s),1562(s),1 498(w),1 428(s),1 359(s),1 251(s),1 221(w),1 166(m),840(m),757(m),722(m),680(m)(Fig.S2g);7,3 405(m),2 943(w),1 713(m),1 675(s),1 618(s),1 606(s),1 581(s),1 551(m),1 437(s),1 380(s),1 265(m),1 165(m),1 146(m),1 100(m),759(m),722(s),682(m)(Fig.S2h).
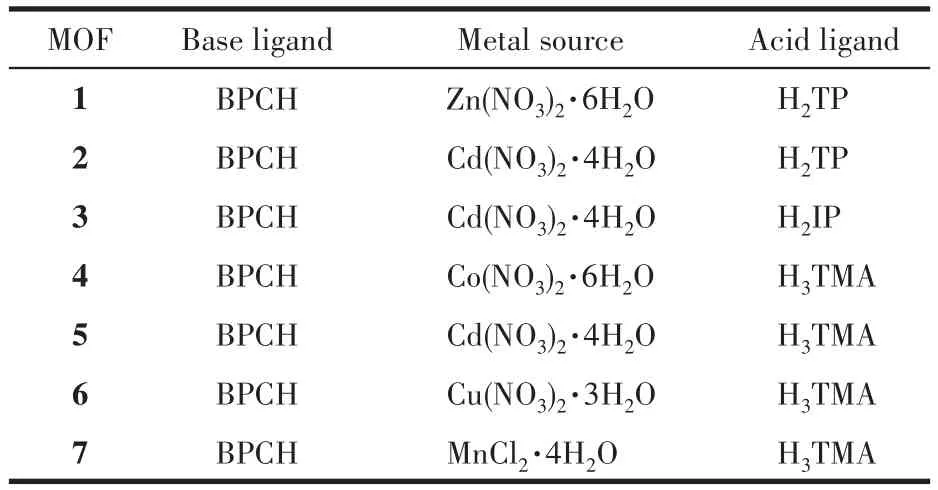
Table 1 Reactants for MOFs 1-7
1.4 X-ray crystallography
Suitable single crystals of BPCH and MOFs 1-7 were selected and X-ray diffraction analysis data were collected on an Oxford Diffraction Gemini R Ultra diffractometer with graphite-monochromated Cu Kα (λ=0.154 184 nm)or Mo Kα (λ=0.071 073 nm).Structures were solved by direct methods and refined on F2by full-matrix least-squares methods using the SHELXTL package[24].Crystal data and structure refinements of BPCH and MOFs 1-7 are summarized in Table S1.Selected bond lengths and angles are listed in Table S2.
2 Results and discussion
2.1 Structural characterization of MOF 1
X-ray single crystal diffraction analysis reveals that MOF 1 crystallizes in the monoclinic crystal system with the C2/c space group.The asymmetric unit of MOF 1 contains two crystallographically independent Zn(Ⅱ)ions,one BPCH molecule,and two TP2-anions(Fig.1a).The two Zn(Ⅱ)ions are five-coordinated with oxygen atoms(O2,O4A,O6,O8A and O3,O5A,O7,O9A)from four TP2-anions and nitrogen atoms(N2A and N1)from one BPCH molecule(Fig.1b).Additionally,the adjacent Zn1 and Zn2 ions give rise to a[Zn2(CO2)4]secondary building unit(SBU1)established by four bridging carboxylate oxygen atoms of four TP2-anions,and the Zn—O and Zn—N bond distances are in the ranges of 0.202 0(2)-0.206 6(2)nm and 0.202 7(2)-0.203 3(2)nm,respectively(Table S2).The binuclear SBU1 units are connected by the carboxylates to compose a 2D layer structure,which is further linked through BPCH molecules to achieve a 3D framework(Fig.1c).The topological analysis indicates that MOF 1 has two interpenetrating nets with Schläfli symbol{812.123}{8}3rationalized by TOPS 4.0(Fig.1d).The porosity is 39.8% and the total solvent-accessible volume is 3.522 nm3,which is estimated by PLATON[25].
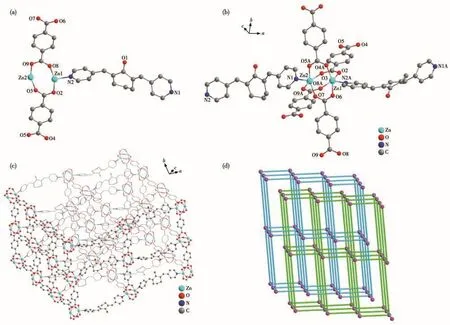
Fig.1 Structure of MOF 1:(a)asymmetric unit;(b)coordination environment of Zn2+ion;(c)3D structure;(d)topology
2.2 Structural characterization of MOF 2
MOF 2 crystallizes in the triclinic crystal system with the Pspace group.The asymmetric unit of MOF 2 contains two crystallographically independent Cd(Ⅱ)ions,two BPCH molecules,and two TP2-anions(Fig.2a).Those two Cd(Ⅱ)ions are all six-coordinated with four oxygen atoms(O2,O4A,O8A,O9A,and O3,O5A,O6,O7)from three TP2-anions and nitrogen atoms(N1A,N2 and N3,N4A)from two BPCH molecules(Fig.2b).Additionally,the adjacent Cd1 and Cd2 ions give rise to a[Cd2(CO2)4]secondary building unit(SBU2)established by four chelating-bridging and four bridging carboxylate oxygen atoms of four TP2-anions,and the Cd—O and Cd—N bond distances are in the ranges of 0.220 1(1)-0.246 8(8)nm and 0.232 5(2)-0.237 1(2)nm,respectively(Table S2).The binuclear SBU2 units are connected by the carboxylates to compose a 2D layer structure,which is further linked through BPCH ligands to form a 3D framework(Fig.2c).The topological analysis indicates that MOF 2 is a pcu topology with two interpenetrating nets and the Schläfli symbol is{412}{63}rationalized by TOPS 4.0(Fig.2d).The porosity is 7.7% and the total solvent-accessible volume is 0.213 nm3,which is estimated by PLATON.
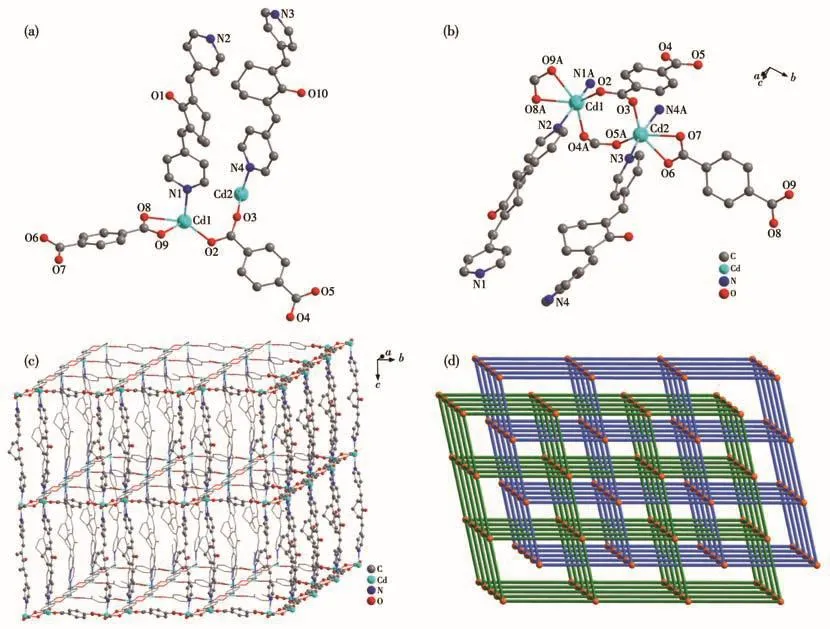
Fig.2 Structure of MOF 2:(a)asymmetric unit;(b)coordination environment of Cd2+ion;(c)3D structure;(d)topology
2.3 Structural characterization of MOF 3
MOF 3 crystallizes in the monoclinic crystal system with the C2/c space group.The asymmetric unit of MOF 3 contains one crystallographically independent Cd(Ⅱ)ion,one BPCH molecule,and one IP2-anion(Fig.3a).The coordination mode of the Cd(Ⅱ)ion is the same as Cd(Ⅱ)ion in MOF 2 except TP2-anions replaced by IP2-anions(Fig.3b).Additionally,the two adjacent Cd1 ions give rise to a[Cd2(CO2)4]secondary building unit(SBU3)established by four chelating-bridging and four bridging carboxylate oxygen atoms of four IP2-anions,and the Cd—O and Cd—N bond distances are in the ranges of 0.226 9(3)-0.239 4(3)nm and 0.232 0(4)-0.232 8(4)nm,respectively(Table S2).The binuclear SBU3 units are connected by the carboxylates to compose a 2D layer structure,which is further linked through BPCH ligands to construct a 3D framework(Fig.3c).The topological analysis indicates that MOF 3 is a cds topology with Schläfli symbol{65}{8}rationalized by TOPS 4.0(Fig.3d).The porosity is 22.5% and the total solvent-accessible volume is 1.269 nm3,which is estimated by PLATON.
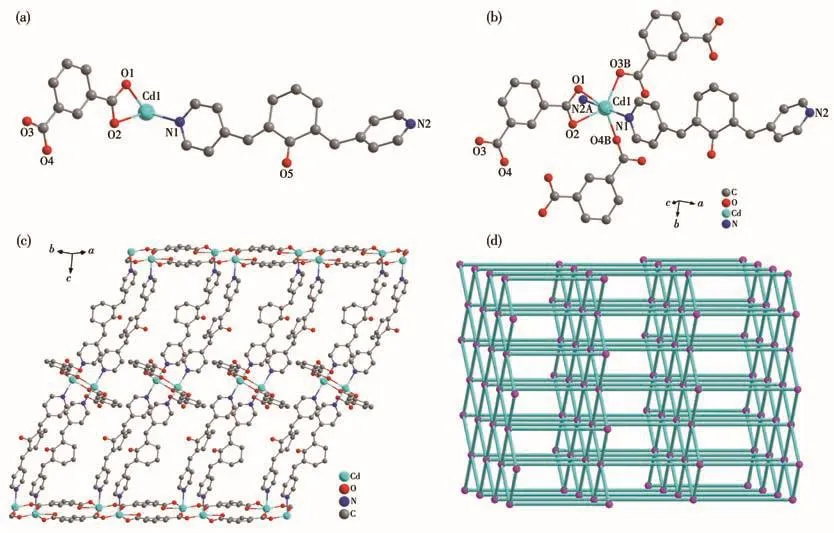
Fig.3 Structure of MOF 3:(a)asymmetric unit;(b)coordination environment of Cd2+ion;(c)3D structure;(d)topology
2.4 Structural characterization of MOFs 4-7
X-ray single crystal diffraction analysis shows that MOFs 4-7 crystallize in the triclinic crystal system with the P1 space group and they are isomorphic.Thus,the structure of MOF 6 is described as a common example.The asymmetric unit of MOF 6 contains one crystallographically independent Cu(Ⅱ)ion,one BPCH molecule,and one HTMA2-anion(Fig.4a).The Cu(Ⅱ)ion is six-coordinated with four oxygen atoms(O1,O2B,O3A,O4A)from three HTMA2-anions and two nitrogen atoms(N1,N2A)from two BPCH molecules(Fig.4b).In addition,the two adjacent Cu1 ions form a[Cu2(CO2)4]secondary building unit(SBU4)established by four chelating-bridging and four bridging carboxylate oxygen atoms of four HTMA2-ligands,and the Cu—O and Cu—N bond distances are in the ranges of 0.197 7(4)-0.219 8(4)nm and 0.200 1(5)-0.200 7(5)nm,respectively(Table S2).The binuclear SBU4 units are connected by the carboxylates and BPCH molecules to form a 2D layer structure(Fig.4c).The topological analysis indicates that MOF 6 is a sql topology with Schläfli symbol{44}{62}rationalized by TOPS 4.0(Fig.4d).
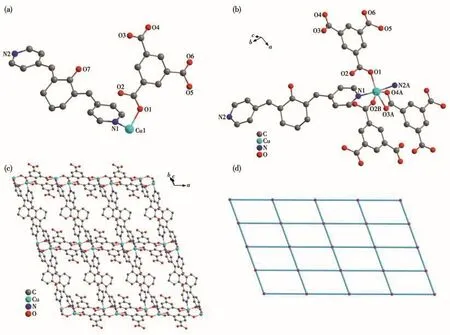
Fig.4 Structure of MOF 6:(a)asymmetric unit;(b)coordination environment of Cu2+ion;(c)2D structure;(d)topology
2.5 PXRD patterns and TGA
Fig.S3 displays the simulated PXRD results and the experimental patterns of BPCH and MOFs 1-7.The main peak positions of the experimental results were consistent with their simulated patterns,demonstrating the high single-phase purity of those crystalline products.
As shown in Fig.S4,TGA curves of MOFs 1-7 showed two major steps of weight loss.In this series of MOFs,the first step of weight loss should be attributed to the removal of guest solvent molecules(DMF and H2O).Then,MOFs 1-7 began to collapse at 379,354,346,291,273,241,and 242℃,respectively,demonstrating their certain thermal stability.
2.6 Fluorescence behaviors
MOFs constructed by d10metal ions and conjugated organic ligands could have fluorescence performance[26-28].Since MOFs 1-7 contain d10metal ions(Zn(Ⅱ) ,Cd(Ⅱ))and π -conjugated aromatic ligands(BPCH,TP2-,IP2-,HTMA2-),the fluorescence properties of MOFs 1-7 were examined in DMF solution at room temperature.As displayed in Fig.S5,the emission bands were observed at 463 nm(λex=274 nm)for BPCH,373 nm(λex=276 nm),415 nm(λex=274 nm),403 nm(λex=275 nm),435 nm(λex=274 nm),410 nm(λex=275 nm),373 nm(λex=275 nm),321 nm(λex=291 nm)for MOFs 1-7 respectively.
The fluorescence detection experiments of various metal ions were carried out in EtOH suspension.The samples of MOFs 1-7 were fully milled and immersed in EtOH solution,then the mixture was under ultrasonic treatment for about one hour to receive a stable suspension.In order to explore the fluorescence sensing behavior of MOFs 1-7 towards metal ions,6µL 0.1 mol·L-1different metal ions solution were added in 2 mL EtOH suspension of 3 mL quartz cuvette(metal ions:Li+,Na+,K+,Ag+,Mg2+,Ca2+,Co2+,Ni2+,Zn2+,Cd2+,Al3+,Fe3+,Nd3+,Eu3+,Gd3+,Dy3+and Er3+,0.3 mmol·L-1).As illustrated in Fig.5 and S6,after the addition of different metal ions,most of the ions performed a relatively weak effect on the fluorescence emission intensity of MOFs 1-7,whereas the Fe3+ion displayed a fluorescence quenching effect.The results demonstrate that MOFs 1-7 have certain selectivity toward Fe3+ions.
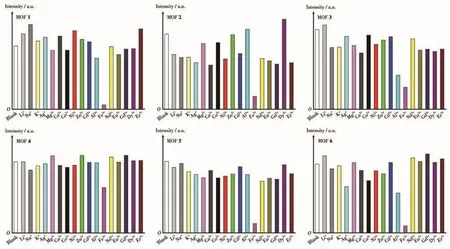
Fig.5 Fluorescence relative intensities of MOFs 1-6 in EtOH suspension with different metal ions
To further explore the selectivity towards Fe3+,using MOF 6 as the representative complex,competition experiments have been done in the presence of other metal ions(Fig.6a).These results demonstrated that MOF 6 had certain sensitivity for Fe3+ion,and its fluorescence sensing was not affected by the coexisting metal ion.To evaluate the detection limit of MOF 6 towards Fe3+,the fluorescence spectra were recorded by gradually adding the Fe3+ion with the concentration range from 0 to 1.9×10-8mol·L-1(Fig.S7).The emis-sion intensity of suspension decreased consecutively when increasing the Fe3+concentration.The detection limit calculated through 3σ/k(k:the slope,σ:the standard error)was about 3.02 µmol·L-1(Fig.6b).
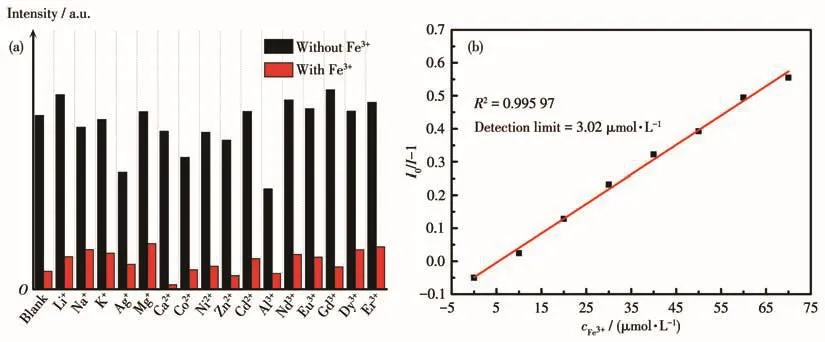
Fig.6 (a)Fluorescence intensity ratio histograms of MOF 6 dispersed in EtOH with different metal ions(black)and subsequent addition of 1 Equiv.of Fe3+ions(red);(b)Linear relationship between I0/I-1 andcFe3+for MOF 6
In general,the luminescence quenching caused by the Fe3+ion may associate with(1)the collapse of the crystal skeleton,(2)cation exchange,or(3)competitive absorption.As shown in Fig.7a,PXRD experimental results showed that the crystal skeleton kept its integrity during the experiment.On the other hand,neutral MOFs could achieve fluorescence sensing through cation exchange to a lesser extent.As shown in Fig.7b,the fluorescence excitation spectrum of MOF 6 overlapped with the UV-Vis absorption spectrum of Fe3+(300-400 nm),indicating that Fe3+may affect the absorption of excitation energy and consequently,resulting in the observed fluorescence quenching effect.Therefore,the mechanism of fluorescence quenching of MOFs for Fe3+ions may arise from the competitive absorption.
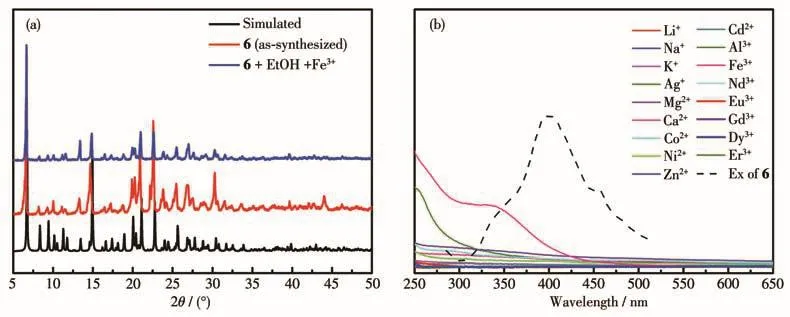
Fig.7 (a)PXRD patterns of MOF 6 before(red)and after(blue)the Fe3+-detection experiment;(b)Excitation spectra of MOF 6 and UV-Vis absorption spectra of different metal cations
2.7 Ability of MOFs 1,2,3,and 6 toadsorb dye molecules
MOF 1(5.0 mg)was added into a 10.0 mL aqueous solution of parafuchsin hydrochloride(PH,10µmol·L-1),methylene blue(MB,10 µmol·L-1),rhodamine B(RhB,40 µmol·L-1),rhodamine 6G(R6G,10µmol·L-1),methyl orange(MO,10 µmol·L-1),respectively and the solution was magnetically stirred for 14 h at room temperature,then the solution was centrifugated and analyzed by UV-Vis spectrum(Fig.8).MOFs 2,3,and 6 all followed the same procedure as MOF 1(Fig.S8-S11).
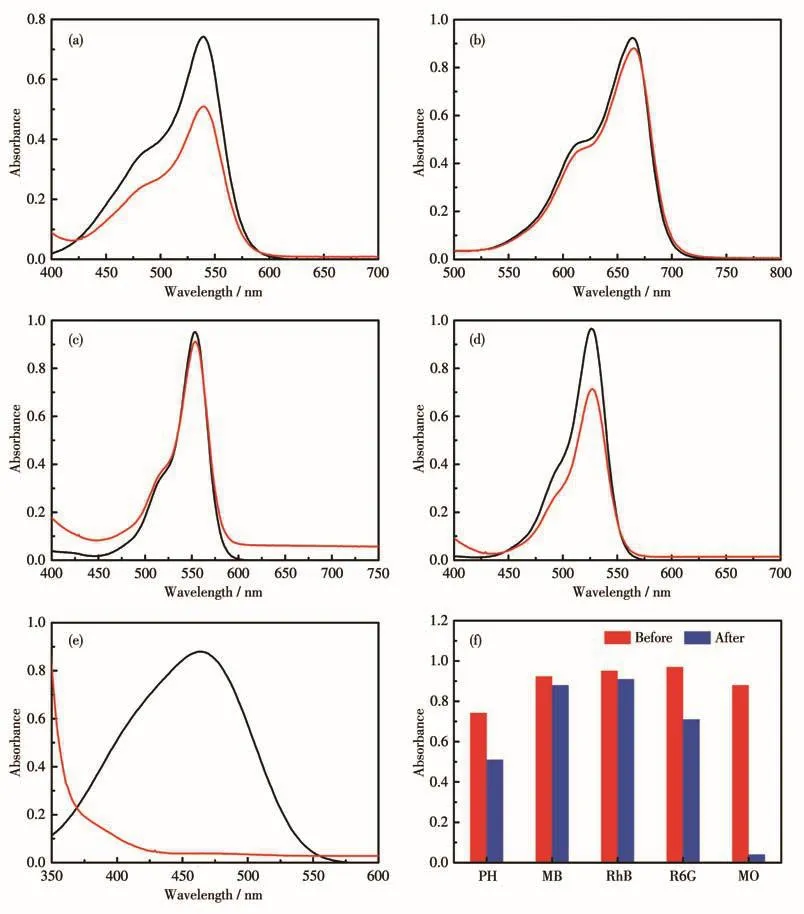
Fig.8 UV-Vis spectra of dye molecules during the adsorbing process of 1(14 h):(a)PH,(b)MB,(c)RhB,(d)R6G,and(e)MO;(f)Comparison of MOF 1 for five dyes adsorbing
The results show that MOFs 1,2,3,and 6 had certain adsorbing activity for MO,while MOF 3 also had an effect on RhB and MOF 6 had an effect on MB.The amount of uptaken dyes was derived from the equation:Qad=(c0-cad)V/m,where Qad(mmol·g-1)is the amount of uptaken dyes by MOF,c0is the initial concentration of dye in aqueous solution(mmol·L-1),cadis the concentration of dye after the uptake process(mmol·L-1),m is the mass of MOF(g),and V is the volume of the solution(L).Therefore,it was calculated that 1 g MOFs 1,2,3,and 6 could absorb 0.095 6,0.095 8,0.092 0,and 0.095 9 mmol MO respectively;1 g MOF 3 could absorb 0.019 7 mmol RhB;1 g MOF 6 could absorb 0.008 1 mmol MB.
3 Conclusions
In summary,taking advantage of a basic ligand and three kinds of acid ligand,seven MOFs have been obtained feasiblely.MOFs 1-7 display relatively good thermal and chemical stability.Fluorescence measurements indicate that 1-7 displayed high selectivity toward Fe3+ions.More strikingly,those MOFs showed certain activity for adsorbing some organic dyes,indicating they have the potential to be used as functional materials.Recently,our group has worked on MOFs by using new ligands such as chalcone dicarboxylic acid ligand and the key step in controlling structural characters involves combining different lanthanide or transition metals with a single ligand[29-31].Apparently,by using the mixed-ligand synthetic strategy,various MOF materials could be achieved systematically and then set the stage for the application of multifunctional materials.Moreover,by the impressive abilities such as high effective mass-and heat-transfer of the emergent technique-flow chemistry[32-35],diverse MOFs could be fabricated with high yields by finely controlling reaction rates,speeding up the experimental process.Compared with traditional batch-type synthetic strategy(hydrothermal or solvothermal methods),flow chemistry has a high surface area-to-volume ratio and as a consequence,it could achieve MOFs materials efficiently and cost-effectively,control the size of the target molecules in a time-efficient manner and meanwhile,may significantly improve their physical properties.For example,the catalytic performance could be improved dramatically.We are actively utilizing this technique to achieve new crystalline MOF materials.
Supporting information is available at http://www.wjhxxb.cn

