Non-carcinogenic health risk assessment of nitrate and fluoride contamination in the groundwater of Noyyal basin, India
Karung Phaisonreng Kom, Balasubramanian Gurugnanam, Swaminathan Bairavi
Centre for Applied Geology, Gandhigram Rural Institute - Deemed to be University, Gandhigram, Dindigul 624302, Tamil Nadu, India
Keywords:Nitrate Fluoride Non-carcinogenic Hazard quotient Total hazard index Noyyal basin
ABSTRACT The study aims to assess the nitrate and fluoride concentration in groundwater and its adverse effects on human health. In 2019, 42 groundwater samples were collected from various bore wells within the western Noyyal basin, India. Sodium and chloride are the dominant cation and anion, respectively. The nitrate concentration in groundwater samples varies from 2 to 89 mg/L, of which 33.33% are above the permissible limit of 45 mg/L for drinking water. The fluoride concentration ranges from 0.2 to 2.4 mg/L,with 28.57% of the samples exceeding the safe value of 1.5 mg/L for drinking water. Correlation plots demonstrate that the potential of hydrogen(pH),electrical conductivity(EC),total dissolved solids(TDS),Na+ and HCO-3 are positively correlated with F-, whereas Ca2+ is negatively correlated. Mixed Ca-Mg-Cl is the most common water type in the investigated region.The Gibbs diagram demonstrates that the interaction between rock and water impacts the groundwater chemistry.Using the method of the United States Environmental Production Agency (USEPA), this study assesses the non-carcinogenic health risk posed by nitrate and fluoride in different age groups (infants, children, and adults). The values of total hazard index (THI) vary from 0.59 to 10.07 (mean = 4.76) for infants, 0.36 to 6.23 (mean = 2.95) for children, and 0.19 to 3.32 (mean = 1.57) for adults. Furthermore, 97.62%, 92.86%, and 73.81% of the samples surpass the recommended limit(THI=1)for infants,children,and adults,respectively.Thus,the health risk assessment (HRA) indicates that infants and children are more susceptible to noncarcinogenic health hazards than adults. The THI spatial variation map shows that central and southern regions of the study area have been identified as high health risk areas(THI >3.0)for all age groups.
1. Introduction
Groundwater is a significant resource for drinking and irrigation, especially in dry and semi-arid regions where precipitation and surface water are scarce[1].With the growth of population and the lack of alternative water sources,the demand for groundwater is growing every day. An estimated 2.5 billion people worldwide depend heavily on groundwater to meet their daily water needs[2,3].It is generally accepted that the groundwater is safe to drink,so most rural residents rely on private or public wells to meet their water needs.However,shallow groundwater in the alluvial plain is vulnerable to contamination from various sources. Climate change and anthropogenic activities, such as rapid industrialization, urbanization, excessive fertilizer use, and unscientific effluent discharge, significantly contribute to groundwater pollution [4].Other processes affecting groundwater quality include the cation exchange during oxidation or reduction, mineral dissolution/precipitation,biological activities,and the composition of rainfall[5,6].In addition, over-exploitation of groundwater resources has sped up the process of rock weathering, resulting in more significant geogenic contamination of groundwater[7].Prolonged ingestion of excessively polluted groundwater (inorganic or organic) may be harmful to the health of nearby residents[8].Human health may be negatively affected by nitrates and fluorides in groundwater.These two toxic ions are listed as non-carcinogens by USEPA, which has received worldwide attention because of their devastating effect on human health.
Nitrate (NO3-)is an inorganic ion that naturally occurs as part of the nitrogen cycle. It is a nutrient primarily used as plant fertilizer.The primary source of nitrate in groundwater is attributed to human activities[9,10].There are many different ways for nitrates to end up in groundwater, but most of them come from point and non-point sources. The most common point sources where NO3-can get into the groundwater, like septic tanks, dairy lagoons, wastewater effluents percolation, and livestock waste [11,12]. Elevated nitrate levels in groundwater could result from a non-point source like fertilizers,pesticides, and manure application [13]. Nitrates may be released into the groundwater by natural processes through rock-water interaction from the weathering of tobelite, nitratine, and nitritebearing rocks [14]. In addition, nitrate contamination in groundwater can also be derived from the nitrogen fixation by legume plants and microbes [15]. Fertilizers are extensively used in agricultural land areas, as plants get their nitrogen from nitrate, an oxidized form of dissolved nitrogen.When soil is extensively farmed,it loses the natural ability to hold it. Nitrogen fertilizers are widely used to replenish depleted soil nutrients.But these nitrates are toxic to human health when they enter the food chain via groundwater and surface water. The Bureau of Indian Standards (BIS) recommended that the maximum permissible NO3-concentration in drinking water is no more than 45 mg/L. When the nitrate level in water is high,it can cause blue infant disorder(methemoglobinemia)in infants(under 6 months of age).In adults,it increases cancer risks like stomach tumours and colorectal cancer, non-lymphoma Hodgkin's lymphoma [16], hypertension and thyroid dysfunction [17].Over 108.2 million people in India consume water with nitrate levels exceeding the permissible limit of 100 mg/L[18].
Fluoride (F-) is another harmful substance that can adversely affect human health and other organisms. It is primarily found in natural waters as a free ion. Fluoride in groundwater is typically naturally occurring or artificially affected [19]. The minerals like cryolite, hornblende, biotite, amphiboles, apatite, fluorite, and muscovite(fluoride-bearing minerals)are the natural source of F-[20,21].These minerals are abundant in granitic terrain[22].When the minerals mentioned above are exposed, they break down,releasing fluoride into the soil. Hence, fluoride is excreted by this process and seeps into the groundwater via soil moisture or rainfall infiltration. Numerous factors that influence the quantity of naturally occurring fluoride in groundwater include climate,more bicarbonate, pH and sodium concentration, water temperature, and host rock composition (availability of fluorine-bearing minerals) [23]. Among the anthropogenic sources, phosphatic fertilizer plants, brick manufacturing, steel production, coal combustion, sewerage, over-withdrawal of groundwater, etc., are the major cause of elevated fluoride levels in groundwater [24,25].Fluoride levels of 0.6-1.5 mg/L in drinking water are considered safe by the World Health Organization (WHO). The level of F-in drinking water less than the recommended value (<0.6 mg/L) is susceptible to dental caries. However, fluorosis (dental and skeletal) can be caused by fluoride levels greater than 1.5 mg/L in drinking water [26-28]. In addition, the thyroid, arthritis, osteoporosis, infertility, and abortion have been linked to excessive fluoride intake[29,30].Fluorosis affects approximately 200 million people worldwide [31].
The countries that are extensively reported about the noncarcinogenic health issues include China [19,32], India [33-35]Iran [36,37], Mexico [38], and Pakistan [39,40]. Several studies on non-carcinogenic health risks have been carried out in most states in India. Nawale et al. [41] examined the non-carcinogenic health hazards due to the high concentrations of nitrate and fluoride contamination in the groundwater resources of the Wardha subbasin, central India. Duvva et al. [42] reported elevated amounts of NO3-and F-in the groundwater of Telangana state. They also evaluated the health risk associated with high levels of NO-3and F-,and found that children in the study area have a higher risk of health problems than adults. Similarly, in the state of Punjab(northern India), Singh et al. [43] performed the potential noncarcinogenic impacts of NO3-and F-on human health through groundwater ingestion and found that children are more vulnerable than adults. Raja and Neelakantan [44] evaluated the noncarcinogenic hazards possed by NO3-and F-in the groundwater of Ramanathapuram, South India, and they concluded that the central and northeast region people have a higher noncarcinogenic health risk. Jandu et al. [45] studied the source of fluoride and nitrate and the related non-carcinogenic health risk in the groundwater of Rajasthan. They found that the anthropogenic and geogenic sources are the leading causes of high nitrate and fluoride levels in groundwater, respectively. Further,they revealed that the evaluation of non-carcinogenic hazards for children,women and men exceeded the allowable limit.
Coimbatore is known as the Textile City of South India for its extensive textile industry. Due to the rapid urbanization, industrialization, intensive agricultural and mining activities, the groundwater quality in this area has been degraded. Groundwater contamination in urban areas is primarily caused by textile effluents and sewage [46]. Kom et al. [47] examined the groundwater chemistry and the suitability for drinking and agriculture in the current investigation region. Further, the study concluded that human interference in the groundwater system is endangering the quality of the water. Kumar [48] studied the groundwater fluoride contamination and related human health hazards in Coimbatore district, Tamil Nadu. The study concluded that both natural and anthropogenic effects cause a high level of F-in groundwater and all age groups (infants, children and adults) possess noncarcinogenic risks. Karunanidhi et al. [49] evaluated the impacts of nitrate and fluoride contamination on human health at the Sulur Taluk (located in the eastern portion of the current research area).However, no comprehensive study on the non-carcinogenic hazards posed by NO-3and F-contamination in groundwater has been published in this study area.The main objectives of this study are to provide information about the hydrochemical composition that governs the groundwater chemistry, ascertain the spatial distributions of nitrate and fluoride, and evaluate the non-carcinogenic health risk for infants, children, and adults.
2. Study area
2.1. Research location and characteristics
The investigated region is a part of the Noyyal basin,situated in the northwest Coimbatore,South India.It lies in latitudes 10°56'22″~11°6'27''N and longitudes 76°39'22″~77°17'36''E, covering an area of 1519.11 km2(Fig.1).Topographically,most of the study area is flat,while hill ranges of the Ghats(Nilgiris hills)are located in the western regions.The elevation of the study region ranges between 0 and 1972 m(Fig.1).The present investigated region is bisected by the Noyyal River,flowing east-west.It is one of the most important tributaries of the Cauvery River.The upper part of the river is used for drinking and agricultural purposes. However, due to the untreated direct discharge of municipal sewage and effluents from the industries, it is polluted along with the tanks when it enters the urban areas(Coimbatore city) [50].

Fig.1. Location map of the study area showing the sampling point, stream order and elevation.
Additionally, the water quality deteriorates due to dyeing textiles and tanning leather[27].The geomorphic features of the study area include pediment, pediplain complex, bajada, dissected hills,and valleys.The drainage pattern is mainly dendritic.The soil types of the study area comprise colluvial, alluvial, calcareous, noncalcareous, red soil, brown soil, and forest soil. The present study area experienced the northeast monsoon delivering more rainfall than the southwest. The average annual rainfall is 647 mm, and temperatures vary between 14°C and 40°C, experiencing a subtropical climate[51,52].
2.2. Geology and Hydrogeology
Fig. 2 depicts the geology of the study area. The dominant lithologies are fissile hornblende-biotite gneiss,granites,gypseous clay,and charnockite.The aquifers range from Archaean to recent alluvium. The alluvium deposits, considered to be significant water-bearing formations, are found along the course of the Noyyal River.However, the essential aquifer systems in the study area are Archaean crystalline rocks associated with weathered fractures and joints. Gneissic formations are more weathered,while charnockite formations are moderately weathered[53].The highly weathered formation extends up to 15 m in the granitic gneiss area, but only up to 8 m and sometimes up to 10 m in the charnockite area [51].
3. Materials and methods
3.1. Sampling and analysis
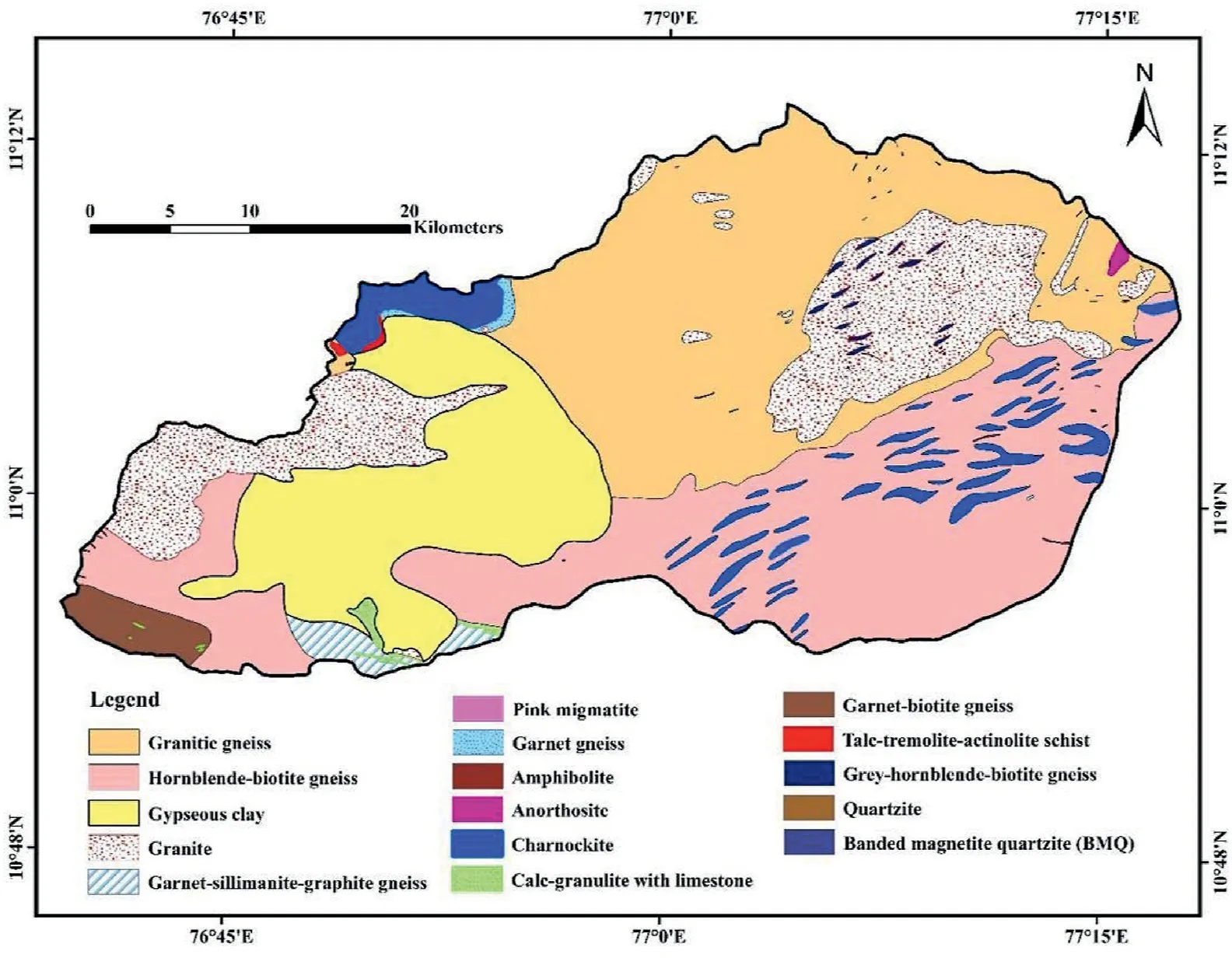
Fig. 2. Geology map of the study area.
We collected 42 groundwater samples from different locations in the textile city (Coimbatore) of South India for quality and health-related issues caused by F-and NO3-. The samples were obtained from various borewells in the study area between November and December 2019.Polyethene bottles with a capacity of 1.0 L were used to gather the samples. The wells were pumped for about 10-15 min before collecting samples to avoid the adverse effects of stagnant water. Immediately after sampling, a portable digital metre (HANNA, HI 9828) was used to determine the potential of hydrogen(pH),total dissolved solids(TDS),and electrical conductivity (EC) of the samples. A standard method recommended by the American Public Health Association is strictly followed when collecting, transporting, and analyzing groundwater samples [54]. Before chemical analysis, each sample was labelled and stored in a refrigerator at 4°C.
The concentrations of magnesium (Mg2+), calcium (Ca2+) and total hardness (TH) were estimated by EDTA titrimetric method.The chloride(Cl-)and bicarbonate(HCO3-)levels were measured by titrimetry technique with standard solutions of H2SO4and AgNO3,respectively.The ions of Na+and(K+)were measured using a flame photometer.The concentration of NO3-and sulfate SO42-were estimated with the help of UV-visible spectrophotometer,while the fluoride is by the ion-selective electrode. The base map and spatial variation maps were prepared in ArcGIS 10.5, and Microsoft Excel was used to analyze the chemical data.
3.2. Health risk assessment (HRA)
HRA is an effectual probabilistic technique to determine the highly toxic chemical concentrations in our drinking water and the related human health consequences.This technique was developed by the USEPA [55]. It has been widely used to assess the noncarcinogenic health risk of groundwater contaminants over the last 30 years [56-60]. Drinking contaminated water can cause serious health problems in humans [61]. According to the USEPA[55], toxic substances are categorized as carcinogenic and noncarcinogenic, whereas nitrate and fluoride are listed under the non-carcinogen category.However,water contaminated with noncarcinogens can endanger human health through direct ingestion and skin contact [62]. Oral ingestion is the critical exposure route[63]. Therefore, this study evaluated the human health risk posed by nitrate and fluoride via oral pathway for three different age groups: infants (<2 years), children (2-16 years), and adults (≥16 years). The following equation calculates the non-carcinogenic health hazards associated with drinking water [64].
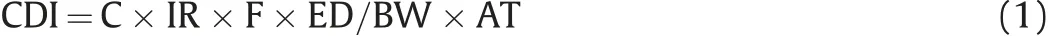
Where CDI represents the chronic daily intake (mg/kg × day) of NO3-and F-via oral ingestion,C is the concentration of NO3-and F-in groundwater in mg/L, IR signifies the ingestion rate, F indicates the exposure frequency,ED denotes the exposure duration,BW represents the average body weight, and AT denotes the average exposure time.
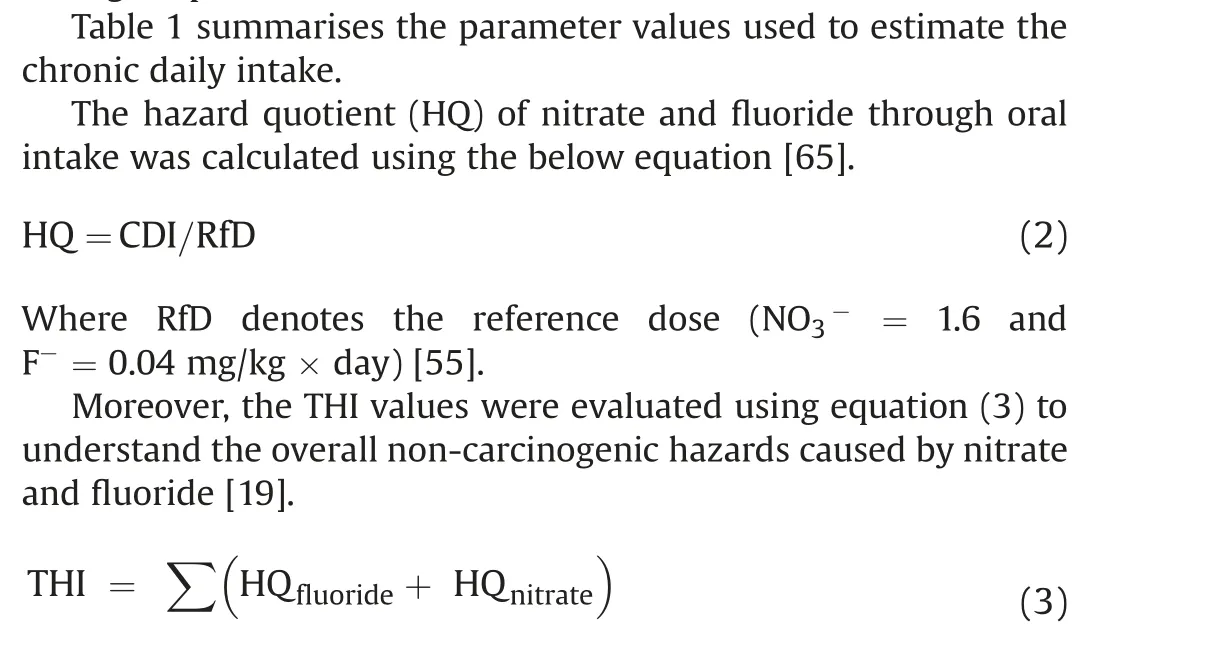
THI ≤1 indicates no health risk to humans,while THI >1 signifies a higher level of hazard [66,67].
4. Results and discussion
Table 2 shows the statistical outcomes of various physical and chemical analyses. All parameters are denoted by mg/L, except pH and EC, expressed as the number and μS/cm, respectively. Permissible limits for various parameters in the samples were determined using the drinking water guidelines of BIS [70]and WHO [71].
4.1. Physico-chemical parameters of groundwater
Groundwater resources can be studied using hydrogeochemical methods to identify the processes that influence the groundwater chemistry [72]. The hydrochemical analysis shows that the pH values of groundwater samples vary from 7.03 to 8.7(average 8.0),indicating alkaline groundwater (Table 2). Around 23.81% of the samples have a pH value above the BIS permissible limit[70].Longterm consumption of high pH water causes skin, eye, and mucous membrane irritation.EC concentration is between 635 and 6124 μS/cm (average 2771 μS/cm). Langenegger [73] proposed five distinct categories for the classification of groundwater based on its EC concentration. About 66.67% of the groundwater samples are classified as saline water,19.05% fall into the permissible category,and 14.29% are classified as brackish water. Furthermore, the EC value in 71.43%of samples exceeds the WHO recommended limit of 1500 μS/cm.However,based on the EC classification by Todd(1980)[74],11.90%,28.57%,16.67%,and 42.86%of the samples are classified as good, permissible, doubtful, and unsuitable category, respectively. The EC values may rise in groundwater with the increase of temperature and available geogenic salt[75].Total dissolved solids(TDS)varied from 320 to 4870 mg/L(mean 1864 mg/L),with 33.33%of the samples exceeding the BIS standard value (2000 mg/L). The TDS classification based on Davis and De Wiest [76] shows that 9.52%, 30.95%,40.48%, and 19.05%of groundwater samples are fall under the desirable for drinking (TDS<500), permissible for drinking (TDS between 500 and 1000), useful for irrigation (TDS between 1000 and 3000) and unfit for drinking and irrigation(TDS>3000) categories, respectively. The groundwater with high TDS causes intestinal annoyance, kidney stones, heart problems,bad taste,and bad odor.The concentration of total hardness(TH)inthe samples ranges between 220 and 1785 mg/L(mean 1032 mg/L).Based on Sawyer and McCarty's [77] classification, 9.52% and 90.48%of samples are hard and very hard.The analysis reveals that 78.57% of samples exceed the BIS standard limit of 600 mg/L for drinking water. The intake of high TH causes body tissue calcification and kidney diseases [78].

Table 1 Parameters and input assumptions for the evaluation of non-carcinogenic health risk.
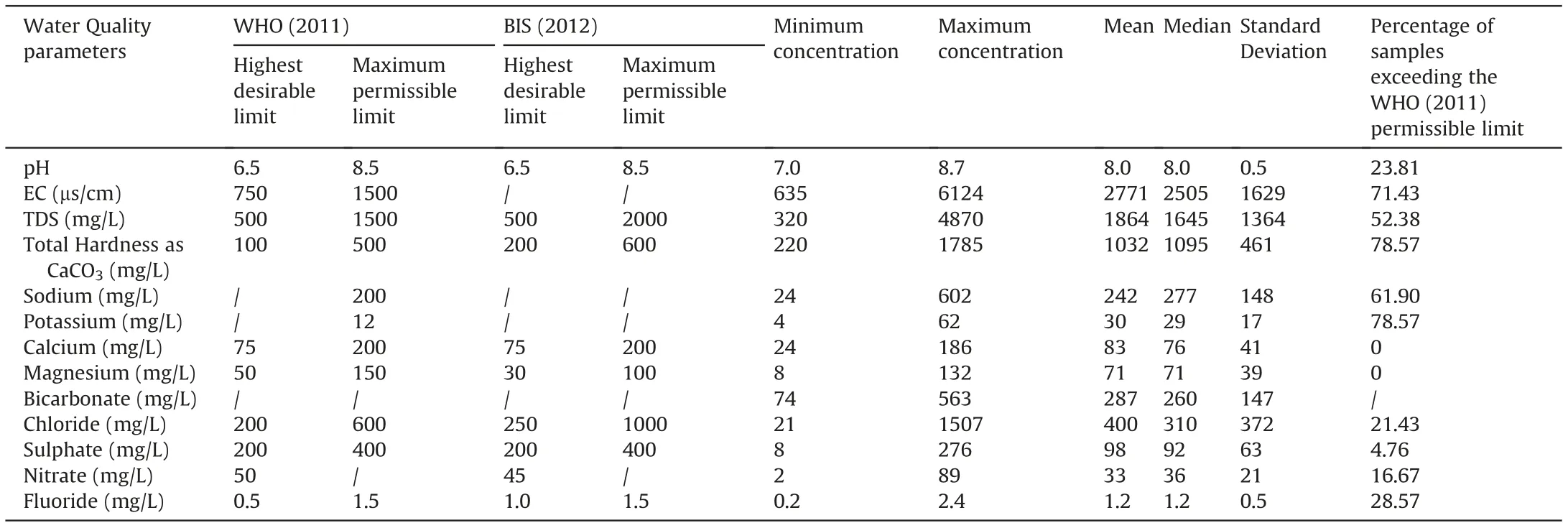
Table 2 The descriptive statistical analysis of groundwater samples with comparison to drinking water standards of WHO (2011) and BIS (2012).
The hydrochemical analysis found that Na+and Cl-are the abundant cation and anion in the groundwater of the study area.The level of Na+is between 24 and 642 mg/L (average 242 mg/L),indicating that the permissible limit of WHO (200 mg/L) is surpassed by 61.90% of the groundwater samples. Consuming Na+in excess of the recommended amount can cause vomiting, heart disease, and stroke. High Na+levels in the groundwater can be caused by silicate (albite, NaAlSi3O8) and clay minerals, which are abundantly occurred in the study area[79]. The samples have calcium concentrations ranging from 24 to 602 mg/L(average 242 mg/L and median 277 mg/L). The result shows that all samples are within the WHO-recommended calcium limit of 200 mg/L. Colorectal cancer,kidney stones,osteoporosis,and hypertension can be caused by excessive calcium consumption in drinking water [64].Calcium in groundwater is derived from gypsum, limestone, dolomite, and silicate minerals, including feldspar, pyroxene, and amphibole. The average concentration of magnesium (Mg2+) is 71 mg/L, and it varies from 8 to 132 mg/L, and the permissible threshold of 100 mg/L is exceeded by 30.95% of the samples. It occurs naturally in various rock types, including pyroxenites, talc,gypsum, dolomite, dunites, basalt, amphibolite and tremoliteschists [80]. In these groundwater samples, the K+varies from 4 to 62 mg/L (average 30 mg/L). The analysis also indicates that the potassium content in the samples surpasses the WHO [71] prescribed value of 12 mg/L by 78.57%. Naturally, the source of K+in the groundwater could be the presence of silicate minerals due to the dissolution process in the region.Other sources could be animal wastes and excessive potassium fertilizers in agricultural land[81].
Chloride is the highest concentration among all the anions. It diverges from 21 to 1507 mg/L(averaging 400 mg/L)in the samples,indicating 21.43%of the samples exceed the drinking water limit of WHO (600 mg/L). High concentrations of Cl-in groundwater are caused by anthropogenic sources like household waste, landfills,septic tank leaks,and agricultural fertilizers[82].Another potential source of Cl-contamination in the groundwater is the abundant occurrence of clay minerals [83]. Excessive chloride consumption can result in osteoporosis, hypertension, and asthma [84].74-563 mg/L of bicarbonate is found in groundwater samples,but HCO-3has no recommended guideline value. However, long-term intake of HCO-3groundwater can cause aesthetic problems and even kidney failures. The dissolution of carbolic acid and chemical weathering of carbonate are typical sources of HCO-3in groundwater [85]. Concentrations of SO42-exhibited a wide range of 8-276 mg/L(mean=98 mg/L).The analysis shows that only 4.76%of samples have sulphate concentrations higher than the BIS recommended level (200 mg/L) for drinking water. However, the mineral sulphate can be found in all types of rock: igneous, sedimentary, and metamorphic, in the form of naturally occurring minerals such as metallic sulphides [86].
4.2. Nitrate in groundwater (non-carcinogen)
Table 2 shows a wide range of nitrate in the groundwater,varying from 2 to 89 mg/L(average 33 mg/L).Nitrate levels in water should not be higher than 45 mg/L,as per the BIS(2011).In addition,with nitrate level between 45 and 100 mg/L, the risk of health complications rises significantly,and it is hazardous above 100 mg/L[87]. The study found that 33.33% of the samples exceeded the recommended value. Orogenic and anthropogenic sources of NO-3groundwater can be identified, but the natural process of contamination is much slower compared to that in [88]. The primary anthropogenic sources include agriculture fertilizers, leakages of septic tanks, municipal sewage, industrial outlets, poultry waste disposal, leachate from landfills, and urbanisations [89-92]. Fig. 3 depicts the groundwater NO3-distribution in the study area. It demonstrates that the NO3-concentration of the central and eastern regions is the highest.The regions with high NO3-level are plains, where settlement and farmland are prevalent. Rainfall and groundwater nitrate concentrations may be inversely related since nitrate is transported to the soil via infiltration[93]. Nitrogen concentrations can be elevated by undiluted irrigation return flow during periods of low rainfall [26]. Excess nitrate intake can be dangerous, especially for infants. Syndromes like congenital disabilities and methemoglobinemia (blue baby syndrome) are the most common in infants.When infants are fed milk containing high levels of NO3-, they are more likely to develop this syndrome. On the other hand, gastric cancer, goitre, and high blood pressure in adults are all possible side effects of nitrate contaminants in drinking water [94]. In addition, pregnant women who ingest excessive amounts of nitrate-rich groundwater increases the risk of complications[95].In contrast,a lack of nitrate concentration may result in the inability of tissues, muscles, and bones to function properly[96].
Bivariate plots between nitrate, chloride, and potassium are utilized to identify the underlying source of NO-3contamination in groundwater [97,98]. Fig. 4(a) demonstrates that the plot between NO3-and Cl-has a strong correlation. Based on these findings,animal and human waste are likely to be a source of groundwater nitrate contamination [88]. However, Fig. 4(b) reveals the nonsignificant correlation between NO3-and K+, suggesting that high nitrate could result from anthropogenic activities like sewage,manure, and excessive fertilizer application in the agricultural areas.
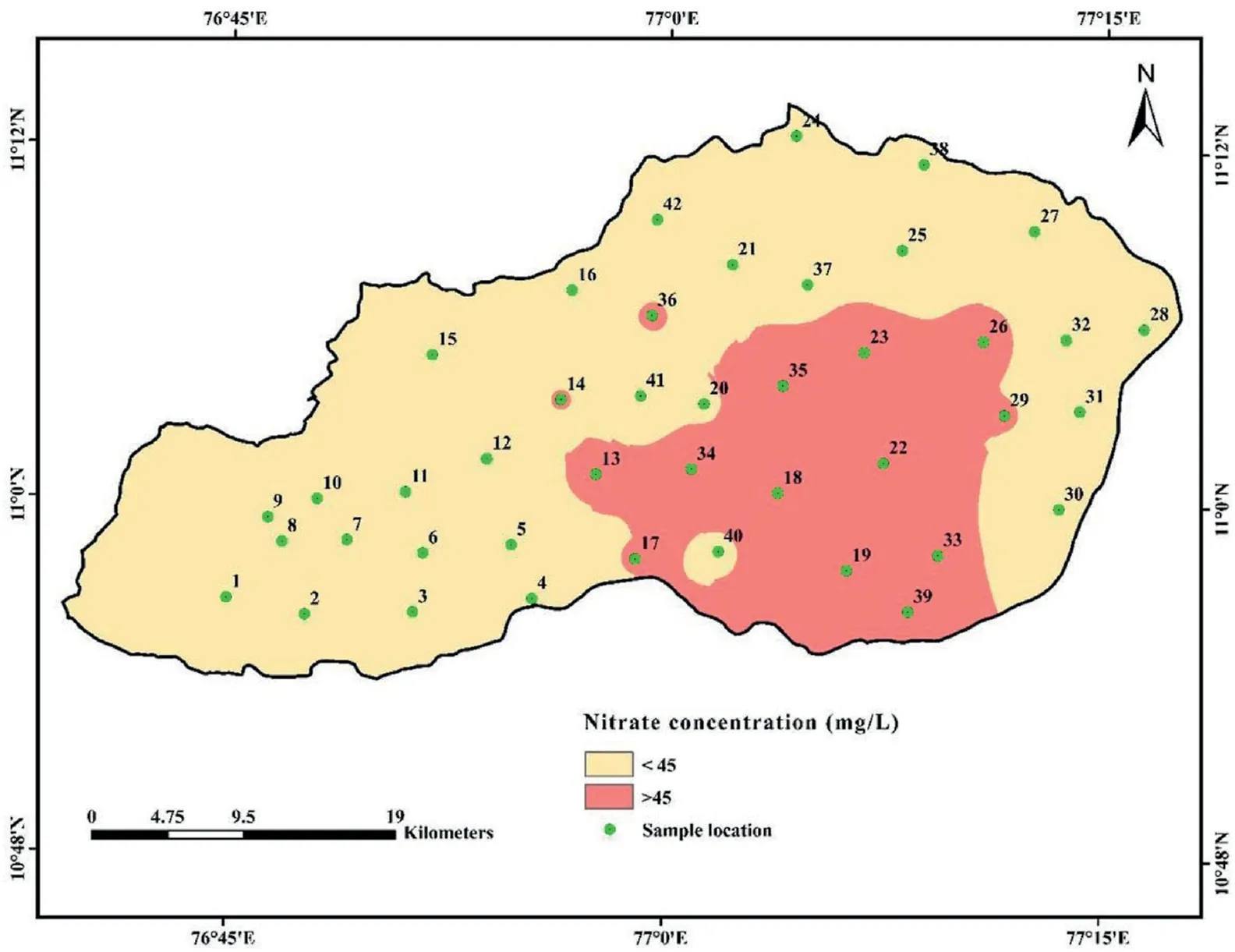
Fig. 3. Spatial distribution map of nitrate in groundwater.
4.3. Fluoride in groundwater
A range of 0.2-2.4 mg/L of F-is found in the samples,as shown in Table 2. According to the WHO (2011) [71], the threat of dental carries rises when the level of F-is less than 0.6 mg/L in drinking water. However, when it stays between 0.6 and 1.5 mg/L (the accepted level), there is no evidence of a health issue. The level of F-above 1.5 mg/L is associated with the development of fluorosis[28,70].The spatial variation map of fluoride(Fig.5)demonstrates that fluoride concentrations in groundwater were higher in the central, southern, and eastern parts of the study area. Fluoride in groundwater is primarily derived from the weathering and dissolution of fluoride-bearing minerals,such as fluorite,biotite,apatite,hornblende,and amphibole[99-101].These minerals are common in granitic and gneissic terrains, and the geogenic source contributes vast quantities of F-to groundwater [102]. The elevated fluoride regions (central, southern, and eastern regions) are dominated by granitic gneiss,charnockite,granite and hornblendebiotite gneiss (Fig. 2). Percolation of rainwater through these weathered rocks may contaminate the groundwater with excessive fluoride [103]. It is possible to replace fluoride ions from fluoride bearing minerals with hydroxyl ion ( OH-) under alkaline conditions [104]. For example, equations (4) and (5) explained the replacement mechanism in biotite and hornblende, respectively[105].

In addition, anthropogenic sources that increase the F level in the groundwater include the excessive use of pesticides and potash fertilizer in the agricultural areas.
4.4. Correlation between fluoride and other parameters
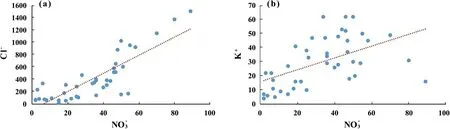
Fig. 4. Bivariate plot showing the relationship of (a) nitrate-chloride and (b) nitrate-potassium. Parameters are expressed in mg/L.
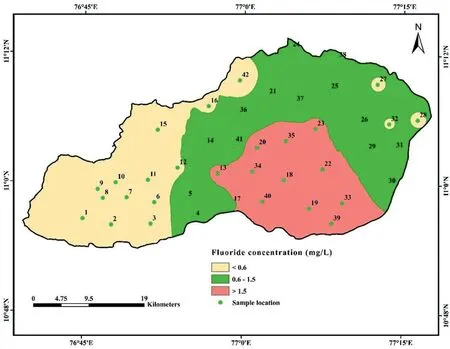
Fig. 5. Spatial distribution map of fluoride in groundwater.
The relationship between fluoride and other parameters was examined using bivariate plots (Fig. 6). These parameters were chosen based on previous researches and their importance in Fmobilization. Numerous studies have demonstrated the importance of pH in water fluorine content[106].The scatter plot shows that pH and F-are positively correlated.As the pH value increases,fluoride concentration varies positively in most groundwater samples. Fluorine is mainly dependent on the pH of the groundwater. Fluorite (CaF2) dissolves more easily in alkaline and slightly alkaline water. Therefore, the level of F-in groundwater increases as the pH value rises.Similarly,in the case of F-and TDS,a positive correlation is observed like pH. Increasing ion concentrations due to high TDS levels will hasten F-dissolved into the groundwater, causing contamination [107]. A strong relation between F-and EC is shown in Fig. 6 (c), implying that F-increases with rising amounts of EC. Fig. 6(d) shows that Na+and F-ions have a positive correlation. Anthropogenic influences and the weathering of silicate minerals can contribute to elevated sodium levels in groundwater[108].Fig.6(e)demonstrates that the content of F-is negatively correlated with the content of Ca2+. Fluoride level decreases as Ca2+amount increases,which may be due to the formation of insoluble fluorite(CaF2),apatite Ca5(PO4)3F,and other solid materials[109].The following reaction(Eq.(6))can be used to determine the solubility of calcite and fluorite in groundwater[110].


4.5. Piper diagrams

Fig. 6. The relationship between the fluoride concentration and (a) pH/(b) TDS/(c) EC/(d) Na+/(e) Ca2+/(f) HCO3- (all parameters are expressed in mg/L).
The Piper Trilinear Diagram(1953)can identify a specific type of groundwater [112]. A diamond field separates the two triangles in the diagram (the right triangle represents anions and the left triangle as cations).A diamond plot comprises six important zones,representing the various groundwater types (Fig. 7). Generally,recharge zones include Ca-HCO3water type; however, there is a greater chance of higher mineral composition in the discharge zone due to more extended interaction with the aquifer composite. As illustrated in Fig. 7, most of the samples were mixed Ca-Mg-Cl(reverse ion exchange water) type water, followed by Ca-Cl(leachate water) type.
4.6. Hydrogeochemical process
Gibbs plots reveal the mechanism that governs groundwater chemistry [113]. The Gibbs diagram has three critical zones: (i)Precipitation dominance,(ii)Rock dominance,and(iii)Evaporation dominant. These zones are the primary determinant of the hydrochemical composition of groundwater. Many samples are found in the rock dominance field, as shown in Fig. 8. However,there is also evidence of gradual shifts towards the evaporation zone.The results indicate that rock-water interactions and mineral dissolution are primarily responsible for determining the groundwater quality in this area.Many researchers[114-118]asserted that high levels of F-in groundwater were caused by interactions between rock and water.
4.7. Evaluation of non-carcinogenic health risk
As discussed above,the Noyyal river and its tanks are polluted,and the groundwater becomes the most important source to meet the daily requirements in the study area. However, the most prevalent contaminants and toxicants like NO3-and F-are present in groundwater samples at concentrations of 33.33% and 28.57% over the BIS [70] recommended limits. Drinking this contaminated groundwater can be hazardous to human health. As a result, this study examined the health effects of non-carcinogenic contaminants(NO3-and F-) on infants, children, and adults, with water consumption as a key exposure pathway. The HQnitrateconcentrations vary between 0.12 and 5.21 (mean = 1.95), 0.07 and 3.22(mean=1.20),and 0.04 and 1.72(mean=0.64)for infants,children and adults, respectively (Table 3). The results of HQnitrateshow that the exceedance rates are 73.81%,57.14%and 14.29%for infants,children,and adults,respectively.On the other hand,the HQfluorideranges from 0.47 to 5.62(mean=2.82)for infants,0.29 to 3.47(mean=1.74)for children,and 0.15 to 1.85(mean=0.93)foradults.About 90.48%of groundwater samples for infants,85.71%for children,and 45.24%for adults surpassed the safe limit (HQfluoride= 1), suggesting the possibility of non-carcinogenic human health hazards.
The total hazard index (THI) of nitrate and fluoride ions on human health was evaluated using equation(4).Table 3 shows that the THI levels vary from 0.59 to 10.07 (average: 4.76) for infants,0.36 to 6.23(average:2.95)for children,and 0.19 to 3.32(average:2.21) for adults. The recommended safe limit for THI is 1.0, and higher than this specified limit is considered to pose a noncarcinogenic risk to humans [55]. The computed THI values indicate that about 97.62%,92.86%,and 73.81%of infants,children,and adults, are at risk of non-carcinogens (Fig. 9). Furthermore, the results reveal that infants are more prone to non-carcinogenic causing health hazards than children and adults, which may be due to the lower body weight of infants compared to children and adults. Based on the intensity of health risk, the study area is primarily categorized into two zones,namely the safe zone(0<THI≤1)and vulnerable zone (THI>1). Furthermore, the vulnerable zone is subdivided into three:(i)THI between 1 and 2 as moderate health risk zone,(ii)THI between 2 and 3 as high health risk zone,(iii)THI higher than 3 as very high health risk zone.The spatial maps of THI for infants, children and adults show that the safe zone regarding non-carcinogenic health hazards is mainly located in the western and northern portions of the research area. However, the vulnerable intensity zones are concentrated in the southern and central parts.This result corroborates the NO3-spatial distribution map of NO3-and F-ions(Figs.3 and 5),in which high and very high levels of NO3-and F-are found in the THI vulnerable intensity zones and vice versa.
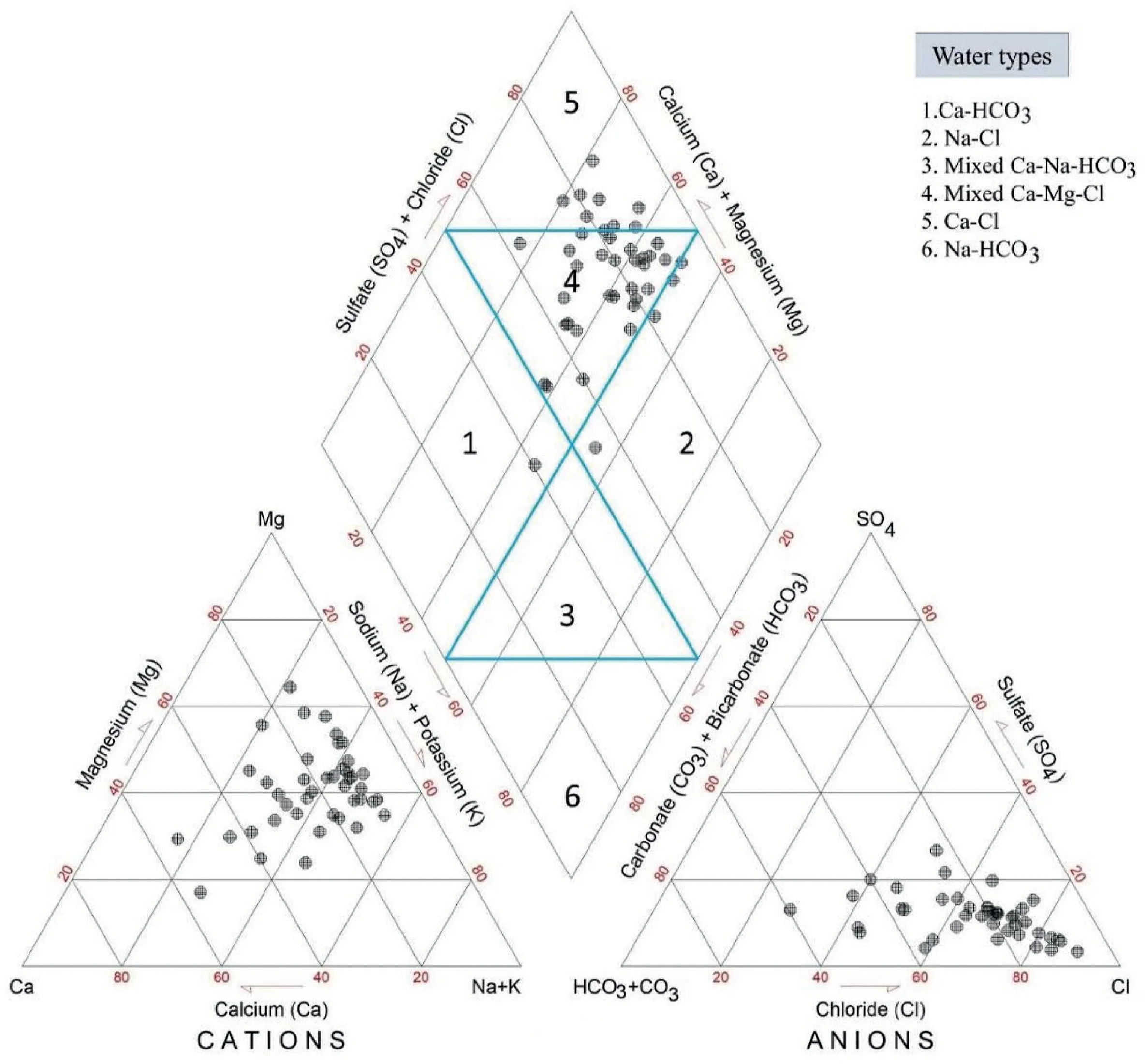
Fig. 7. The piper diagram showing the water types of the study area.
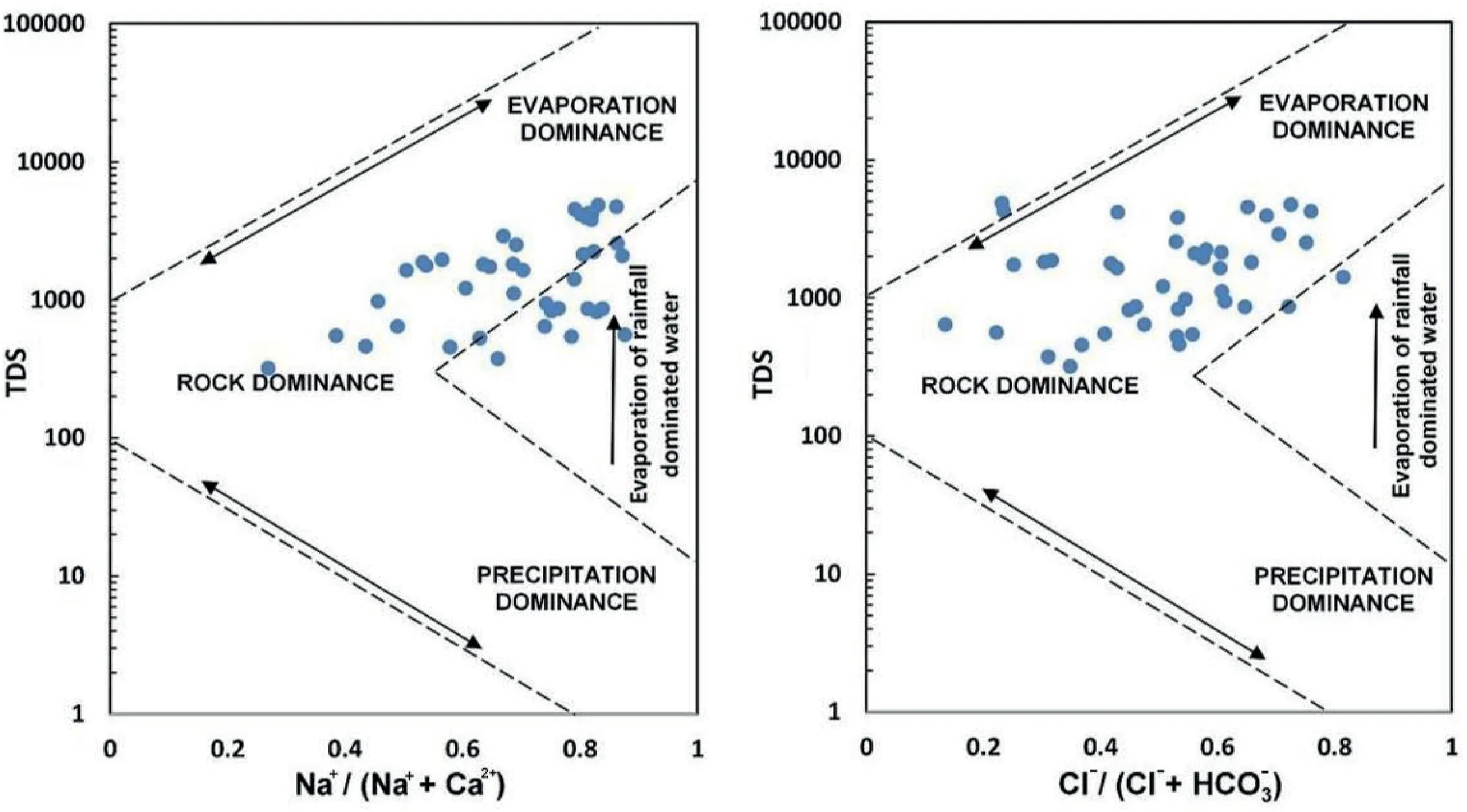
Fig. 8. The Gibbs diagram demonstrating the mechanism controlling the groundwater chemistry of the study area.
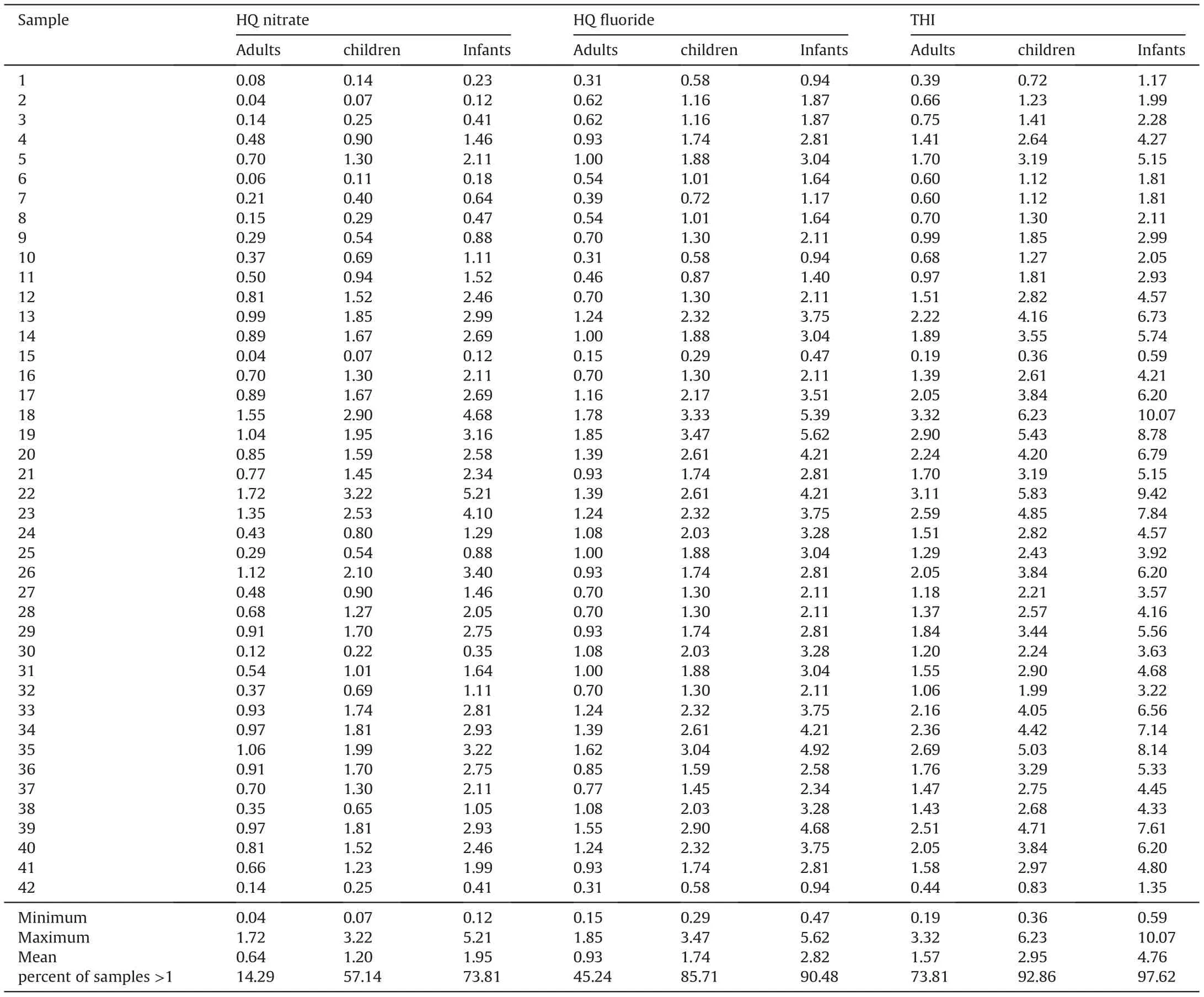
Table 3 Human health risk assessment of nitrate and fluoride in groundwater by Hazard Quotient (HQ) and Total Hazard Index (THI).
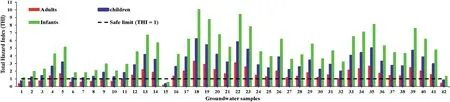
Fig. 9. Total hazard index of groundwater sample for infants, children, and adults.
Similar findings were reported globally by several researchers.A study by Adimalla et al. [63] found that infants and children are more vulnerable to the non-carcinogenic health risks of NO-3and F-contamination in Telangana, India. Karunanidhi et al. [95]evaluated the health hazards posed by nitrate and fluoride in groundwater of Shanmuganadhi basin,South India.They concluded that 87% and 69% of the samples surpassed the standard limit(THI=1)for children and adults,respectively,which indicates that children are more prone to non-carcinogenic risks than adults. Su et al. [19] investigated fluoride and nitrate contamination and the potential health risks in Jiaokou district, China. They found that non-carcinogenic health hazards of F-and NO3-are more susceptible to infants and children than teenagers and adults. In another similar study, Qasemi et al. evaluated fluoride and nitrate contamination in the groundwater of Sabzevar city,Iran,and found higher health risks for children and infants than adults [119].
The present study recommends the following points to improve the quality of drinking water and minimise potential health hazards: (i) a proper water management plan like reverse osmosis treatment, (ii) adequate construction of landfills and septic tanks,(iii) avoiding the excess use of fertilisers and pesticides, and (iv)construction of artificial recharge systems.
5. Conclusion
This study aims to assess the non-carcinogenic risk of drinking nitrate and fluoride contaminated groundwater. For this purpose,42 groundwater samples from different sites of the western Noyyal basin in Tamil Nadu were collected.The hydrogeochemical analysis indicates that cations are in the order of Na+>Ca2+>Mg2+>K+,while anions are Cl->HCO-3>SO2-4>NO-3>F-.Most of the tested sample parameters such as pH(23.81%),EC(66.67%),TDS(33.33%),TH (78.57%), Na+(61.90%), K+(78.57%), Mg2+(30.95%), Cl-(21.43%), NO3-(33.33%) and F-(28.57) are above the drinking standards of WHO. The concentration of NO3-and F-in groundwater varies from 2 to 89 mg/L and 0.2 to 2.4 mg/L,respectively.The Piper diagram demonstrates that groundwater in the study area is predominantly a mixed Ca-Mg-Cl type. The Gibbs diagram indicates that the interaction between rock and water is the most important mechanism for controlling the groundwater chemistry.A strong correlation between NO-3and Cl-in bivariate plot reveals an elevated nitrate could result from human influences. A positive correlation between F-and other quality parameters(pH,TDS,EC,Na+and HCO3-)is shown in the scatter plot.However,a negative correlation with Ca2+is shown in the bivariant plot, indicating Flevel decreases as Ca2+increases, which could be due to the formation of insoluble fluorite, apatite,and other solid materials.
The USEPA method was used to assess the non-carcinogenic health hazards associated with NO3-and F-exposure from drinking water consumption. The ranges of HQnitrateare 0.12-5.21(average 1.95), 0.07-3.22 (average 1.20) and 0.04-1.72 (average 0.64) for infants, children and adults, respectively, while HQfluoridevaries from 0.47 to 5.62 (average 2.82), 0.29 to 3.47 (average 1.74)and 0.15 to 1.85 (average 0.93) for infants, children and adults,respectively. The overall non-carcinogenic health risk evaluation(THI-based assessment) shows that 97.62%, 92.86%, and 73.81% of the samples surpass the recommended limit (THI = 1) for infants,children, and adults, respectively. Thus, the evaluation of HRA reveals that infants and children are more vulnerable to noncarcinogenic health hazards than adults in the research area.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors declare no conflicts of interest in the current research work.
Acknowledgments
The authors express their sincere appreciation to the editor-inchief and anonymous reviewers for their insightful comments and suggestions, significantly enhancing the manuscript.
 Geodesy and Geodynamics2022年6期
Geodesy and Geodynamics2022年6期
- Geodesy and Geodynamics的其它文章
- Models (form) of long-, medium- and short-term earthquake precursors
- Testing of new ionospheric models along the meridian 110° E over the Northern Hemisphere
- Possibilities of mapping neotectonic elements based on the interpretation of space images: A study of Fergana Depression
- Crustal structure of the Qiangtang and Songpan-Ganzi terranes(eastern Tibet) from the 2-D normalized full gradient of gravity anomaly
- Thermospheric density responses to Martian dust storm in autumn based on MAVEN data
- Coastal transgression and regression from 1980 to 2020 and shoreline forecasting for 2030 and 2040, using DSAS along the southern coastal tip of Peninsular India
