Potential for sodium-glucose cotransporter-2 inhibitors in the management of metabolic syndrome: A systematic review and meta-analysis
Abdulbaril Olagunju,Naser Yamani,Dorothy Kenny,Martina Mookadam,Farouk Mookadam, Samuel Unzek
Abstract
BACKGROUND
Landmark trials have established the benefits of sodium-glucose cotransporter-2 inhibitors (SGLT2-Is) in cardiovascular disease including heart failure with reduced and preserved ejection fraction and renal diseases regardless of the presence of diabetes mellitus. However, studies evaluating the role of SGLT2-Is in metabolic syndrome (MetS) are limited.
AIM
This study primarily aimed to evaluate the impact of SGLT2-Is on the components of MetS.
METHODS
Two independent reviewers and an experienced librarian searched Medline,Scopus and the Cochrane central from inception to December 9, 2021 to identify placebo controlled randomized controlled trials that evaluated the impact of SGLT2-Is on the components of MetS as an endpoint. Pre- and post-treatment data of each component were obtained. A meta-analysis was performed using the RevMan (version 5.3; Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration).
RESULTS
Treatment with SGLT2-Is resulted in a decrease in fasting plasma glucose (–18.07 mg/dL; 95%CI: -25.32 to –10.82), systolic blood pressure (–1.37 mmHg; 95%CI: -2.08 to –0.65), and waist circumference (–1.28 cm; 95%CI: -1.39 to –1.18) compared to placebo. The impact on highdensity lipoprotein cholesterol was similar to placebo (0.01 mg/dL; 95%CI: -0.05 to 0.07).
CONCLUSION
SGLT2-Is have a promising role in the management of MetS.
Key Words: Metabolic syndrome; Sodium-glucose cotransporter 2 inhibitors; Dapagliflozin; Empagliflozin;Cardiovascular disease
INTRODUCTION
Sodium-glucose cotransporter-2 inhibitors (SGLT2-Is) are a relatively novel and revolutionary class of medications that reduce the reabsorption of glucose from the proximal tubules in the kidneys[1-4]. Their glycosuric effect led to their initial use in the management of patients with type 2 diabetes mellitus (DM)[1-4]. However, recent large, randomized control trials (RCTs) have highlighted the extension of their benefits to cardiovascular diseases (CVD) including heart failure with reduced and preserved ejection fraction and renal diseases regardless of the presence of DM[5-15]. However, to date, studies on the impact of SGLT2-Is in the management of metabolic syndrome (MetS) and its components remain inadequate. Metabolic syndrome is an emerging pandemic[16-19]. Its prevalence has risen from approximately 25% to 38% between the early 1990s to 2010s in the United States[16-19]. The prevalence has increased by 29.1% in people aged 40-60 years[16-19]. It has been defined according to the National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III as the presence of 3 of 5 entities: (1) Waist circumference (WC) ≥ 102 cm in men and ≥ 88 cm in females; (2) Serum triglycerides(TGL) ≥ 150 mg/dL or on drug treatment for hypertriglyceridemia; (3) Serum high-density lipoprotein(HDL) cholesterol < 40 mg/dL in males and < 50 mg/dL; (4) Blood pressure (BP) ≥ 130/85 mmHg or on drug treatment for hypertension (HTN); and (5) Fasting plasma glucose (FPG) ≥ 100 mg/dL or on drug treatment for elevated blood glucose[20]. A growing body of evidence exists supporting the association of MetS with the development and progression of CVD[17-20]. In a meta-analysis by Mottilloet al[19] a 2-fold increase in the risk of CVD and CV mortality in patients with MetS was noted. DM is a component of the MetS and affords a 2-4-fold increase in CVD Risk[21]. Hence, there is an urgent need to improve the management of MetS, which currently ranges from lifestyle interventions such as physical activity and caloric restriction through dietary modification to pharmacological and surgical approaches that address components of the MetS[4]. The primary aim of this study is to evaluate the impact of the SGLT2-Is on the MetS parameters noted in NCEP ATP III criteria. The secondary aim is to highlight the effect of SGLT2-Is on other cardiometabolic parameters including hemoglobin A1C(HbA1c), body weight (BW) and uric acid (UA). This study is derived from placebo controlled RCTs that have evaluated the impact of these medications on CVD and its risk factors, as well as reported pre/post treatment values of MetS components.
MATERIALS AND METHODS
Data sources and searches
Two authors independently searched the electronic library database in Medline, Scopus and the Cochrane central from inception to December 9, 2021, using the following keywords: SGLT2-I,metabolic, cardiometabolic, TGL, FPG, BP, HDL, waist, abdominal, circumference, lipids, waist-toheight ratio, hypertriglyceridemia, HTN, MetS, RCT, random allocation, randomly allocated, random,and allocated randomly. Additionally, different combinations of these keywords were applied in each database search. The search was extended to ClinicalTrials.gov. An independent search was also conducted by a qualified librarian using similar search terms.
Study selection
The eligible studies were RCTs, allocated patients to an SGLT2-I group (that received either Dapagliflozin or Empagliflozin) or a placebo group, reported baseline and post-treatment values ≥ 1 component of MetS, had a treatment duration 6 mo and were published in the English language. Studies not meeting these criteria were excluded. Disagreements on study selection were either resolved by consensus or by Farouk Mookadam. The study adhered to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) reporting guideline[22] (PRISMA checklist).
Data extraction
Extracted data included duration of follow-up, sample size and dose of dapagliflozin and empagliflozin studied. Demographic and biomarker characteristics extracted at baseline and follow up included mean age, gender, race, DM, mean WC, FPG, TGL, HDL, systolic BP (SBP), diastolic BP (DBP), HbA1C, BW and UA.
Quality assessment
The methodologic quality of the RCTs was assessed using the Jadad score. Points were allocated for randomization, blinding and accountability of the study participants, with a total score range from 0 to 5[23] (Table 1).
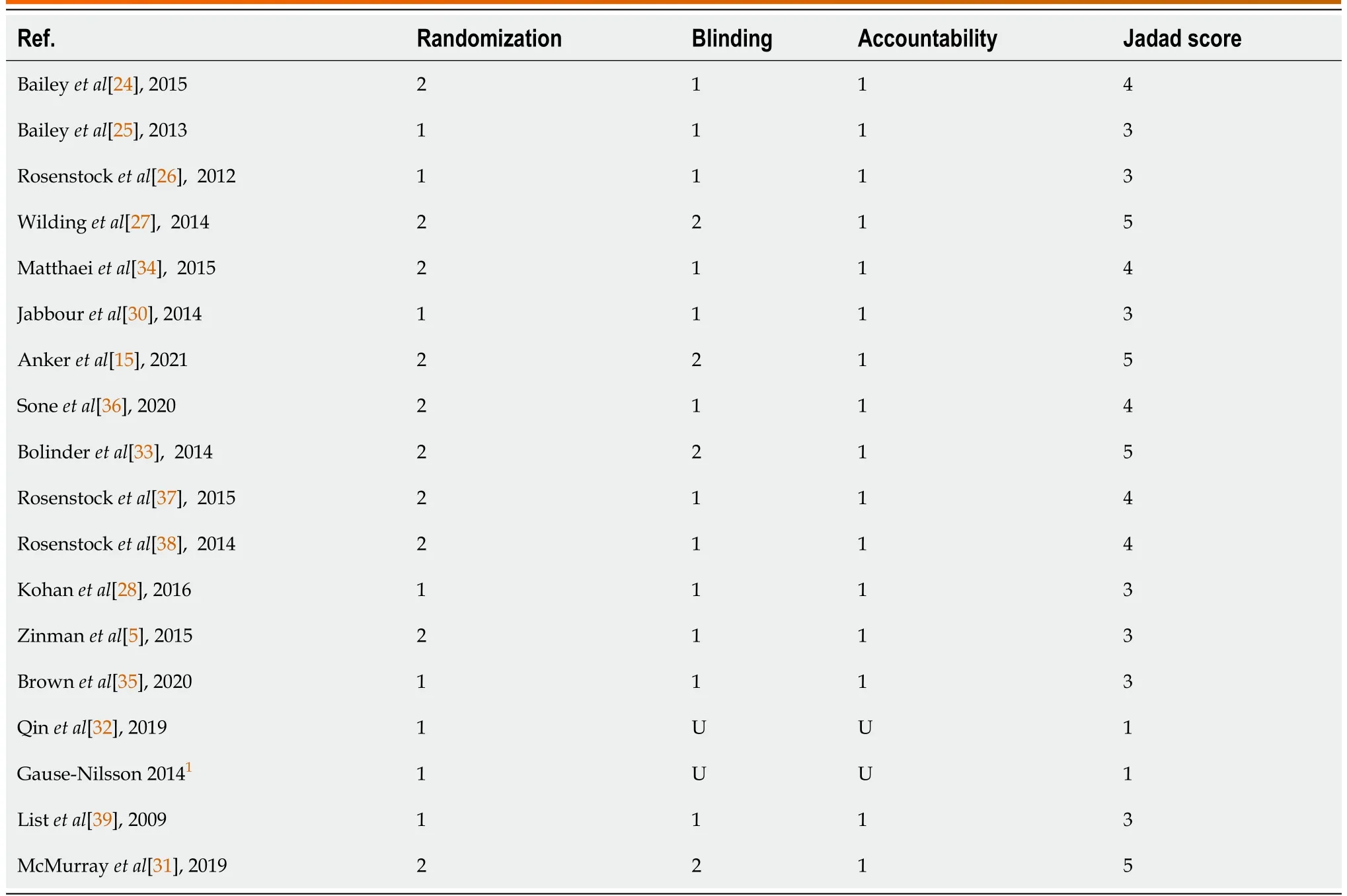
Table 1 Jadad score of included studies
Outcomes
The primary outcomes of this study are post-treatment changes in WC, FPG, TGL, HDL, and BP. The secondary outcomes are post-treatment changes in BW, HbA1C and UA.
Statistical analysis
All outcome data were reported as mean with standard deviation and were converted to conventional units. Data analysis was performed using the RevMan (version 5.3; Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration). The forest plots of the above outcomes were visually represented after pooling the mean differences using the random-effects model. Heterogeneity was assessed with theI2test. Post-hoc subgroup analyses including doses and/or SGLT2-I type were performed if there was significant heterogeneity.
RESULTS
Search results and study inclusion
The initial literature search identified a total of 2636 potentially relevant studies, 14 of which were gathered from ClinicalTrials.gov. After excluding 1042 duplicates, a total of 1594 studies were screened.Of these, 235 studies were selected for abstract and/or full text review. An additional 217 studies were excluded either because they did not meet the above inclusion criteria, precursors of long-term studies,had a cross-over design or had no published results. A total of 18 studies[5,15,24-39] were eligible for meta-analysis (Figure 1). Of these, 3 studies reported WC[30,33,36], 9 reported FPG[24-27,29,30,34-36,39], 4 reported TGL[5,30,35,38], 3 reported HDL[34,37,38], 7 reported SBP[12,15,28,29,33,35,36] and 6 reported DBP[24,26,28,33,35,39] (Table 2 and 3).

Figure 1 PRISMA flow diagram showing outcomes of databases and registers search. SGLT2-I: Sodium-glucose cotransporter 2 inhibitor; Mets:Metabolic syndrome.

Table 2 Characteristics of included studies
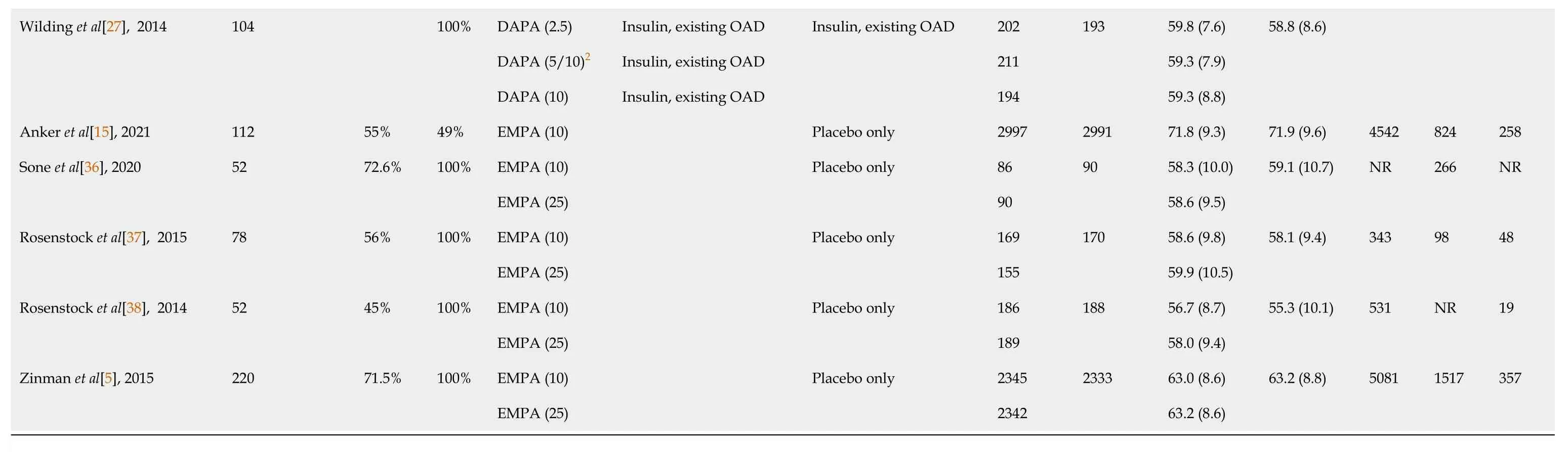
Data reported as mean (SD).1Nobaseline data reported for Gause-Nilsson2014.25 for 48 wk, 10 for 56 wk.NR: Not reported; OAD: Oral antidiabetic drugs; SGLT2-I: Sodium-glucose cotransporter 2 inhibitor; DM: Diabetes mellitus.

Table 3 Baseline values for the MetS components

15 mg for 48 wk, 10 for 56 wk.HDL: High-density lipoprotein; DBP: Diastolic bloodpressure; SBP: Systolic bloodpressure; SGLT2-I: Sodium-glucose cotransporter 2 inhibitor.
Participant characteristics
A total of 26427 patients were included in the analysis. The SGLT2-I group comprised a total of 15914 patients. Of these, 7355 patients received dapagliflozin and 8559 received empagliflozin. The placebo group comprised a total of 10513 patients (Table 2 and 3). 59.4% were men. Among studies with reported data, 75% were White, 19.9% were Asian and 4.8% were Black. The mean treatment duration was 79 wk. The mean age in the SGLT2-I group was 53.4 years, and 54.8 years in the placebo group. The vast majority (78.6%) were DM patients. The baseline and post-treatment values of MetS components and the cardiometabolic variables are presented in Tables 3 and 4.
SGLT2-Is andFPG:Nine RCTs in whicha total of 1474 patients received SGLT2-Is were analyzed. The randomeffect model demonstrated a mean reductioninFPG of –18.07 mg/dL(95%CI: -25.32 to –10.82;I2=99%) (Figure 2A). The significant heterogeneitypersisted after a subgroup analysis basedondose of SGLT2-Is (2.5 mgvs10 mg). 463participants received the 2.5mgdose whichhad a similar impact as placebo on FPG: -1.45mg/dL (95%CI: -8.73 to 5.82;I2= 71%) (Figure 2B). The 10 mg dose resulted in a higher reductioninmeanFPG of –30.02 mg/dL(95%CI: -38.97 to –21.08;I2= 87%) (Figure 2B).
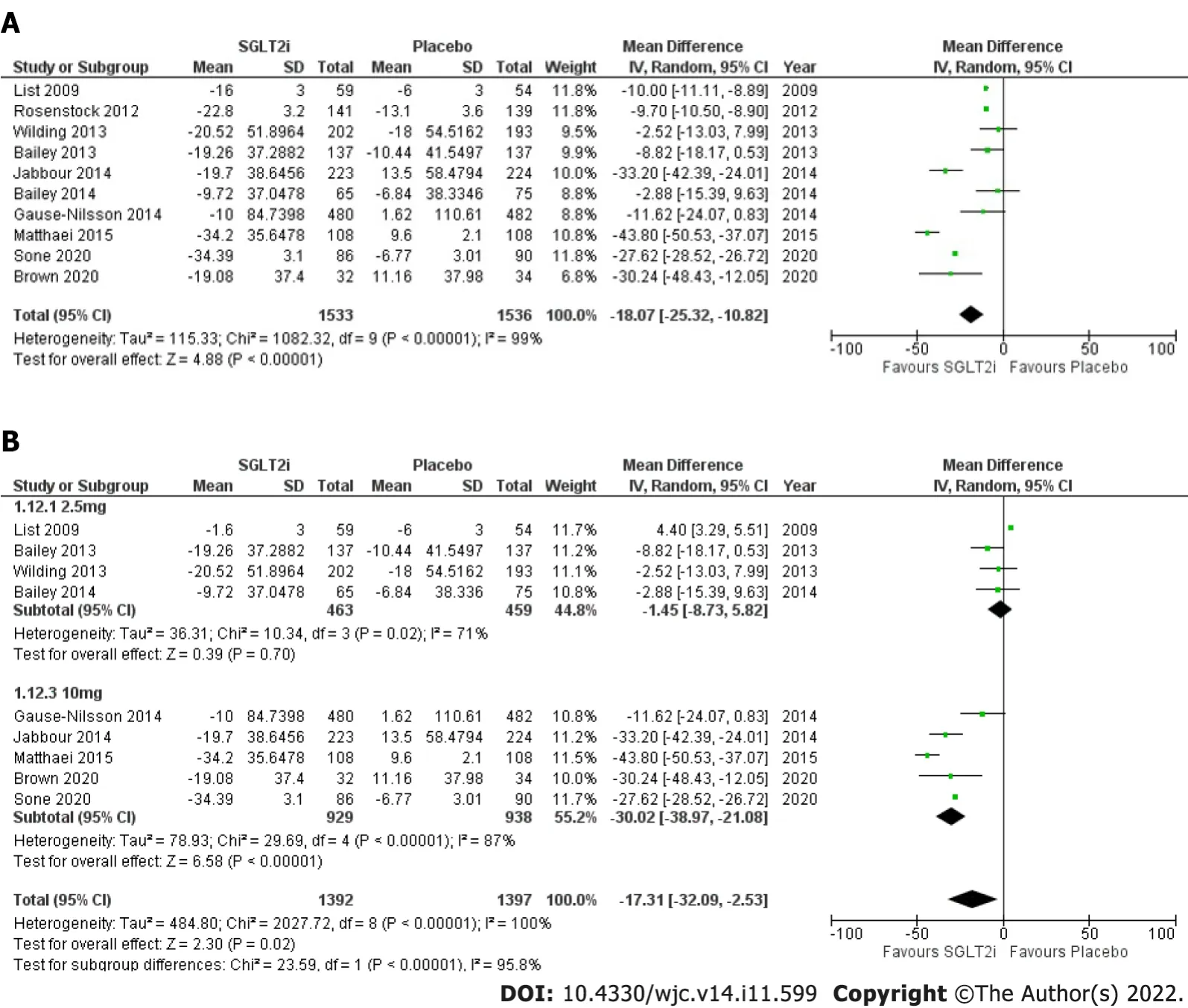
Figure 2 Forest plot. A: Highlighting impact of SGLT2-I on FPG compared to placebo; B: SGLT2-I dose subgroup analysis performed for FPG. SGLT2-I: Sodiumglucose cotransporter 2 inhibitor; FPG: Fasting plasma glucose.
SGLT2-Is andBP:The analysis for SBPincludeda total of 6662 participants from sevenRCTs. There was a modest mean reductioninSBP of –1.37mmHg(95%CI: -2.08to–0.65,I2= 85%) (Figure 3A). A subsequent post-hoc analysis basedonSGLT2-I type demonstrated theempagliflozin RCTs were responsible for the high heterogeneity. The mean reductionnoted with empagliflozinwas not statisticallysignificant: -0.70mmHg(95%CI: 1.72 to 0.32;I2= 97%). Dapagliflozinuse was associated with a higher mean SBPreduction of -2.03 mmHg (95%CI: -2.83to–1.24;I2= 8%) (Figure 3B). The analysis of 6 RCTs that comprised1018 total patients demonstrated no reductioninDBP with SGLT2-I use compared toplacebo: -0.50 mmHg (-1.76 to 0.75;I2= 97%) (Figure 4).

Figure 3 Forest plot. A: Highlighting impact of SGLT2-I on SBP compared to placebo; B: SGLT2-I Type subgroup analysis performed for SBP. SGLT2-I: Sodiumglucose cotransporter 2 inhibitor; SBP: Systolic blood pressure.
SGLT2-Is andWC:Atotal of 378patients from 3RCTs received an SGLT2-I. The random effect model highlighted ameanreduction in WC of –1.28 cm (95%CI: -1.39 to –1.18;I2= 0%) (Figure 5).

Figure 5 Forest plot highlighting impact of SGLT2-I on WC compared to placebo. SGLT2-I: Sodium-glucose cotransporter 2 inhibitor; WC: Waist circumference.
SGLT2-Is andHDL:Atotal of 1080 patients from 3RCTs were analyzed for the impact of SGLT2-Is on HDL. There was no significant difference in post-treatment HDLbetween the SGLT2-I andplacebo groups: 0.01 mg/dL(95%CI: -0.05to0.07;I2= 100%) (Figure 6).

Figure 6 Forest plot highlighting the absence of significant impact of SGLT2-I on HDL compared to placebo. SGLT2-I: Sodium-glucose cotransporter 2 inhibitor; HDL: High-density lipoprotein.
SGLT2-Is andTGL:TGLlevels pre or post treatment were notreported in all the trials. Hence this component of the MetS couldnot be analyzed in this meta-analysis.
SGLT2-Is andother cardiometabolic parameters:HbA1C, BW andUA.
SGLT2-Is resulted in amodest mean reductioninHbA1C:-0.68%(95%CI: -0.88to–0.48;I2= 89%)(Figure 7A). Asubgroupanalysis basedondoses (2.5 mg and10 mg) demonstrated no change in heterogeneity andstatistical significance. Both the 2.5 mg and10mgdoses ofSGLT2-I resulted in a statistically significant improvement in A1C(Figure 7B). There was a reductioninmeanBWof –1.79kg(95%CI: -2.07 to -1.51;I2= 97%) with SGLT2-I use (Figure 8A). This improvement in BW was notedregardless of SGLT2-I dose. The subgroup analysis basedondose andSGLT2-I type couldnot highlight the potential cause of the significant heterogeneity(Figures 8B andC). UA decreasedwiththe use of SGLT2-I: -1.03 mg/dL (95%CI: -1.14 to –0.93;I2= 98%) (Figure 9A). This reductionwas greater within the dapagliflozin subgroup: -4.52 mg/dL(95%CI: -8.96to–0.08;I2= 100%)vs–0.20 mg/dL(95%CI: -0.51to0.12;I2= 88%)the empagliflozinsubgroup. The impact on UA also appears to be dose-dependent: -1.05mg/dL(95%CI: -1.98to–0.12;I2= 99%) with 10 mg and–0.18 mg/dL(95%CI: -1.4 to 1.05;I2= 0%) (Figures 9B and C). Table 4 provides asummary of the placeboadjustedtreatment effect of SGLT2-Is on metabolic parameters: HbA1C, BW andUA.

Table 4 Baseline data for HbA1C, BW, and UA

Figure 7 Forest plot. A: Highlighting impact of SGLT2-I on HgbA1C compared to placebo; B: SGLT2-I Dose subgroup analysis performed for HgbA1C. SGLT2-I:Sodium-glucose cotransporter 2 inhibitor.

Figure 8 Forest plot. A: Highlighting impact of SGLT2-I on BW compared to placebo; B: SGLT2-I Type subgroup analysis performed for BW; C: SGLT2-I Dose subgroup analysis performed for BW. SGLT2-I: Sodium-glucose cotransporter 2 inhibitor; BW: Body weight.

Figure 9 Forest plot. A: Highlighting impact of SGLT2-I on UA compared to placebo; B: SGLT2-I Type subgroup analysis performed for UA; C: SGLT2-I Dose subgroup analysis performed for UA. SGLT2-I: Sodium-glucose cotransporter 2 inhibitor; UA: Uric acid.
DISCUSSION
This meta-analysis of 18 placebo controlled RCTs was designed to primarily evaluate the impact of SGLT2-Is on the components of the MetS as defined by the NCEP ATP III criteria. In addition, it evaluated their impact on other cardiometabolic parameters including HbA1c, BW and UA. The major findings include: (1) An improvement in MetS components (FPG, WC and BP) in the SGLT2-I group compared to the placebo group, and (2) an improvement in HbA1c, BW and UA in the SGLT2-I group compared to the placebo group.
Previous meta-analyses[40-50] that evaluated the cardiometabolic effects of SGLT2-Is have only included at most four of the five components of MetS. Teoet al[40] evaluated WC, BP, FPG; Choet al[49]analyzed WC, BP, HDL and Zaccardiet al[47] evaluated FPG, BP, HDL and TGL. In addition, the results of these studies have been inconsistent. While our study aimed to evaluate all components, we only had enough data for four components (FPG, BP, WC and HDL) owing to our inclusion criteria. In contrast to these studies[42,43,50], our study did not highlight a significant improvement in HDL with the use of SGLT2-Is. The reason behind this might be an inadequate statistical power; this study analyzed only 3 RCTs owing to the inclusion criteria compared to 47, 5 & 15 RCTs by Sánchez-Garcíaet al[41], Chenet al[42], and Shiet al[50] respectively. This study also evaluated the effect of low-dose SGLT2-Is on HDL,however it is unlikely this played a role in the outcome as the analysis by Chenet al[45] demonstrated a dose-independent impact. While this study has a higher mean treatment duration of 79 wk compared to prior meta-analyses which have a mean duration of 29 wk[40-50], the magnitude of the improvement in FPG, WC and BP appear similar between this study and its counterparts. This might suggest that SGLT2-Is have a ceiling effect on the components of MetS.
A high heterogeneity is noticed across all outcomes except for WC. This could be related to the differences in baseline diabetic medications taken by the patients, different doses, inclusion of more than one type of SGLT2-I and differences in the severity of hyperglycemia among the patients.However, the subgroup analysis for FPG based on dose revealed a significantly elevated heterogeneity with all doses evaluated. This study could not adjust for the differences in baseline diabetic medications and severity of hyperglycemia because these were universally different across the included RCTs, and a patient level meta-analysis would be needed for this. The heterogeneity associated with the SBP outcome in the empagliflozin subgroup may be due to the significant difference in sample size between the analyzed RCTs. A further sub-analysis based on the sample size was not completed because there were only 2 studies in the empagliflozin subgroup for SBP. The difference in efficacy between both SGLT2-Is on SBP appears to be largely due to the significant difference in the number of RCTs that constitute both SGLT2-I subgroup (2 RCTs in the empagliflozin subgroupvs5 RCTs in the dapagliflozin subgroup). The small number of RCTs in the empagliflozin subgroup is due to this study’s inclusion criteria. The differences between the patients' baseline antihypertensives could also be contributory to the high heterogeneity in the empagliflozin subgroup for SBP. The significant difference in treatment duration between the studies that evaluated DBP might explain the significant heterogeneity associated with the 10 mg dose of dapagliflozin. Inadequate power might explain the lack of statistical significance in the reduction of DBP.
The mechanism by which SGLT2-Is lead to improvement in the components of MetS and other cardiometabolic parameters have been partially elucidated[1,51-55]. The glucosuria, osmotic diuresis and natriuresis induced by the inhibition of SGLT-2 and the sodium hydrogen exchanger appears to play an important role in the improvement of FPG, HTN and HbA1c[51,52]. Their impact on HTN also stems from their ability to reduce arterial stiffness and endothelial dysfunction[51-53]. Furthermore, the improvement in UA noted with SGLT2-Is has been associated with the upregulation of the glucose transporter 9, a major urate transporter that secretes UA in the proximal kidney[1,53]. Interestingly,SGLT2-Is' cardiometabolic benefits have been linked to modification of certain genes involved in homeostasis[51,55]. These include a potential upregulation of Angiotensin 1-7 which leads to improvement in HTN and arterial stiffness[51]. The upregulation of genes involved in lipid metabolism including peroxisome proliferator-activated receptor alpha, acetyl-CoA carboxylase, fibroblast growth factor 21 and adenosine monophosphate-activated protein kinase have been associated with the improvement in TGL, HDL and BW[50]. SGLT2-Is have also been associated with increased levels of glucagon-like peptide 1, which is known to slow gastric emptying and reduce weight gain[54].
Perhaps through the improvement in MetS components, the combination of the above mechanisms might explain the improvement in CV mortality and heart failure hospitalization associated with SGLT2-Is in landmark trials[1-4,6,7,9-13]. In addition to its role in CVD, MetS is an independent risk factor in the development of DM[55,56]. Patients with MetS are approximately three to five times more likely to develop type 2 DM[55,56]. This highlights the complex yet incompletely understood connection between MetS, type 2 DM and CVD. Although the improvement in MetS components in this study appears to be modest, our findings anticipate a possible role for SGLT2-Is in the management of MetS.Hence, it highlights the need for RCTs to evaluate the impact of SGLT2-Is on MetS compared with current management modalities including lifestyle modification.
Limitations
The findings of this study should be interpreted cautiously bearing several limitations. First, the mean baseline HDL and DBP of included RCTs did not meet threshold values for MetS. This is likely because our primary objective was mostly a derivative of the secondary outcomes of the included RCTs. Second,owing to our inclusion criteria, only two of the included RCTs recruited patients without DM which limits the external validity of our study. Furthermore, we did not conduct a patient level analysis in those without DM. Third, this study limited its analysis to only dapagliflozin or empagliflozin and did not thoroughly compare the efficacy of both. Fourth, the improvement in MetS components noted by our analysis might be confounded by other medications taken by the RCTs’ participants. Therefore, our analysis could not quantify the absolute effect of SGLT2-Is. This might imply the need for the evaluation of SGLT2-Is as a first line pharmacotherapy in treatment of MetS components. Additionally, MetS has multiple causes besides sedentary lifestyle, and unhealthy eating; it is usually heterogenous in its presentation due to the different possible combinations of its components; this study did not address these in its analysis. Lastly, not all included RCTs are open labelled and hence the risk of bias could not be reliably assessed by the Cochrane risk of bias tool.
CONCLUSION
SGLT2-Is were associated with an improvement in all components of MetS. There appears to be a role for their use in the management of patients with MetS regardless of the presence of DM and HF.Prospective studies are needed to further evaluate the role of SGLT2-Is in patients with MetS either as first-line agents and/or add-on pharmacotherapy. This study, to the best of our knowledge, is the first to fully explore a possible role for SGLT2-Is in the management of MetS.
ARTICLE HIGHLIGHTS

FOOTNOTES
Author contributions:Olagunju A, Mookadam M and Mookadam F designed the research; Olagunju A, Kenny D,Yamani N performed the research; Olagunju A, Kenny D, Yamani N, Mookadam M, Mookadam F and Unzek S analysed the data; Olagunju A, Kenny D, Yamani N and Mookadam F wrote the paper.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
PRISMA 2009 Checklist statement:The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United States
ORCID number:Abdulbaril Olagunju 0000-0001-9255-602X.
S-Editor:Liu XF
L-Editor:A
P-Editor:Liu XF

