Shugan Huoxue Huayu Fang (疏肝活血化瘀方) attenuates carbon tetrachloride-induced hepatic fibrosis in rats by inhibiting transforming growth factor-β1/Smad signaling
LIU Lei,GUO Hanbin,SHAO Cuiping,WANG Lin,XU Youqing,ZHOU Yiming
LIU Lei,GUO Hanbin,SHAO Cuiping,WANG Lin,XU Youqing,Department of Gastroenterology,Beijing Tiantan Hospital,Capital Medical University,Beijing 100070,China
ZHOU Yiming,Department of Hepatology,the seventh medical center of the People′s Liberation Army General Hospital,Beijing 100700,China
Abstract OBJECTIVE: To investigate the potential mechanism by which Shugan Huoxue Huayu Fang (疏肝活血化瘀方,SGHXHYF) ameliorates liver fibrosis.METHODS: Liver fibrosis was induced in rats by intraperitoneal injection of carbon tetrachloride (CCl4) in peanut oil solution (40%,3 mL/kg body weight) twice a week for 8 weeks.A normal control group received the same volume of peanut oil alone.During weeks 5-8,the CCl4-injected rat groups were administered saline(vehicle control),colchicine (0.1 mg/mL,1 mg/kg,positive control),or SGHXHYF (0.1 mg/mL;0.3,0.6 and 1.2 mg/kg)once daily by oral gavage.Rats were sacrificed 24 h after the last treatment.Blood samples were collected for measurement of serum alanine aminotransferase (ALT),aspartate aminotransferase (AST),alkaline phosphatase(ALP),albumin (ALB),collagen Ⅰ,and collagen Ⅲ levels.Liver samples were analyzed by histopathological staining,Masson’s staining of extracellular matrix proteins,and immune-ohistochemical staining of αsmooth muscle actin (α-SMA).TGF-β1/Smad protein and mRNA levels were analyzed by Western blot and quantitative reverse transcription-polymerase chain reaction analysis,respectively.In vitro experiments were also performed using rat hepatic stellate cells (HSCs).RESULTS: Compared with the control animals,CCl4-exposed rats exhibited elevated serum levels of ALT,AST,ALP,collagen Ⅰ,and collagen Ⅲ;reduced serum levels of ALB;and increased collagen deposition and α-SMA expression in liver sections,reflecting liver fibrosis.CCl4also increased expression of TGF-β1 and the activated (phosphorylated) forms of Smad2 and Smad3 but reduced expression of the negative regulator Smad7 in the liver.Notably,concomitant administration of SGHXHYF to CCl4-exposed rats was found to significantly reverse or abolish the pro-fibrotic effects of CCl4in the liver and reduced serum transferase levels.Analysis of HSCs in vitro confirmed that,mechanistically,SGHXHYF inhibited activation of the TGF-β1/Smad signaling pathway by downregulating phosphorylated Smad2 and Smad3 and upregulating Smad7 levels.CONCLUSION: SGHXHYF ameliorated CCl4-induced liver fibrosis by inhibiting the TGF-β1/Smad signaling pathway.These findings suggest that SGHXHYF may have clinical utility for the treatment or prevention of liver fibrosis.
Keywords: transforming growth factor beta;Smad proteins;signaling transduction;liver cirrhosis;Ito cells;Shugan Huoxue Huayu Fang
1.INTRODUCTION
Liver fibrosis is a serious health problem worldwide and has extremely high mortality and morbidity.1,2Liver fibrosis is a common feature of hepatic injury caused by various etiological factors,including hepatitis B and C virus infection and alcohol consumption,3and can lead to cirrhosis and hepatocellular carcinoma.The early stages of liver fibrosis are typically reversible,whereas latestage cirrhosis is often incurable.4,5
Traditional Chinese Medicine (TCM) has been used to treat a variety of diseases for many years and continues to play a vital role in modern medicine.6One example is salvianolic acid [from Danshen (Radix Salviae Miltiorrhizae)],which can be used as an adjuvant treatment for hepatic fibrosis.7During liver fibrosis,smooth muscle α-actin (α-SMA) expression and collagen production are upregulated,leading to excessive deposition of extracellular matrix (ECM) and liver damage.8Hepatic stellate cells (HSCs) are activated by liver damage and differentiate into myofibroblast-like cells,which are the primary source of collagen and play pivotal roles in liver fibrosis.Transforming growth factor-β1 (TGF-β1) produced by hepatocytes and Kupffer cells is considered the most important activator of HSCs and acts by promoting the Smad (Sma and Mad homologue) signaling pathway.9,10TGF-β1-receptor binding induces Smad2 and Smad3 phosphorylation and recruitment of Smad4,and the complex is then transported to the nucleus to activate transcription of liver fibrosis-associated genes.11Therefore,inhibition of HSCs activation is a potential therapeutic target for liver fibrosis.12
Shugan Huoxue Huayu Fang (疏肝活血化瘀方,SGHXHYF) is a TCM used for the treatment of liver fibrosis;however,the mechanisms underlying its therapeutic effects are still unclear.Here,we investigated whether TGF-β1/Smad signaling is involved in mediating the therapeutic effects of SGHXHYF in liver fibrosis.
2.MATERIALS AND METHODS
2.1.Preparation of SGHXHYF
SGHXHYF was prepared from the following herbs:Biejia (Carapax Trionycis) 30 g,Honghua (Flos Carthami) 15 g,Taoren (Semen Persicae) 15 g,Danggui(Radix Angelicae Sinensis) 20 g,Chishao (Radix Paeoniae Rubra) 10 g,Zhiqiao (Fructus Aurantii Submaturus) 15 g,Chuanxiong (Rhizoma Chuanxiong)15 g,and Xiangfu (Rhizoma Cyperi) 15 g (all from Beijing Tong Ren Tang Co.,Ltd.,Beijing,China).The herbs were soaked in water (1∶8 wt∶vol) at room temperature for 30 min and boiled to dryness for 1 h.The dried extract was then mixed with 10 mL of vinegar,diluted with six volumes of water,and boiled for 1 h.The suspension was filtered and the remaining liquid was concentrated to a relative density of 1.0 at 60 ℃ to give a crude SGHXHYF preparation (0.1 mg/mL).SGHXHYF was stored at 0-4 ℃ and was shaken before use.The purity and main components of the preparation were analyzed by high resolution time-of-flight mass spectroscopy (HRTOF-MS).
2.2.Experimental drugs and reagents
Peanut oil and colchicine were from Sinopharm (Beijing,China).Carbon tetrachloride (CCl4) was from Beihua(Beijing,China).PCR primers for α-SMA,TGF-β1,Smad2,Smad3,Smad7,and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were from Invitrogen(Shanghai,China).Antibodies against α-SMA,TGF-β1,Smad2,Smad3,and Smad7 were from Cell Signaling Technology (Danvers,CO,USA).
2.3.Animals and treatments
Wistar rats [males,weighing (200 ± 20) g] were obtained from the Institute of Experimental Animals,Chinese Academy of Sciences (Beijing,China).Five groups of rats (n=8/group) were injected intraperitoneally (IP)with CCl4(40% in peanut oil;3 mL/kg body weight)twice a week for 8 weeks to induce hepatic fibrosis.A sixth normal control group (n=8) received peanut oil alone on the same schedule.Starting at week 5,the CCl4-injected animals were administered normal saline (model group),colchicine (1 mg/kg;positive control),or SGHXHYF (0.3,0.6,or 1.2 mg/kg) once daily by oral gavage.The study protocol was approved by the Animal Experiments Ethics Committee of Beijing Tiantan Hospital.
2.4.Measurement of serum liver function markers and collagen I and Ⅲ levels
Rats were sacrificed 24 h after the last administration.Serum alanine aminotransferase (ALT),aspartate aminotransferase (AST),alkaline phosphatase (ALP),and albumin (ALB) levels were measured using an automatic biochemical analyzer (HITACHI,Tokyo,Japan).Serum collagen Ⅰ and Ⅲ levels were measured using a specific enzyme-linked immunosorbent assay kit(Yiyan Biological Technology Co.,Ltd.,Shanghai,China).
2.5.Histological and immunohistochemical analyses
Liver tissues were paraffin-embedded,sectioned (5-μmthick),and stained with hematoxylin and eosin (HE) or Masson’s stain,or subjected to α-SMA immunohistochemical staining as previously described.13,14
2.6.Western blot analysis
α-SMA,TGF-β1,Smad2,phosphorylated Smad2,Smad3,phosphorylated Smad3,and Smad7 protein levels were analyzed by western blotting as described previously.15
2.7.Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was extracted from liver samples using TRIzol reagent (Ambion,Austin,TX,USA).Aliquots of 1 μg RNA were reverse transcribed using a First Strand cDNA Synthesis Kit (Thermo Fisher Scientific,Waltham,MA,USA).Quantitative PCR was performed on a Step One Real-Time PCR System (Applied Biosystems,Warrington,UK) using SYBR Green PCR Master Mix (Thermo Fisher Scientific).Relative quantification of target genes was performed by the 2-ΔΔCtmethod.Data are presented as normalized expression relative to GAPDH mRNA.PCR primer sequences are listed in Table 1.
2.8.Cell culture and treatments
The HSC-T6 cell line was obtained from the Cell Bank of Institute of Oncology,Chinese Academy of Medical Sciences and cultured in Dulbecco’s modified Eagle’s Medium (Gibco-Life Technologies,Grand Island,NY,USA) supplemented with 100 U/mL penicillin and 10% fetal bovine serum.Cells were maintained at 37 °C in a 5% CO2atmosphere.For experiments,cells were serum-starved overnight and then treated with 10 ng/mL of TGF-β1and vehicle or SGHXHYF for 24 h.
2.9.Statistical analysis
Data were analyzed with SPSS version 22.0 software using one-way analysis of variance followed by Tukey’s post hoc test.P< 0.05 was considered statistically significant.
3.RESULTS
3.1.Chemical analysis of SGHXHYF
HRTOF-MS analysis of SGHXHYF extracts identified 20 main components (Figure 1,Table 2).
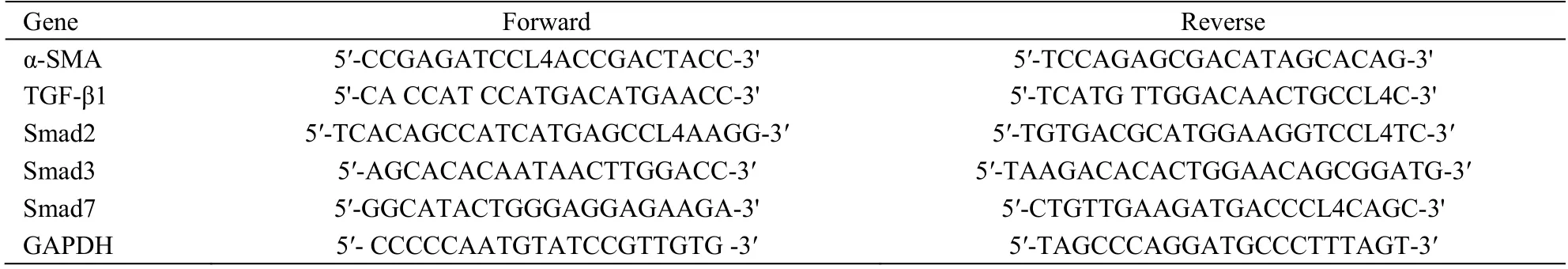
Table 1 Primer sequences for PCR
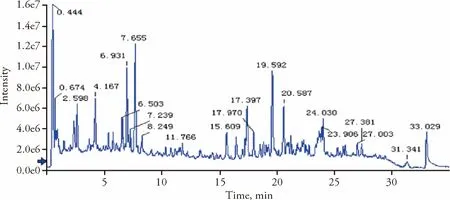
Figure 1 Total ion chromatogram of Shugan Huoxue Huayu Fang
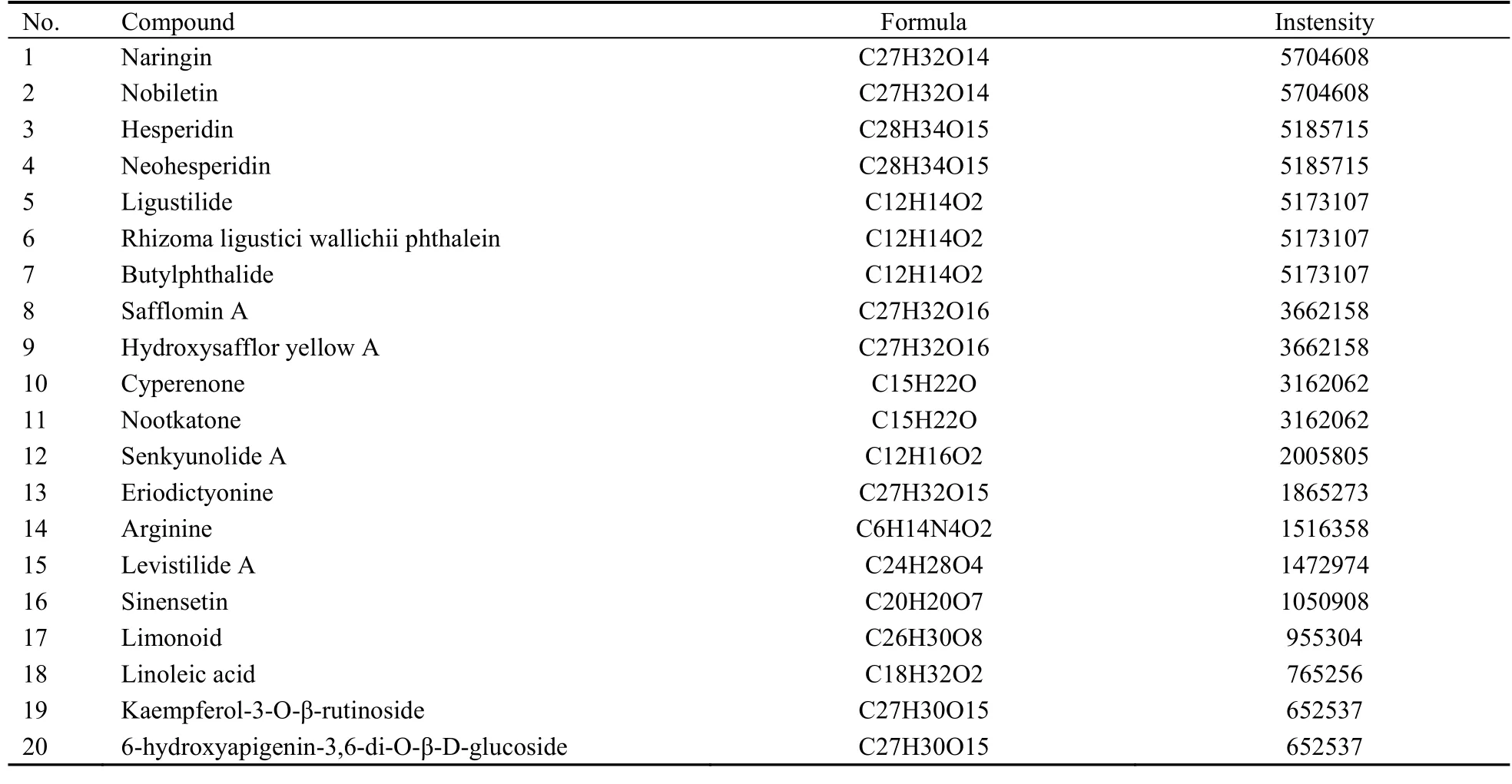
Table 2 Main chemical constituents of Shugan Huoxue Huayu Fang
3.2.Effect of SGHXHYF on liver function in rats with CCl4-induced fibrosis
As shown in Table 3,CCl4injection significantly increased serum ALT and AST levels compared with the normal group;however,the increase in both enzymes was dose-dependently attenuated by SGHXHYF to the level observed in rats treated with colchicine.CCl4exposure also significantly increased serum ALP levels and significantly decreased serum ALB levels.The CCl4-induced changes in both factors were significantly reduced by SGHXHYF treatment at high doses.
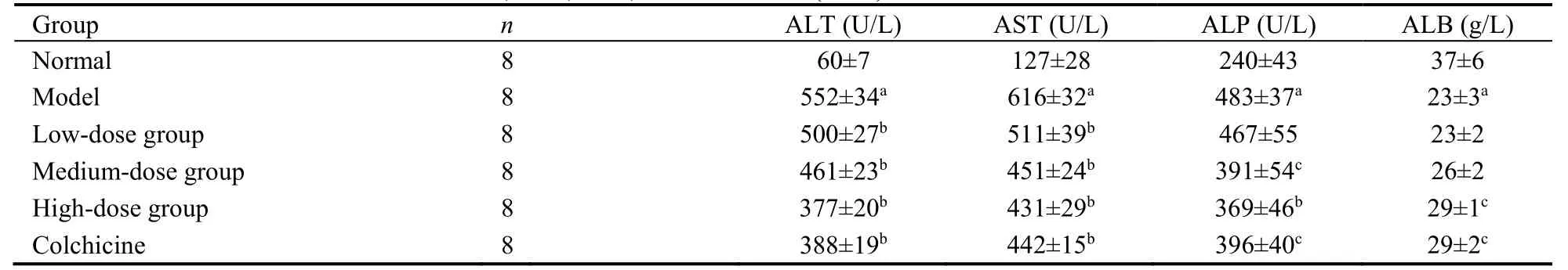
Table 3 Effect of SGHXHYF on serum ALT,AST,ALP,and ALB levels (x± s)
3.3.SGHXHYF inhibits CCl4-induced rat hepatic fibrosis
Rats in the model group displayed increased areas of ECM in liver sections (Figure 2A,B) and elevated serum collagenⅠand Ⅲ levels (Figure 2C,2D) compared with normal rats.These effects of CCl4were significantly reduced by co-treatment with SGHXHYF or colchicine.Consistent with these observations,CCl4exposure increased the overall fibrosis score,which was also reduced in SGHXHYF-treated rats (Figure 2E).
3.4.Effect of SGHXHYF on expression of the HSC activation marker α-SMA
Meanwhile,we analyzed the expression of α-SMA by immunohistochemical staining and western blot analysis of liver sections.Both analyses revealed that CCl4-exposed rats had significantly higher levels of α-SMA compared with normal control rats,and that the expression level was significantly reduced in rats administered SGHXHYF (Figure 3A-3C).Comparable results were obtained after qRT-PCR analysis of α-SMA mRNA levels in rat livers (Figure 3D).

Figure 2 SGHXHYF inhibits CCl4-induced liver fibrosis in rats

Figure 3 Effect of SGHXHYF on α-SMA expression in the liver.
3.5.Effect of SGHXHYF on TGF-β1/Smad signaling during liver fibrosis
CCl4-induced liver fibrosis was associated with an increase in the production of TGF-β1,but the effect was diminished in animals treated with SGHXHYF (Figure 4A,4B).CCl4exposure also resulted in activation of Smad signaling,as evidenced by an increase in the ratio of phosphorylated (p-) to total (t-) Smad2 and Smad3;of note,these ratios were also significantly reduced in the livers of rats treated with SGHXHYF (Figure 4A,4C,4D).
Interestingly,the inhibitory effect of SGHXHYF on TGF-β1/Smad signaling was underscored by analysis of the expression of Smad7,an inhibitory regulator of TGFβ1/Smad signaling.In agreement with this function,Smad7 protein levels were significantly lower in the livers of CCl4-exposed rats compared with normal rats,but the CCl4-induced increase was virtually abolished in SGHXHYF-treated rats (Figure 4E).
3.6.SGHXHYF inhibits HSCs activation and TGF-β1-Smad signaling in vitro
As shown in Figure 5A-5D,TGF-β1 treatment significantly upregulated expression of α-SMA,a marker of HSC activation,and increased the p/t-Smad2 and p/t-Smad3 ratios,but these increases were suppressed by SGHXHYF treatment.Finally,we found that TGF-β1reduced Smad7 protein levels,but co-treatment with SGHXHYF largely abolished this effect (Figure 5E).
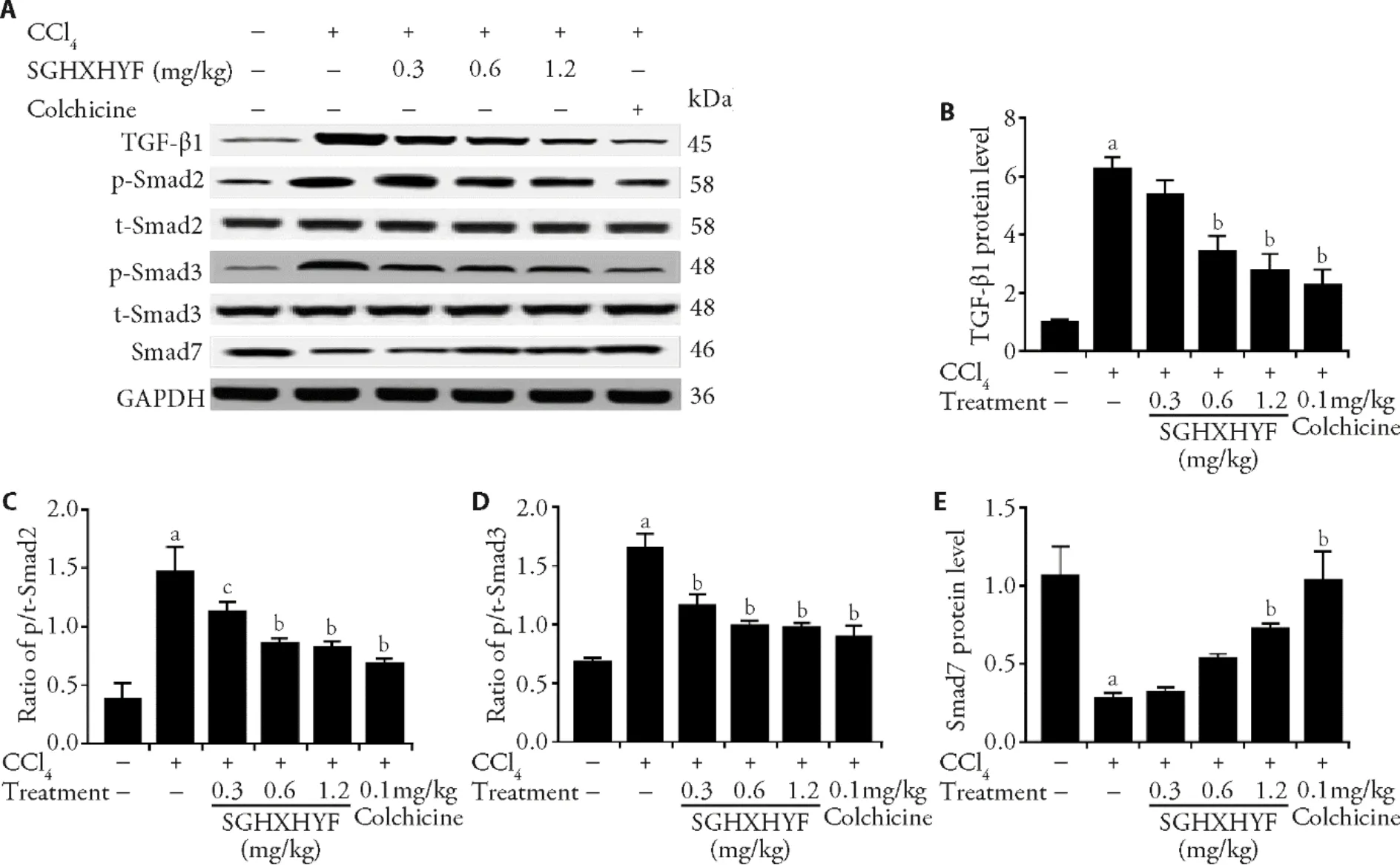
Figure 4 SGHXHYF inhibits activation of the TGF-β1/Smad signaling pathway in the liver
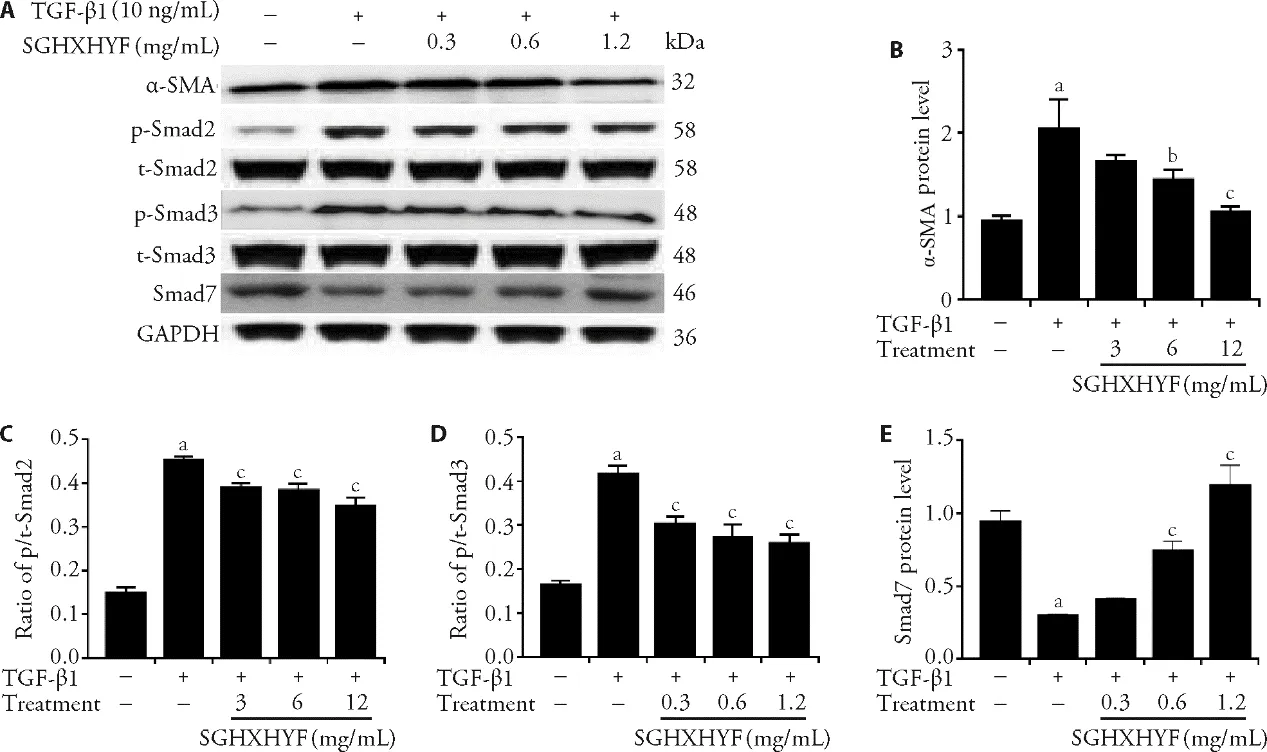
Figure 5 SGHXHYF inhibits activation of the TGF-β1-Smad signaling pathway in HSCs in vitro
4.DISCUSSION
Liver fibrosis resulting from the deposition of excessive levels of ECM can cause severe damage and lead to cirrhosis and hepatocellular carcinoma.1Therefore,interventions that prevent liver fibrosis at an early stage would represent an important advance in the treatment of liver diseases.Although a range of small molecules and biological agents have been developed to treat liver fibrosis,16,17TCM may have advantages over the traditional drugs because they can act simultaneously on various targets,18,19thereby delaying or suppressing liver fibrosis.Hence,considerable attention has focused on TCM as potential anti-fibrotic agents.20-22SGHXHYF is recognized in China as a highly efficacious anti-fibrosis agent.Here,we investigated the potential molecular mechanisms underlying the protective effects of SGHXHYF against CCl4-induced liver fibrosis in rats.We found that SGHXHYF significantly reduced and/or abolished many of the hallmarks of fibrosis;most notably,elevated serum transferases and collagenⅠ andⅢ,reduced serum ALB,and increased ECM deposition in the liver.
During liver fibrosis,the normal ECM composition is altered by enrichment of fibrillar collagen Ⅰ and Ⅲ,which are primarily produced by activated HSCs that differentiate to become myofibroblast-like cells.23Our observation that SGHXHYF administration attenuated CCl4-induced increases in serum collagen Ⅰ and collagenⅢ levels and production of α-SMA and ECM in the liver suggests that SGHXHYF may suppress liver fibrosis by inhibiting HSCs activation,differentiation to myofibroblasts,and production and deposition of collagens.
TGF-β facilitates the transformation of HSCs into myofibroblasts and promotes ECM formation.TGF-β1is the main isoform involved in liver fibrosis and exerts its biological effects predominantly by activating Smad signaling and transcription of Smad target genes.24Smads have well-known roles in regulating TGF-β1-induced collagen production and can affect the formation of liver fibrosis.Consistent with these functions,we observed that CCl4exposure increased phosphorylation and activation of Smad2 and Smad3 and reduced expression of Smad7,a negative regulator of TGF-βinduced responses.25However,these effects of CCl4were significantly reduced in SGHXHYF-treated rats.These results are consistent with previous findings.13Meanwhile,we validated our results by directly examining TGF-β1-induced Smad signaling in the rat HSC-T6 cell linein vitro.Thus,our results imply that inhibition of TGF-β1/Smad signaling may be the key mechanism by which SGHXHYF reduces liver fibrosis in CCl4-exposed rats.
In conclusion,this is the first study to elucidate the possible molecular mechanisms of the anti-fibrotic effect of SGHXHYF.In future work,it will be of interest to determine whether SGHXHYF affects the activity of other factors that regulate TGF-β1/Smad signaling,such as miRNAs that modulate TGF-β1 expression,26or signaling pathways involved in the development of liver fibrosis,such as the platelet-derived growth factor27and Wnt28signaling pathways.
5.ACKNOWLEDGEMENTS
We thank Anne M.O’Rourke,PhD,from Liwen Bianji,Edanz Group China (www.liwenbianji.cn/ac),for editing the English text of a draft of this manuscript.
 Journal of Traditional Chinese Medicine2022年1期
Journal of Traditional Chinese Medicine2022年1期
- Journal of Traditional Chinese Medicine的其它文章
- Development and evaluation of short form of constitution in Chinese medicine questionnaire: a national epidemiological survey data of 21 948 cases
- Registration of intervention trials of Traditional Chinese Medicine for four neurological diseases on Chinese Clinical Trial Registry and ClinicalTrials.gov: a narrative review
- Acupuncture for low back pain: a clinical practice guideline from the Hong Kong taskforce of standardized acupuncture practice
- Applying latent tree analysis to classify Traditional Chinese Medicine syndromes (Zheng) in patients with psoriasis vulgaris
- Efficacy of glucocorticoids,chloroquine and vitamin A on cytokine release syndrome: a network pharmacology study
- Mechanism underlying the effect of Liujunzi decoction (六君子汤) on advanced-stage non-small cell lung cancer in patients after first-line chemotherapy
