Safety and efficacy of the SeparGateTM balloon-guiding catheter in neurointerventional surgery: A prospective, multicenter, single-arm clinical trial
Hun Liu, Rufeng Ji, Ynyn He, Tengfei Zhou, Lingfu Zhu, Yonghong Ding,Juh Antero Hernesniemi, Tinxio Li,**, Yingkun He,*
a Department of Cerebrovascular Disease,Zhengzhou University People's Hospital,Henan University People's Hospital,Henan Provincial People's Hospital,Henan Provincial Neurointerventional Engineering Research Center, Henan International Joint Laboratory of Cerebrovascular Disease and Henan Engineering Research Center of Cerebrovascular Intervention, Zhengzhou, 450003, China
b Department of Radiology, Mayo Clinic, Rochester, MN, USA
c“Juha Hernesniemi” International Center for Neurosurgery, Henan Provincial People′s Hospital, Zhengzhou, China
Keywords:SeparGateTM balloon guiding catheter Safety and efficacy Neurointerventional surgery
ABSTRACT
1. Introduction
Cerebrovascular disease is the second leading cause of death.1In recent years,neurointerventional therapy has significantly improved the procedural success and favorable prognosis rates of these patients.2,3However, some technical problems remain unresolved. Plaques that rupture during surgery escape to the distal vessel and cause ischemic events in atherosclerotic patients.4Intraoperative aneurysm rupture results in subarachnoid hemorrhage with high morbidity and mortality rates.5Vascular tortuosity renders the interventional device inaccessible to the target vessel.This can preclude some patients from interventional therapy.6
The balloon-guiding catheter(BGC)can both deliver the intervention device as a support catheter and temporarily block blood flow by inflating the balloon, which can prevent or reduce the above complications.However,the use of BGC is controversial considering puncture site hematoma, iatrogenic dissections, other complications, and additional medical expenses.7,8The limited domestic BGC product options also impede its clinical use.
The domestic SeparGate™BGC (Hunan Ruikangtong Technology Development Co., Ltd.) is registered with the China Food and Drug Administration (CFDA). This trial is the first to evaluate the safety and efficacy of SeparGate™BGC in endovascular therapy for cerebrovascular disease.
2. Method
2.1. Study design and patients
This prospective multicenter single-arm study aimed to verify the safety and efficacy of the SeparGate™BGC in neurointerventional surgery (registration number: ChiCTR1800014459). Between February 28,2018, and September 28, 2019, 129 patients from seven hospitals in China were enrolled. Eligible patients were aged 18–80 years and required temporary blocking of the blood flow of the supra-aortic arch arteries and their branch vessels during interventional therapy.Patients were excluded if they were diagnosed with heart, lung, liver, kidney failure, or other serious diseases; generalized infection; severe coagulation disorders;or severe known contrast allergy.Before implementation,the ethics committee of each participating center approved the trial protocol. Written informed consent was obtained from patients or their legally authorized representatives before enrollment. Follow-up was performed preoperatively,intraoperatively,and at 7 days postoperative.The details are described elsewhere.9
2.2. Study device and procedure
The SeparGate™BGC is a double-cavity catheter with steel wire mesh reinforcement including the catheter cavity to allow access to the interventional device and the balloon cavity to inject the contrast agent and water. The distal part of the catheter was equipped with radiopaque markers and a compliant balloon. When filled, the balloon can temporarily block the blood flow from the proximal to the distal vessel. The proximal portion of the BGC is a Y-joint connecting the catheter cavity and the balloon cavity.Fig.1 shows the detailed structure of the device.
Patients who underwent BGC adjuvant therapy for arterial embolization, stent angioplasty, recanalization of arterial occlusion, or other procedure according to their medical history and the investigators’clinical diagnosis were included. The contrast agent and normal saline were mixed in a 1:1 ratio as a balloon inflation medium. To reduce the complications caused by air embolism, the balloon cavity was degassed until no air bubbles were aspirated. Next, the balloon was inflated and deflated to check for leaks or shape abnormalities. Intraoperatively, the catheter lumen was flushed with heparinized saline and the air was evacuated.According to surgical requirements,the BGC was inserted into the target vessel with the guiding wire under X-ray fluoroscopy,and then the guiding wire was removed.Angiography was performed to measure the vessel size and determine the balloon diameter for expansion and the required filling fluid volume.The filling fluid was then injected into the balloon, and occlusion was simultaneously observed through digital subtraction angiography.After the necessary operation,the balloon was deflated to avoid prolonged ischemia of the distal blood vessels. The balloon was completely deflated prior to catheter withdrawal. Fig. 2 shows a typical case treated with the SeparGate™BGC.
2.3. Outcome measures
The primary endpoint was the immediate operation success rate defined as successful delivery of the interventional devices to the target location, temporary blockage of the blood flow, and successful withdrawal. The secondary endpoints were intraoperative product performance evaluated by the operators on a scale of 1–5 of its rigidity,smoothness, and fracture resistance of the catheter wall, catheter push performance and compatibility,radiopaque display of the catheter under X-ray guidance, catheter integrity and adhesion thrombus after withdrawal,and incidence of balloon rupture.
The safety endpoints were adverse and serious adverse events.Adverse events were defined as unfavorable medical events occurring during a clinical trial,whether or not related to the test device,including hematoma or bleeding at the puncture site, vasospasm, vascular dissection, vascular perforation, air embolism, acute or subacute thrombosis,vascular occlusion,distal vessel embolism,infection,adverse reactions to antiplatelet/anticoagulant drugs or contrast agents, intracranial hemorrhage,stroke and death,and device defect rate including device fracture,poor compatibility,and error markers.Serious adverse events can result in death or serious health deterioration.
2.4. Statistical analysis
The sample size was determined by the assumption of an immediate procedural success rate of 99%with an evaluation standard of 94%,onesided statistical significance level of 0.025, test power of 80%, and maximum dropout rate of 5%. Categorical data are summarized as numbers and percentages,while continuous variables are shown as mean±SD.The primary endpoint was analyzed based on the full analysis set(FAS) and per protocol set (PPS). The asymptotic normal or exact probability method was used to analyze the immediate procedural success rate and 95% confidence interval (CI). The lower-bound CI was compared with the preset target value with a one-sided significance level of 0.025 to judge whether the test product met clinical application needs.Baseline data, secondary endpoints, and safety endpoints were descriptively analyzed based on the FAS,with a two-sided significance level of 0.05. Adverse events are described as number and incidence, while the relationship between specific presentation,severity, and device implantation are described in detail.
3. Result
3.1. Patient and vascular disease characteristics

Fig. 1. Description of balloon guiding catheter structure. Radiopaque marker (1), balloon (2), catheter (3), catheter reinforcement structure (4), balloon filling seat(5), catheter seat (6).
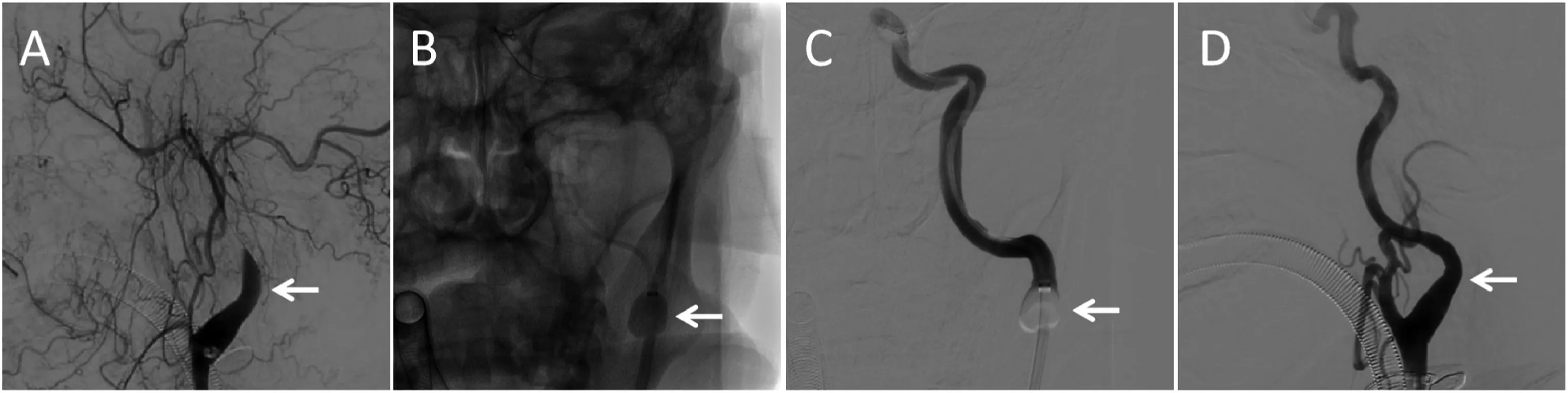
Fig.2. Balloon guiding catheter use in an acute stroke patient with a left internal carotid artery occlusion(A).The balloon guiding catheter was dilated(B and C),and no dissection occurred at the site after recanalization (D).
According to our statistical calculations, this study required at least 128 patients. A total of 129 patients were included; of them, 128 were included in the FAS and PPS,while one patient was excluded for no use of a matching arterial sheath. The mean patient age was 60.57 ± 12.37 years, and 87 (67.97%) patients were male. Preoperative diagnoses included intracranial aneurysm in 4.7% (6/128), intracranial artery stenosis or occlusion in 20.3% (26/128), carotid artery stenosis or occlusion in 38.3% (49/128), and other diseases such as acute cerebrovascular disease and cavernous sinus fistula in 78.9%(101/128)patients.The patients’ baseline characteristics and medical history are summarized in Table 1.
3.2. Procedure
Intracranial aneurysm embolization was performed in 3.9% (5/128)of patients,intracranial stent angioplasty for arterial stenosis in 3.1%(4/128), recanalization and stent angioplasty for intracranial arterial occlusion in 8.6% (11/128), recanalization for acute cerebrovascular occlusion in 25.8%(33/128),stent angioplasty for carotid arterial stenosis in 22.7%(29/128),recanalization for carotid arterial occlusion in 18.8%(24/128),and other in 52.3%(67/128).Right femoral artery access was established in 96.9%of patients versus left femoral artery access in 3.1%patients.The target vessel location included the common carotid artery,internal carotid artery, vertebral artery, and other vessels. All BGC products successfully delivered interventional devices, including microguide wires, micro-catheters, guiding catheters, stent retrievers, and other devices. Temporary blockage of the blood flow failed in 2.3% of cases. All test devices were successfully withdrawn. The procedure details are summarized in Table 2.
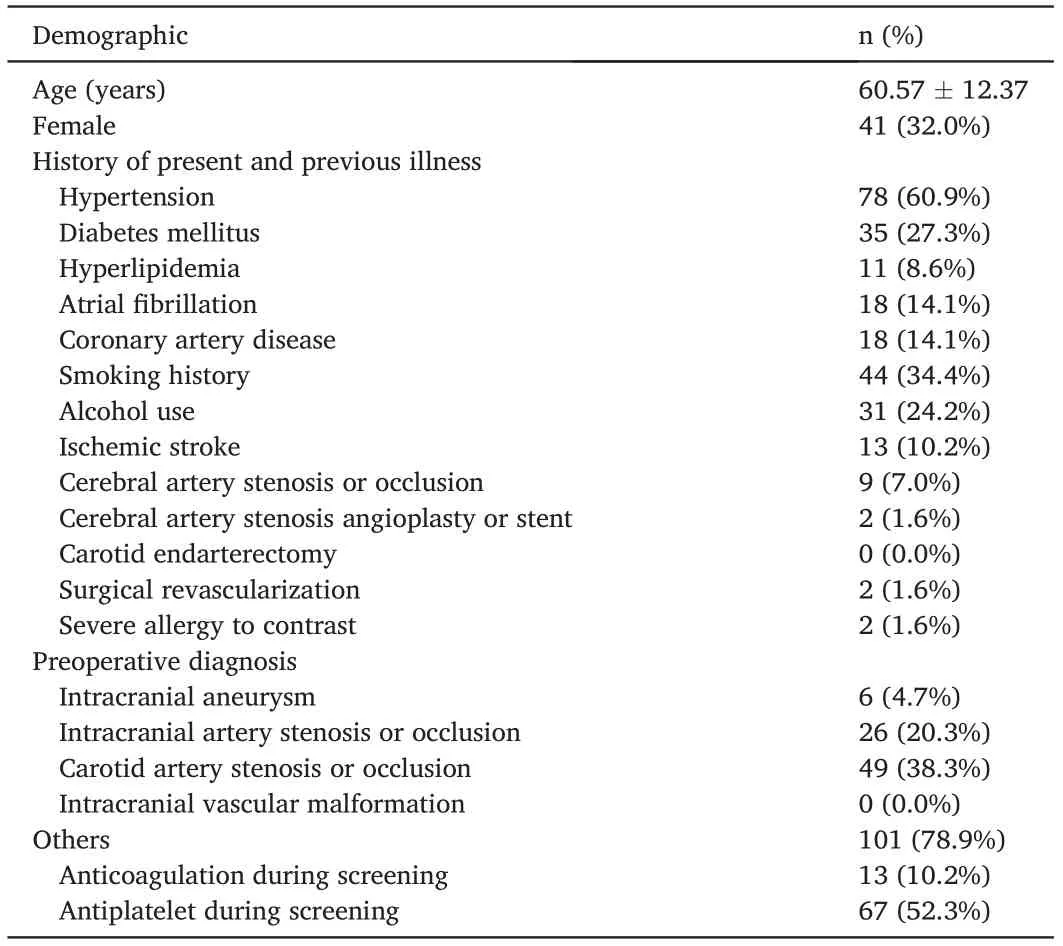
Table 1 Patient baseline characteristics (n = 128).
3.3. Efficacy outcomes
For the primary outcome,97.7%(125/128)(95%CI,94.6–100.0%,P= 0.0146) of patients achieved immediate procedural success rates in terms of FAS and PPS.The lower bound of the 95%CI was 94.6%,which is greater than the preset target value of 94%, indicating that the test device meets the clinical application requirements. For the secondary endpoints,most patients had a score of 4–5 in operation,radiopaque,and withdrawing performance with average scores of more than 4.5 and no cases of balloon rupture. Table 3 shows the details of the efficacy outcomes.
3.4. Safety outcomes
Adverse events occurred in 78.9% (101/128) cases, while serious adverse events occurred in 21.1%. Two (1.6%) were determined to be possibly related to the test device but not the technique. In one patient with acute ischemic stroke and left middle cerebral artery occlusion caused by an internal carotid dissection of moderate severity, the test device aggravated the dissection during balloon inflation and blood flow blockage. Stent angioplasty was performed for the dissection, and the lesion had disappeared by the 1-month follow-up. In the other devicerelated adverse event,vasospasm occurred in the C1 segment of the left internal carotid artery (ICA) during the delivery of a 7.5F balloon catheter,but it spontaneously disappeared.No device defects were observedduring the trial.The mortality rate was 8.59%(11/128),and none of the deaths were related to the test device or procedural technique.
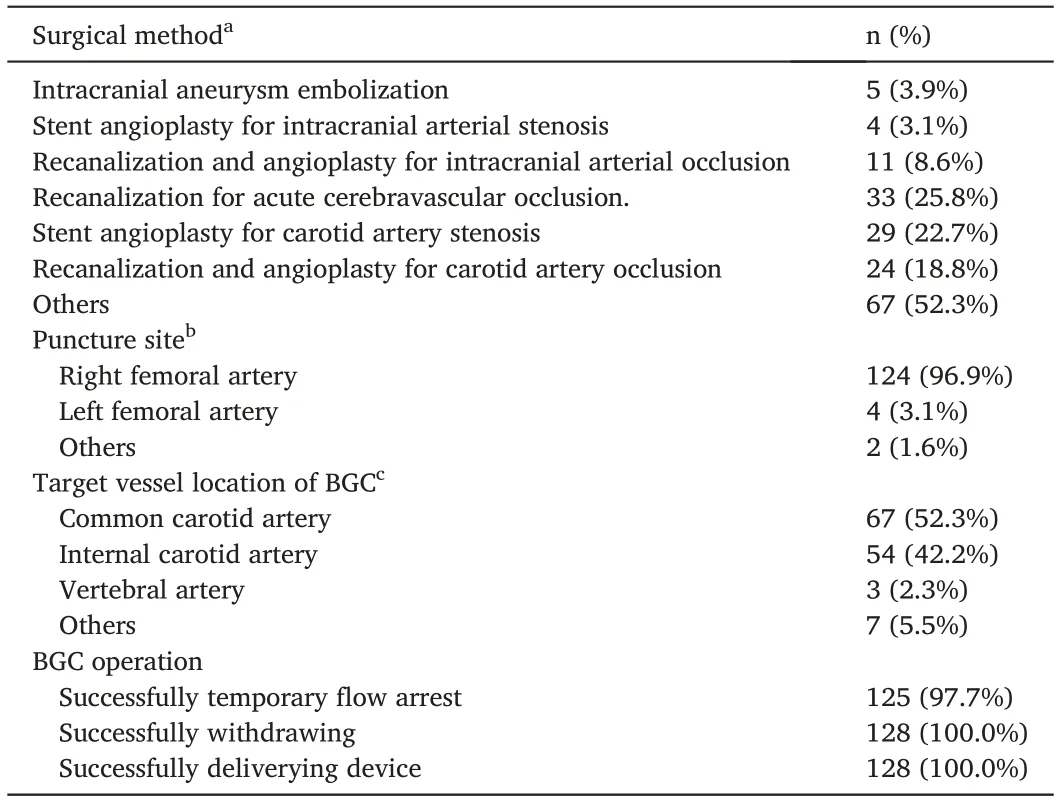
Table 2 Procedural details(n = 128).
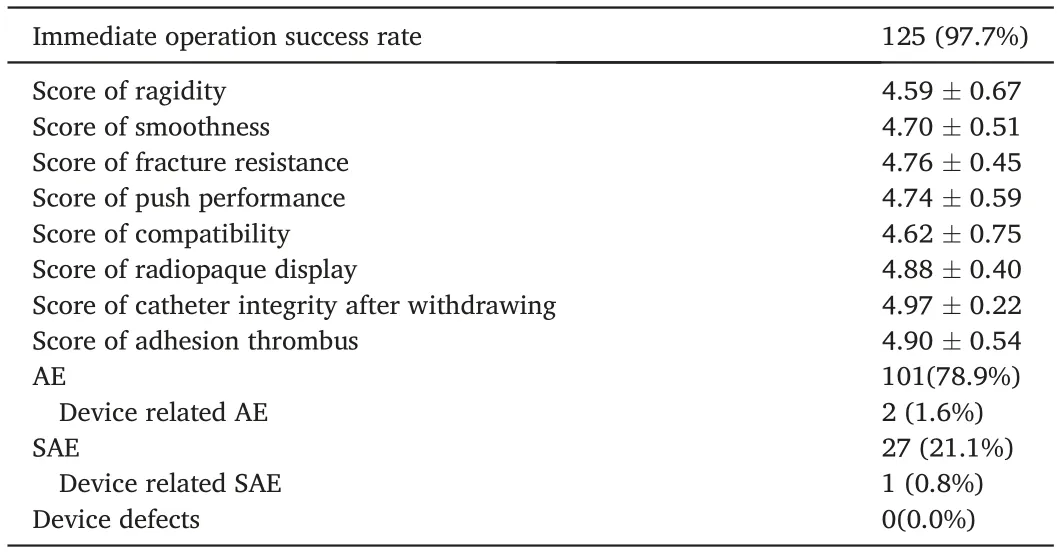
Table 3 Outcome details(n = 128).
4. Discussion
This prospective multicenter single-arm study showed the efficacy of the SeparGate™BGC at blocking anterograde blood flow and delivering an interventional device, with good product performance, radiopacity,and withdrawal processes during the procedural. The incidence of adverse events and severe adverse events associated with the test devices was low,suggesting that BGC is a safe and effective adjuvant therapy for cerebrovascular disease.
The immediate procedural success rate is the basic effectiveness index for evaluating surgical instruments,but few studies have reported it.The immediate procedural success rate was 97.7%, with a low boundary of 95%CI,higher than the preset efficacy margin.A Korean study published in 2019 using a similar BGC for interventional treatment of stroke showed an immediate procedural success rate of 97.2% (70/72),10consistent with our results. This result primarily demonstrates the efficacy of BGC for blocking anterograde blood flow and delivering interventional devices and indicates that the tested BGC can meet the basic clinical application requirements. The main purpose of temporarily arresting the blood flow is to prevent ischemic events caused by clot fragmentation and distal embolization,11,12improve coil stabilization during aneurysmal coiling and offload to utilize aneurysmal neck-remodeling balloons for additional adjunctive techniques or deploy rescue stents,13,14and control bleeding caused by intraoperative vessel perforation or aneurysm rupture. This trial also enrolled patients with different cerebrovascular diseases. Although the additional benefits of BGC adjuvant therapy have not been analyzed, the device procedural success rate provides theoretical feasibility for the trial design of the BGC that verifies the adding benefit of improved patient prognosis for different cardiovascular diseases.
Device performance is an important factor affecting its popularity and application, and it determines the procedural success, risk of complications,and surgeons’consideration of specific devices.Compatibility and flexibility are common reasons for surgeon reluctant to use the BGC in previous investigations.7,8In our trial,device performance was set as the secondary outcome,and more than 90%of researchers scored 4–5 points for each performance, indicating the good performance of the Separ-Gate™BGC for interventional diagnosis and treatment.Delayed therapy and increased procedural time are essential reasons for unfavorable outcomes and complications,especially in cases of acute cerebrovascular disease.15,16The time from a puncture to device placement in the target location and the entire operation time are more subjective and comprehensive indicators of device performance and,thus,should be considered for differences in disease types and vessel tortuosity in future studies.
Concerns about potential adverse events prevent the routine use of BGC in neurointerventional procedures.Common device-related adverse events included hematoma or bleeding at the puncture site, vasospasm,and vascular dissection. A larger sheath is needed for BGC use which is reported as a risk factor for access site complications.17,18However, a previous study showed that groin complication rates are very low,even with 8 Fr and 9 Fr sheaths19. In our study, no arteriopuncture-related complications occurred. In a recent report, the sheathless technique of BGC has been supported to reduce access site complications when a large sheath is required, such as for carotid artery stenting and coil embolization.20Therefore,it is unreasonable to abandon BGC adjuvant therapy when considering puncture complications.
Vasospasm and dissection are mainly caused by repeated procedures due to vascular tortuosity,surgeon inexperience,or inappropriate device size. In our trial, two patients (1.56%) had vasospasm (mild adverse events);one case was related to the test device,a finding that is consistent with that of a previous study of a 4.3% vasospasm rate in the Korean population for a similar device.10A dissection in the ICA was aggravated during balloon inflation in one patient (0.8%). Previous studies also showed that BGC use did not increase carotid or groin complications,including carotid dissections, compared to the same procedure without BGC.21,22The distal anchor of the BGC can provide support for the catheter system, which may even contribute to more distal device delivery and reduce surgical complications, especially in an extremely tortuous cervical carotid artery.23Gentle manipulation and suitable device selection can further reduce the risk of complications.
The present study has some limitations.First,it was a single-arm trial lacking a comparison group to compare the outcomes. Second, its nonrandomized selection process may have resulted in selection bias. In addition,a subgroup analysis was not performed to verify the safety and efficacy of BGC for each cerebrovascular disease. Finally, efficacy does not indicate procedural success, favorable patient outcomes and additional benefits of BGC use.
5. Conclusion
The SeparGate™BGC can effectively block blood flow and deliver an interventional device with a low complication rate in neurointerventional surgery. Further results in practical clinical applications can provide a reference for its selection and use.
Ethics approval statements
Before implementation, this trial had been approved by ethics committees of each participating center.
Contributorship statement
Huan Liu and Rufeng Jia analyzed the data and wrote the paper.Tengfei Zhou and Yanyan He selected the data.Yingkun He,Tianxiao Li,Liangfu Zhu, Yonghong Ding, and Juha Antero Hernesniemi were responsible for quality control. All authors read and approved the final manuscript.
Funding
This work was supported by the Co-construction of Provincial and Ministry Youth Project (SBGJ202003004) Scientific and Technological Project of Henan Province (202102310037).
Declaration of competing interest
The authors have no conflicts of interest to declare.
 Journal of Interventional Medicine2022年3期
Journal of Interventional Medicine2022年3期
- Journal of Interventional Medicine的其它文章
- Uterine artery embolization combined with ultrasound-guided dilation and curettage for the treatment of cesarean scar pregnancy: Efficacy and 5–8-year follow-up study
- Microwave ablation of colorectal liver metastases adjacent to the cardiophrenic angle: Parallel versus non-parallel placement of the antenna relative to the diaphragm
- Efficacy of contrast-enhanced ultrasound-guided percutaneous core needle biopsy in anterior mediastinal masses
- Diagnosis of hepatic inflammatory pseudotumor by fine-needle biopsy
- Recent advances of multimoda ultrasound in image-guided prostate-targeted biopsy
- A canine model of aortic arch aneurysm created with autologous pericardium
