Microwave ablation of colorectal liver metastases adjacent to the cardiophrenic angle: Parallel versus non-parallel placement of the antenna relative to the diaphragm
Rui Cui, Xiaowen Liu, Yao Chen, Si Qin, Yimin Wang, Guangjian Liu
Department of Ultrasonography, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, 510000, China
Keywords:Colorectal cancer Liver metastasis Microwave Cardiophrenic angle Interventional ultrasonography
ABSTRACT
1. Introduction
Colorectal cancer is the third most common cancer globally, with 25–30% of patients presenting with or developing colorectal liver metastases (CRLMs) over the disease course.1Surgery is the first-line treatment for CRLMs; however, approximately 75% of patients are not eligible for resection at the time of diagnosis due to the extent of disease or medical condition.2,3For unresectable CRLMs, thermal ablation modalities, including radiofrequency ablation and microwave ablation(MWA),have shown favorable outcomes when combined with standard chemotherapy.4–6Currently,thermal ablation is an established treatment for CRLMs;it is parenchyma-sparing and allows reinterventions.
In clinical practice,CRLMs adjacent to the cardiodiaphragmatic angle are common and difficult to treat with thermal ablation techniques.Previous studies have shown that thermal ablation of liver lesions adjacent to the diaphragm is related to rare diaphragmatic hernias,increased local tumor progression(LTP)rate,and post-procedural pain7,8.Several measures have been proposed to prevent these unfavorable results,including artificial ascites,9one-lung ventilation,10and intra-abdominal carbon dioxide insufflation.11
To date,none of these solutions have focused on antenna placement.As an essential component of pre-ablation planning,antenna placement is vital for treatment safety and efficacy. In most cases, the microwave antenna is placed from the bottom to the top,with the tip pointing to the diaphragmatic surface. This common approach is often associated with risks.It has a risk of puncture or thermal injury to the diaphragm due to the relatively large contact area of the liver and diaphragmatic surfaces.However, the conservative antenna placement depth or ablation parameter setting may contribute to insufficient ablation of the index tumor, which increases the incidence of LTP. From a technical perspective, we considered that the placement of the antenna parallel to the diaphragm,which changes the orientation of the antenna tip,may reduce these risks.Hence,in our center,this approach was also applied to ablate the peripheral CRLMs.
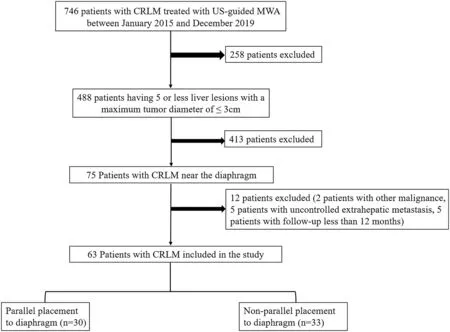
Fig. 1. Flow chart for patient selection.
To preliminary explore the feasibility of this modified technique,we conducted this study to compare its effectiveness and safety with those of the non-parallel placement approach.
2. Methods and materials
2.1. Patients
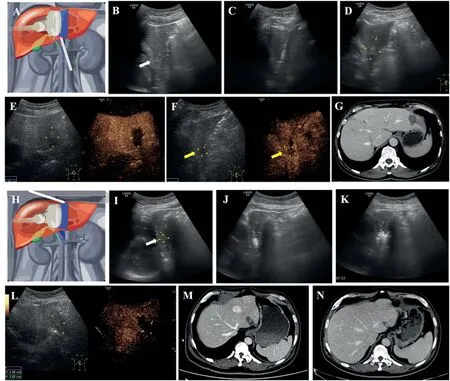
Fig. 2. Imaging evaluation before and after MWA for representative cases of the non-parallel (A-G) and parallel (H-N) groups. (A) Schematic diagram of the nonparallel antenna placement. (B) CRLM is located in S2 with a dimension of 18 * 15 mm in size. (C) The microwave antenna was placed using the non-parallel approach. (D) Two-dimensional sonography of the ablation zone 24 h after MWA. (E) CEUS of the ablation zone 24 h after MWA showed complete ablation. (FG) Two months after MWA, CEUS (F) and contrast-enhanced CT imaging showing growing foci of local tumor progression in the deep side of the ablation zone. (H)Schematic diagram of the parallel antenna placement.(I)CRLM is located in S2 with a dimension of 14*12 mm.(J-K)The microwave antenna was placed using the parallel approach. (L) CEUS of the ablation zone 24 h after MWA showed complete ablation. (M-N) Three months and 12 months after MWA, contrast-enhanced CT imaging showing no local tumor progression.
This retrospective study was approved by the local institutional ethics committee. Written informed consent was obtained from each patient before the procedure. A total of 746 patients with CRLMs underwent ultrasound-guided MWA at our hospital between January 2015 and December 2019. Of these, 63 patients had CRLMs adjacent to the diaphragm. The eligibility criteria were as follows: (a) 5 or fewer liver lesions with a maximum tumor diameter of ≤3 cm, (b) absence of malignancies other than colorectal cancer, and (c) absence of uncontrolled extrahepatic metastasis.The exclusion criteria were as follows:(a)location of the lesion not adjacent to the diaphragm, (b) evidence of direct invasion of the diaphragm or vasculature, and (c) follow-up duration <12 months (Fig. 1). In this study, CRLMs near the diaphragm were located <10 mm from the diaphragm on pretreatment contrast-enhanced computed tomography(CECT)or magnetic resonance imaging (CEMRI). The images were reviewed by blinded radiologists.The distance from the closest lesion to the diaphragm was measured in both the sagittal and coronal planes.The minimal diameter was used as the criterion for identifying lesions near the diaphragm.
2.2. Patient grouping
All patients were classified into parallel and non-parallel groups according to the method of antenna placement.In the non-parallel group,the microwave antenna was placed in the foot–head direction with the antenna tip pointing at the diaphragmatic surface(Fig.2A–C).With this approach,the long axis of the antenna was not parallel to the surface of the diaphragm,and patients with CRLMs ablated using this method were allocated to the non-parallel group.In the parallel group,the microwave antenna was placed in the head–foot direction and parallel to the diaphragm(Fig.2H–J).
2.3. MWA procedures
Before MWA, hydrodissection was performed to alleviate pain and prevent thermal injury to the diaphragm during ablation before antenna placement.To perform hydrodissection,a 22-G needle with a length of 7 cm was inserted into the site between the liver surface and adjacent diaphragm. Subsequently, a 30–50 mL mixture of 2% lidocaine and saline(9:1)was injected for space separation.
Ablation was performed under local anesthesia with lidocaine supplemented by intramuscular injection of pethidine for intraoperative analgesia. Before MWA, all patients received intramuscular injection of 50 mg of Demerol (Chaohui; Shanghai Pharmaceuticals, Shanghai,China). All MWA procedures were performed by a specialized interventional physician with more than 20 years of experience. All ablations were conducted under real-time ultrasonography (LOGIQ E9; GE Healthcare, Milwaukee, WI, USA) using a 2450-MHz MWA system (KY-2000;Canyou Medical Instruments,Nanjing,China),which consists of a microwave generator and 16-G internally cooled antenna with a 5-mm active tip and 10-cm shaft. For lesions with a diameter <2.0 cm, one antenna was inserted.For lesions with a diameter ≥2.0 cm,two antennas were inserted at a distance of 1.0–1.5 cm. The energy output was adjusted between 45 and 60 W at 5–10 min for each session.The needle track was ablated at the end of the procedure to prevent bleeding and tumor seeding. Contrast-enhanced ultrasonography (CEUS) was performed immediately after MWA.On CEUS,complete ablation was characterized by the complete overlap of the non-enhancement ablation zone and target tumor. Additional MWA was performed if CEUS suggested incomplete ablation.
2.4. Data measurement and follow-up
The clinical and laboratory data of the patients before and after MWA were collected by reviewing the electronic medical records. CEUS was performed 1,2,and 3 months after MWA.In the follow-up,CEUS,CECT(Aquilion One, Toshiba Medical System, Tokyo, Japan), or CEMRI (Optima MR360,GE Healthcare,Milwaukee,WI,USA)was performed every 3 months during the first year and every 6 months thereafter.
The primary outcomes were technical success and LTP, assessed according to the standardization of terminology and reporting criteria for image-guided tumor ablation.12Technical success was defined as a complete covering of the tumor by the ablation zone on CEUS,CECT,or CEMRI after tumor treatment according to the protocol.LTP was definedas the appearance of a hyper-enhanced nodule with washout located at the marginal area of the ablated zone after the tumor had been documented as having been completely ablated.For lesions with LTP,further ablation was conducted. In patients with LTP who were not eligible for ablation,systemic chemotherapy was administered whenever possible.
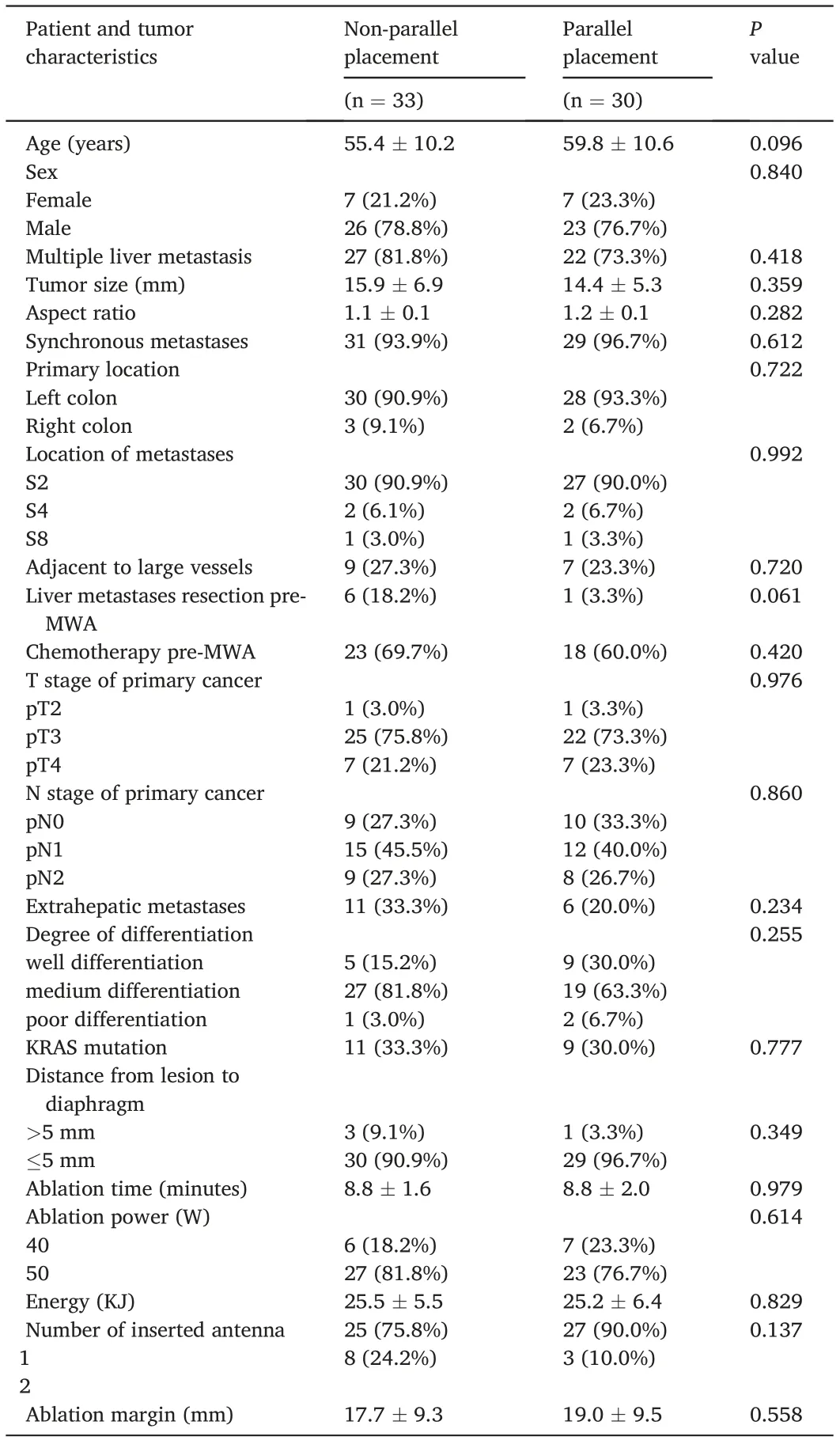
Table 1 Baseline characteristic of the patients.
The secondary outcome was the presence of complications, which were reported using the most recent version of the Society of Interventional Radiology(SIR)classification standard table.Major complications were defined as events that lead to substantial morbidity and disability,which resulted in an increase in the level of care,hospital admission,or substantial prolongation of hospital stay (SIR classifications C–E).12All other complications were considered minor. Regional pain after MWA was recorded according to the Common Terminology Criteria for Adverse Events(CTCAE)v 4.0 of the National Cancer Institute.13
2.5. Statistical analysis
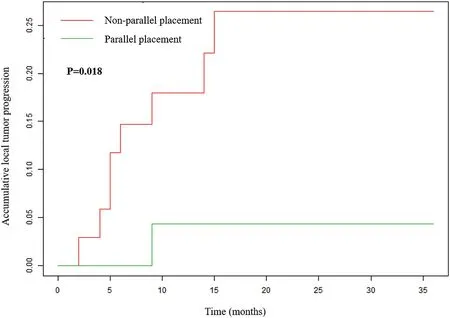
Fig. 3. Local tumor progression of the parallel and non-parallel placement groups.
Data are presented as mean ± standard deviation for continuous variables and percentages for categorical variables according to the antenna placement method.Continuous variables were compared using the Kruskal–Wallis test, and categorical variables were compared using the chi-squared test. The LTP rates were plotted using the Kaplan–Meier method and compared using the log-rank test.The prognostic factors for LTP were assessed using the Cox proportional hazards regression model.Data were analyzed using the R (The R Foundation; http://www.r-pr oject.org; version 3.1.2 2014-10-31) and Empower (R) (www.empow erstatus.com;X&Y Solutions Inc.)statistical packages.
3. Results
3.1. Baseline characteristics of patients
A total of 63 patients with 63 CRLMs adjacent to the diaphragm were included in the study.The mean age was 57.5±10.5 years(range,30–85 years).The mean follow-up duration was 19.7±8.2 months.The mean lesion size was 15.2±6.1 mm.The mean aspect ratio in the longitudinal plane was 1.1±0.1.The mean distance from the lesion to the diaphragm was 1.9 ± 2.4 mm. The baseline characteristics of the parallel and nonparallel groups were well balanced(Table 1).
3.2. Local tumor progression
Fig. 2 shows a representative case before and after MWA for each group.The rate of technical success was 100%(63/63)for CRLMs treated with MWA. During the follow-up, 8 of 33 (24.2%) lesions in the nonparallel group had LTP within 2–14 months, while 1 of 30 (3.3%) lesions in the parallel group had LTP within 4 months(P=0.018,Fig.3).The details of the patients with LTP in the two groups are shown in Table 2. Multivariate Cox regression analysis was conducted in all patients. The results showed that parallel antenna placement was independently associated with LTP (hazards ratio [HR], 0.100; 95%confidence interval [CI], 0.000–0.800; p = 0.034) after adjusting for possible risk factors,including age,sex, tumor size,and KRAS mutation(Table 3).
3.3. Complications
There were no major complications related to the ablation of subphrenic lesions. No arrhythmias occurred during or after MWA.No diaphragmatic injury occurred in any patient. Minor complications developed in one patient with a subphrenic lesion in the non-parallel group. In this patient, there was a small remnant of left pleural effusion 3 days after MWA,and no drainage was observed.Pain was the most common side effect of MWA. Fourteen (42.4%) patients in the nonparallel group and 13 (43.3%) patients in the parallel group complained of local pain with CTCAE grades 1–2 after MWA.
4. Discussion
Thermal ablative techniques, as alternative local treatments to surgical resection recommended by the National Comprehensive Cancer Network guidelines and European Society for Medical Oncology consensus guidelines,are intended to eradicate all visible lesions. However,lesions adjacent to the cardiophrenic angle,which make it difficult to achieve a balance between efficacy and safety, are often difficult to ablate. In our previous study, which included 411 CRLMs, the location adjacent to the diaphragm was identified as a risk factor for LTP,which was associated with three times higher risk than other locations.14To ensure sufficient ablation margins, ablating lesions adjacent to the cardiophrenic angle requires extensive planning and guidance of antenna placement and appropriate preset of ablation parameters.To ensure safe ablation of these lesions, operator caution often contributes to under-treatment,15which may account for the higher incidence of insufficient ablation and LTP than those with lesions in other segments.9,16
In this study, ablation using a parallel approach resulted in a significantly lower LTP rate than that using the non-parallel approach (3.3%vs.24.2%);the parallel approach can reduce the LTP rate of subphrenic lesions. Theoretically, with the long axis of the antenna parallel to the diaphragmatic surface,the subphrenic part of the targeted lesion may be covered by the short axis of the ablation zone,which enlarges regularly with time.With the microwave antenna,it is simpler to predetermine the ablation area using the side direction than that around the antenna tip.Hence, this approach may provide assurance of sufficient ablation of lesions near the cardiophrenic angle to a great extent.
To ensure the feasibility of parallel antenna placement,it is important to fully understand the related anatomy. The part located near the cardiophrenic angle is the bare area of the liver, which is a triangular nonperitoneal area.17,18For lesions in this special anatomical location, the following factors need to be considered for ablation therapy.First,hydrodissection should be performed by injecting diluted lidocaine to distend the space between the liver and diaphragmatic plane before antenna placement,which enhances visibility.Second,for lesions with a long axis that isnot parallel to the diaphragmatic surface,the deepest part of the ablation zone should be monitored carefully in case of injury to other structures.
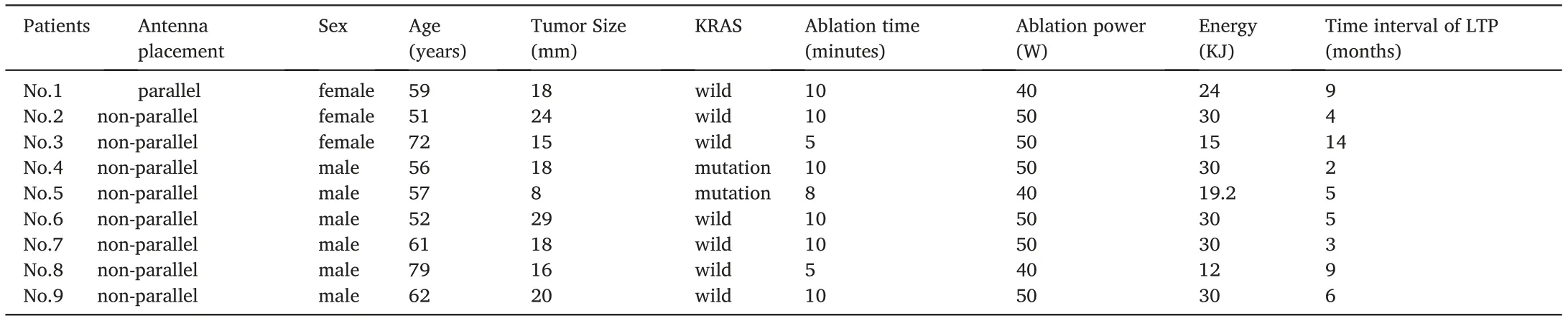
Table 2 The characteristic of the patients with LTP.
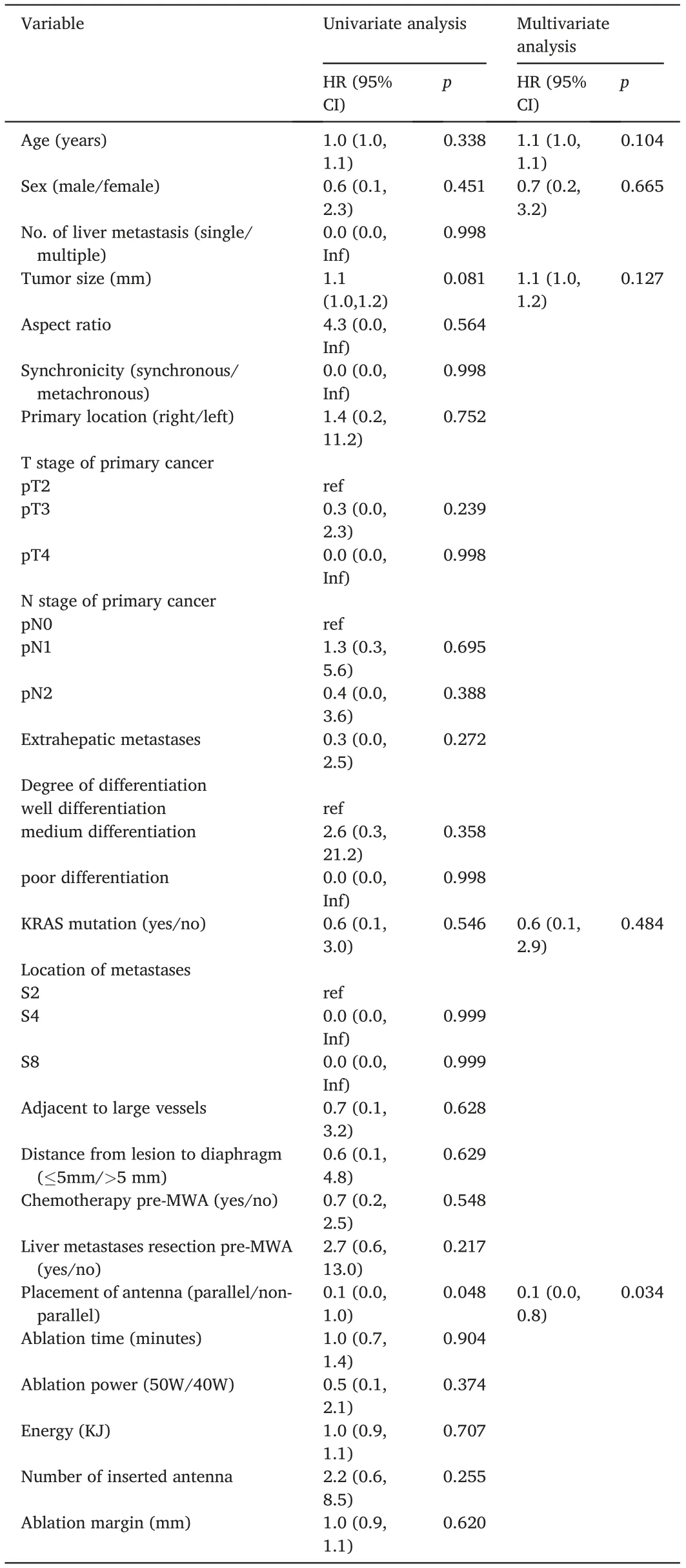
Table 3 Univariable and multivariable analysis of prognostic factors for LTP.
Our results showed no major complications of the ablation in any patient in the two groups.However,there are reports of direct puncture or thermal damage to the paracardiac tissues, resulting in serious complications, including diaphragmatic hernia, cardiac tamponade, and death.7,19,20Unlike the non-parallel approach, the parallel approach prevents the antenna tip from pointing at the subcardiac area, which decreases the risk of damage following a direct puncture. Regarding thermal damage,a larger ablation range may increase the risk of collateral damage to the diaphragm.21In our study, the parallel approach resulted in sufficient ablation of lesions near the cardiophrenic angle without compromising safety.
This study has limitations.First,the sample size was small.However,as a preliminary attempt of the parallel needle approach for CRLMs,the current sample size facilitated the identification of the tendency of a difference in the LTP rate. Second, selection bias was inherent in this retrospective cohort study.When using ultrasound to guide the ablation procedure,the feasibility of antenna placement should be assessed after considering the visibility of the target lesion and safety of the path,which may be influenced by abdominal scars, xiphoid costal angle, and so on.Further prospective studies with larger samples are warranted, and we hope that our initial results will provide practical foundations for these studies.
In conclusion, our results show that the placement of the antenna parallel to the diaphragm is an alternative and effective method for MWA of CRLMs near the cardiophrenic angle and can reduce the LTP rate.
Statements and declarations
Funding:This study was funded by the Science and Technology Program of Guangzhou,China(202102020992).
Declaration of competing interest
We declare that we have no conflict of interest.
 Journal of Interventional Medicine2022年3期
Journal of Interventional Medicine2022年3期
- Journal of Interventional Medicine的其它文章
- Safety and efficacy of the SeparGateTM balloon-guiding catheter in neurointerventional surgery: A prospective, multicenter, single-arm clinical trial
- Uterine artery embolization combined with ultrasound-guided dilation and curettage for the treatment of cesarean scar pregnancy: Efficacy and 5–8-year follow-up study
- Efficacy of contrast-enhanced ultrasound-guided percutaneous core needle biopsy in anterior mediastinal masses
- Diagnosis of hepatic inflammatory pseudotumor by fine-needle biopsy
- Recent advances of multimoda ultrasound in image-guided prostate-targeted biopsy
- A canine model of aortic arch aneurysm created with autologous pericardium
