A high-rate and ultrastable anode for lithium ion capacitors produced by modifying hard carbon with both surface oxidation and intercalation
ZHANG Lu-yao, WANG He,, QIN Nan, ZHENG Jun-sheng,*, ZHAO Ji-gang
(1. Clean Energy Automotive Engineering Center, Tongji University, Shanghai 201804, China;2. College of Automotive Studies, Tongji University, Shanghai 201804, China;3. International Joint Research Center for Green Energy Chemical Engineering, East China University of Science and Technology, Shanghai 200237, China)
Abstract: Due to the difference of energy storage mechanisms between the anode and cathode materials, the power density or rate performance of a lithium-ion capacitor is greatly limited by its anode material. Hard carbon is a promising anode material for lithium ion capacitors, and its modification is an important way to improve the electrochemical performance of lithium-ion capacitors. A commercial hard carbon from Kuraray Inc was modified by oxidation followed by intercalation with ZnCl2 (ZnCl2-OHC).The reversible capacity of a half-cell prepared using this material was 257.4 mAh·g-1 at 0.05 A·g-1, which is obviously higher than the unmodified one (172.5 mAh·g-1). The capacity retention of a full cell prepared using ZnCl2-OHC as the anode and activated carbon as the cathode reached 43.3% when the current density increased from 0.1 to 10 A·g-1, which is more than twice that of the untreated hard carbon. After 5 000 charge-discharge cycles at 1 A·g-1, the capacity retention of the full cell was about 98.4%. The modification of hard carbon by surface oxidation and intercalation is therefore a promising way to improve its anode performance for lithium ion capacitors.
Key words: Lithium ion capacitors;Anode materials;Hard carbon;Intercalation
1 Introduction
Lithium ion capacitors (LICs) are realized by combining two energy-storage-process electrodes(chemical at the negative and physical at the positive)and balance the advantages of both lithium ion batteries and supercapacitors, which can provide high power density and cycle life while maintaining high energy density. That is why LICs are considered to be the outstanding representative of the next generation of supercapacitors and to have wide applications and broad market prospects[1-6]. The biggest challenge for LIC designers is how to reduce the attenuation of capacity at high current densities, and to further improve the power density and cycle life. According to the different reaction mechanisms of the cathode and anode, the intercalation/deintercalation process of lithium ions is a rate control step on the anode. And its materials greatly limit the power density or rate performance of LICs[7,8].
For anodes of LICs, carbon materials display very different morphological and structural characteristics, and it is reasonable to assume that during the cycling process, their different electron transport rates would affect electrochemical performance of LICs.For limited choices of carbon materials, surface modification of anodes is one of the important means to improve the electrochemical performance[9-12]. The introduction of oxygen-containing functional groups on the surface can provide additional active sites and defects, especially the introduction of carbonyl and carboxyl groups can synergically enhance the diffusion control and the storage process of capacitive lithium ions, and improve the electron transport rate. At the same time, intercalation treatment can widen the interlayer spacing, reduce the resistance of lithium insertion/extraction, improve the diffusion kinetics of lithium ions. The utilization of intercalation treatment allows the realization of reducing the accumulation of"dead lithium" formed in the cycling process and improving the electrochemical performance of LICs[13-18]. However, until now, the combined modification with both treatment methods mentioned above has not been carried out to further improve the cycle life and rate performance of LICs. With this aim, the anode enabled by surface oxidation and intercalation treatment of hard carbon should be designed. It is also worth mentioning that for different carbon materials,specific experimental materials and treatment need to be designed correspondingly.
In this paper, hard carbon was used as the anode material, which was treated by surface oxidation and intercalation, aiming at producing a high-rate and ultrastable anode material. Oxygen-containing functional groups such as carboxyl hydroxyl and intercalation modifiers such as zinc chloride were introduced into hard carbon surface through oxidation acid mixtures to testify the function of the combined modification for LICs. The microstructure and performance optimization have been investigated by several analysis methods based on half cells and full cells to explore the effects of oxidation and intercalation.
2 Experimental
2.1 Electrode preparation
Surface modification. A certain amount of commercial hard carbon from Kuraray of 5 μm was added to a 250 mL three-port flask, then an acid mixture(98% H2SO4and 65% HNO3, 1∶1v/vratio) was added, stirred by a magnetic bar for 0.5 h, then put into an ice water bath and ultrasonicated for 3 h. The mixture was poured into a beaker, to which deionized water was added and centrifuged for enough times until the solution was neutral, and dried overnight in an oven at 80 °C. The importance of ultrasound treatment in the modification process was validated by only magnetic stirring without ultrasonication in the oxidation with the acid mixture. The hard carbon without ultrasonic oxidation was named as HC, and the hard carbon prepared by ultrasonic oxidation as OHC.
Intercalation treatment[13]. Anhydrous ZnCl2at a certain mass was mixed with oxidized hard carbon and dried in vacuum at 120 °C for 12 h. The powder mixture was heated in a closed reactor under nitrogen atmosphere for 3 h at a heating rate of 5 °C·min-1. The final calcination temperature was set at 300, 400 and 500 °C for different samples. After cooled to room temperature, the mixture was washed with 0.1 mol L-1HCl and deionized water until the excess chloride was removed and the residue ZnCl2in the carbon materials would not affect their electrochemical performance, and dried in a vacuum oven at 80 °C for 12 h.The sample from the oxidized hard carbon without ultrasonic oxidation but with intercalation treatment was named as ZnCl2-HC, and that from the oxidized hard carbon prepared by ultrasonic oxidation and intercalation treatment as ZnCl2-OHC.
2.2 Cell assembly and electrochemical test
Anode preparation. An appropriate amount of active material (usually 0.2-0.4 g) was mixed with Ketjen black, CMC and SBR with the mass ratios of 90∶2∶2∶6. After ball milled, the slurry was coated on the current collector by a scraping method and dried in a drying box at 80 °C to remove excess solvents. The target thickness was obtained by rolling,and the circular electrode piece with a diameter of 12 mm was obtained by a slicing machine, which was further heated and dried in vacuum and weighed in the glove box.
For full cell test that requires pre-lithiated anode,a certain mass of stabilized lithium metal powder(SLMP) was weighed and placed on the anode surface, and a scraper was used to spread the powder evenly. Then, the lithium powder was pressed with a pressure less than 500 Pa, until the lithium powder on the anode surface appeared bright metallic luster to complete the pre-lithiation.
Cathode preparation. The preparation process of positive electrode was similar to that of the anode, except that the positive electrode adopted NMP system,the paste was prepared with the mass ratios of as-prepared carbon, Ketjen black and PVDF of 8∶1∶1, using aluminum foil as the current collector.
The half cell was assembled with Li-foil as the anode and the modified hard carbon samples as the cathodes. The full cell was assembled with the modified hard carbon samples as the anode and activated carbon as the cathode. The electrolyte was 1 mol L-1LiPF6in a mixture of ethylene carbonate (EC) and diethyl carbonate (DMC) (1∶1v/vratio). The assembly of half cells and full cells was carried out in a vacuum glove box to prevent the oxidation reaction of the electrolyte and the Li-foil by air.
The constant current charge-discharge test was carried out to obtain electrochemical performance such as the capacitive ratio and cyclic performance of the capacitor by a CT2001A battery test system produced by Wuhan Landian Electronics Co., Ltd. The potential window of half cell test was 0.01-2.0 V, and that of full cell test was 2.2-3.8 V. Cyclic voltammetry (CV) was performed on half cells at various scan rates from 0.01 to 10 mV·s-1under a voltage range of 0.02-2.0 V.
2.3 Characterization
The surface morphology of anode material was observed by an FEI 250FEG scanning electron microscope (FEI Company, USA). The phase compositions of the samples were characterized by X-ray diffractometry (XRD,D/Max-2550) with CuKα radiation (λ=0.154 06 nm). The Renishaw inva Raman spectrometer produced by Renishaw Company in the UK was used to analyze the defects of carbon samples. The N2adsorption and desorption behaviors of the modified hard carbon materials were investigated by the Auto-ChemⅡ2020 automatic surface analyzer produced by Micromeritics, USA. By an X-ray photoelectron spectrometer (XPS) from the American Thermo Scientific Escalab 250Xi, the surface elements, the relative contents of oxygen containing functional groups in the samples were quantitativeyl analyzed.
3 Results and discussion
As mentioned before, the ultrasound treatment is expected to play a noticeable role in the modification process. This is confirmed by the SEM images in Fig. 1. It can be seen from Figs. 1 (a-b) that the untreated hard carbon presents an irregular morphology,the particle size is not uniform and aggregation occurs. The surface of the particles is relatively smooth and has fewer ravines. It can be seen that there are certain grooves and surface defects on the surface of the HC without ultrasound after oxidation as shown in Figs. 1(c-d). Figs. 1(e-f) show the surface morphology of OHC with ultrasonic oxidation has considerably more surface defects due to the ultrasound cavitation effect[19,20]. Ultrasound can prevent the hard carbon in the acidic solution from agglomeration and the surface can be fully and uniformly oxidized. At the same time, the instantaneous shock wave generated by collapsed cavitation bubbles will leave more defects on the hard carbon surface, which is beneficial to the electrochemical performance.
The intercalation treatment was carried out at 400 °C, and the reasons are as follows. Fig. 2(e)shows the XRD patterns of hard carbon and the intercalated hard carbon (ZnCl2-HC) at different temperatures. Both (002) and (100) diffraction peaks appear in the vicinity of 2θ= 23° and 43°. The diffraction peak of (002) is mainly related to the local graphite microcrystalline structure of hard carbon[21]. The broadened diffraction peak indicates that there are some short-range ordered amorphous structures in hard carbon rather than completely disordered. The increase of temperature is conducive to the introduction of ZnCl2and a larger layer spacing is conducive to the intercalation and deintercalation of lithium ions, thus favorable for the rate performance. The (002) layer spacing is the widest at 400 °C and drops when the temperature is increased to 500 °C, which may be caused by the destruction of the internal structure of hard carbon due to the excessive introduction of ZnCl2. The SEM images in Figs. 2 (a-d) can also confirm this result. The ZnCl2-HC and ZnCl2-OHC samples are all calcined at 400 °C in the following experiments.
Figs. 3 (a-d) show the surface morphologies of HC, OHC, ZnCl2-HC, and ZnCl2-OHC, respectively, with a magnification of 10 000. The unprocessed hard carbon surface is smooth. After treatment,the hard carbon particles are irregularly shaped, but the oxidation and intercalation treatment change the surface morphology of the hard carbon, thus exhibiting corroded phenomena and leaving defects in hard carbon surface. The rough surface of particles may increase the contact area between hard carbon and electrolyte.
Fig. 3 (e) shows the XRD patterns of different hard carbon samples. It can be seen that the peak at(002) position of ZnCl2-OHC has a wider half-peak width compared with HC, which indicates that the local graphite microcrystalline structure of ZnCl2-OHC is reduced and that the disordered extent is higher. By comparing the change of peak location, it can be found that the peak location of (002) diffraction peak of HC, OHC, ZnCl2-HC and ZnCl2-OHC is 23.1°, 23.0°, 22.5° and 22.8°, respectively. According to the Bragg equation[22,23], the layer spacing of the corresponding materials can be calculated as 0.386,0.387, 0.396 and 0.391 nm respectively, indicating that the intercalation treatment increases the layer spacing of hard carbon.
Further, ZnCl2-OHC shows a slightly narrower layer spacing comparing to the hard carbon without surface oxygen-containing functional groups. Based on these, it is need to mention that ultrasound oxidation has a negative effect on the graphite microcrystalline structure[24]. Considering that the difference is relatively minor and that the performance enhancement offered by surface oxidation can make up for the deficiency of the layer spacing, ZnCl2-OHC still can be seen as a reliable material to bring the superiority of performance.
Specific surface areas and pore structures of different hard carbon samples are determined by N2adsorption, and the results are shown in Fig. 4. After calculation, the pore structure parameters of hard carbon samples are shown in Table 1. Compared with HC,theSBETandSLANGUIRvalues of ZnCl2-OHC are apparently increased to 47.91 and 120.66 m2·g-1, respectively. The specific surface area determines the capacity of electrode materials to store capacitance,and a larger specific surface area is favorable for ZnCl2-OHC material to generate more capacitance.
Fig. 5 shows the Raman spectra of different hard carbon samples. Hard carbon samples have two distinct peaks at ~1 343 and ~1 584 cm-1, corresponding to peakDand peakG, respectively. The intensity ratio of peakDto peakG(ID/IG) can be obtained through calculation to judge the relative defect content of a material. TheID/IGof HC, OHC, ZnCl2-HC and ZnCl2-OHC are 0.91, 0.96, 0.95 and 0.98, respectively. It can be seen that theID/IGincreases after treatment, indicating that ultrasonic oxidation or intercalation treatment will lead to the increase of surface defects of hard carbon samples. At the same time, theID/IGof ZnCl2-OHC is the largest, which shows that the combination of two methods will cause more defects on the hard carbon surface than the single method. This is also confirmed by the results of XRD.
Fig. 6 shows the XPS spectra of HC and ZnCl2-OHC. There are 2 obvious peaks at binding energies 285 and 532 eV in Fig. 6(a), corresponding to C1s and O1s, respectively[10,25-27]. However, the minor peaks of Cl and Zn indicate that the contents of these two elements are relatively low. Table 2 lists the relative contents of each element on the ZnCl2-OHC surface.Figs. 6(b-d) show the high resolution spectra of C, Zn and Cl of ZnCl2-HC. In Fig. 6 (c), 2 peaks at 1 022.3 and 1 045.0 eV are attributed to 2p3/2and 2p1/2electrons of Zn2+, respectively, indicating that the intercalated compounds are Zn2+compounds. The binding energy of Cl2p in ZnCl2is 198.9 eV. The peak in Fig. 6(d) are deconvoluted to 198.4 and 199.9 eV,which correspond to 2p3/2and 2p1/2electrons of Cl-,respectively, indicates that the intercalated compounds have Cl element in the form of Cl-and that C and Cl have certain valence bond and exist in the form of C-C-Cl.

Table 1 Pore structure parameters of hard carbon samples before and after treatment.
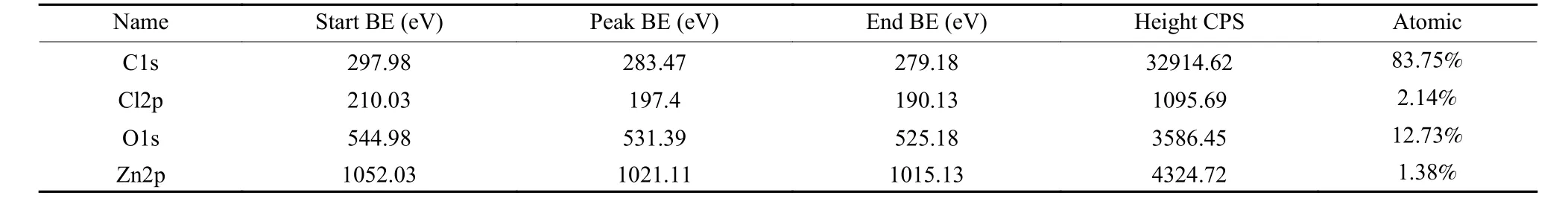
Table 2 Analysis of elements on the surface of ZnCl2-OHC.
Fig. 7 shows the rate performance of half cells of different hard carbon samples. At a current density of 0.05 A·g-1, the specific discharge capacities of HC,OHC, ZnCl2-HC and ZnCl2-OHC anodes are 172.5, 182.1, 210.3 and 257.4 mAh·g-1, respectively.When the current density increases to 0.1 A·g-1, the specific discharge capacities of OHC and ZnCl2-HC electrodes are about 170.0 mAh·g-1, and that of ZnCl2-OHC is up to 246.1 mAh·g-1. When the current density is restored to 0.4 A·g-1, the specific capacity is restored to 184.2 mAh·g-1, and the coulombic efficiency maintains about 100%, which indicates that the ZnCl2-OHC anode has excellent electrochemical stability and that the oxidation and intercalation treatment greatly improves the rate performance of the hard carbon anode. The reasons may be as follows:(1) The synergistic effect of surface oxygen-containing functional groups and intercalators makes more adsorption sites exposed on the surface of lithium ions, which synergistically enhances the diffusion control and capacitive lithium ion storage process.(2) The introduction of the intercalation agent increases the interlayer spacing, widens the diffusion channel of lithium ions and improves the migration rate of lithium ions. (3) The larger pore volume promotes the diffusion of lithium ion and accelerates the reaction kinetic rates.
Fig. 8(a-b) shows the long-term cycling stability of HC, OHC, ZnCl2-HC and ZnCl2-OHC anodes.At a current density of 0.4 A·g-1, the specific capacities of the 4 electrodes increase slightly in the first 15 cycles due to the formation of solid electrolyte interface during activation and the occurrence of electrochemical reactions[28-30]. The initial discharge specific capacities of 4 electrodes are 110.4, 133.4, 144.3 and 184.2 mAh·g-1, respectively. After 1 000 cycles,the specific discharge capacities of 4 electrodes are 55.0, 71.9, 86.0 and 87.7 mAh·g-1, respectively. The coulombic efficiencies are all kept around 100%.Though the cycling performance of the hard carbon material after the combination treatment is weakened and the capacity retention rate is only 47.6%, the discharge specific capacity still remains 87.7 mAh·g-1after 1 000 cycles, due to its largest initial discharge specific capacity. The main reasons for the high discharge specific capacity are that the pseudo-capacitance caused by surface oxygen-containing functional groups and the increase of specific surface area exposes more energy storage sites. At the same time, the capacity of ZnCl2-OHC remains the highest among other materials while the capacity retention rate drops most obviously after certain cycles. Given that the initial specific capacity of ZnCl2-OHC is much more advantageous and after 1 000 cycles the superiority of capacity is less obvious but still unneglectable after 1 000 cycles, the capacity retention is acceptable albeit relatively low.
As can be seen from Figs. 8(c-d), the difference that surface oxidation and intercalation modification bring to the hard carbon anode is quite noticeable within 10 cycles. The initial coulombic efficiency(ICE) reaches 97.4%, which increases by 35.3% compared to the untreated hard carbon. It can be included that a combination of ultrasonic oxidation and intercalation treatment is favorable for improving the performance of lithium ion capacitor by sacrificing capacity retention rate, which is acceptable due to its high discharge specific capacity.
As anode materials, the surface area and porosity are expected to be as low as possible to avoid the undesirable side reactions and irreversible electrolyte decomposition, as represented by the low ICE, which severely restricts the practical commercialization and leads to the consumption of excessive Li from Li-containing cathode materials in a full cell, although meaning a much higher cathode/anode capacity, yet greatly reducing the energy density of the full cell. The modification could usually lead to large surface areas,against the practical applications, especially for lithium ion batteries (LIBs). In this study, it is worth mentioning that the difference between the anodes of LIBs and LICs is key to understand the relatively small impact of ICE on the performance of the latter. Composition of an LIB-type electrode and an electrochemical capacitor (EC)-type electrode (non-Faradic) requests an LIC to complete pre-lithiation on the anode before being first-charged. In order to overcome the disadvantages that may cause by low ICE, various pre-lithiation strategies have been developed to effectively mitigate initial active lithium loss, which is realized by presetting the extra Li sources onto the anode of LICs[31].
Fig. 9 shows CV curves of HC, OHC, ZnCl2-HC and ZnCl2-OHC electrodes at different sweep speeds. The test results show that ZnCl2-OHC electrode shows the highest current density and the largest peak area among the four electrodes. The results indicate that the kinetic processes of electrode charge and discharge, the utilization rate of active sites on the surface of active material and the pseudo capacitance are improved after combined oxidation and intercalation treatment of hard carbon.
Furthermore, considering the surface oxygencontaining functional groups with more defective sites for Li ion adsorption, it can be seen that the potential sloping charge/discharge curves after modification get higher, which not only triggers the low ICE but also is unfavorable for increasing the working voltage of full battery, thus decreasing the power density of the cell.Therefore, it is necessary using the hard carbon modified by surface oxidation and intercalation as the anode to preset lithium as mentioned before to achieve higher energy density as well as better cyclability. Our previous work has proved that the pre-lithiation treatment of the anode has notable improvement on eliminating side effects of the modification by allowing the Li ions to adsorb onto the cathode and to be extracted from anode (which is supplied by pre-lithiated)simultaneously. The electrochemical performance of an LIC composed of edge-carbonylated graphene nanosheets can reach an ultra-high-power density of 53 550 W·kg-1and a cycling stability of 98.9% retention after 50 000 cycles[4]. By this means can the overall electrolyte concentration keep constant and the open-circuit potential of hard carbon be relatively low[32].
4 kinds of LIC devices, LIC-HC, LIC-OHC,LIC-ZnCl2and LIC-ZnCl2-OHC were prepared based on different hard carbon anodes and activated carbon cathode. Fig. 10(a) shows their magnification performance. At a low current density (0.1 A·g-1), the discharge specific capacities of LIC-HC and LIC-ZnCl2-OHC are 35.4 and 41.1 mAh·g-1. When the current density increases gradually, the performance difference between them becomes more obvious.When the current density increases to 10 A·g-1, the discharge specific capacities of LIC-HC and LIC-ZnCl2-OHC are 6.6 and 17.8 mAh·g-1, respectively, which are 18.6% and 43.3% of the initial specific capacities. It can be seen that the capacity retention of LIC-ZnCl2-OHC is 2.7 times that of LIC-OHC, and LIC-ZnCl2-OHC has better rate performance. When the current density decreases to 1.0 A·g-1again, the discharge specific capacities of both immediately return to 29.1 and 36.6 mAh·g-1in the next cycle, and the coulombic efficiencies of both are close to 100%.
Fig. 10(b) shows the cycle life test of four kinds of LICs at a current density of 1 A·g-1. The capacity retention rates of LIC-HC, LIC-OHC,LIC-ZnCl2-HC and LIC-ZnCl2-OHC are 93.8%, 95.0%, 95.5% and 98.4%, respectively, and the coulombic efficiency are all close to 100%. The test results show that the LIC based on the ZnCl2-OHC anode and activated carbon cathode has better cycle stability. It can be included that the hard carbon anode with combined ultrasonic oxidation and intercalation treatment is in favor of improving apparently the rate and capacity performance of the LIC.
4 Conclusion
The oxidation and intercalation treatment of commercial hard carbon will not only reshape the hard carbon surface, showing the phenomenon of corrosion, but also introduce more defects on the hard carbon surface. At the same time, the intercalation broadens the layer spacing of the hard carbon, and the initial specific capacity increases from 172.5 to 257.4 mAh·g-1, due to the oxygen-containing functional groups grafted on the hard carbon surface. In particular, carboxyl and carbonyl functional groups can synergistically enhance the diffusion control and capacitive lithium ion storage process. Meanwhile, the increase of interlayer spacing, pore size and specific surface area after hard carbon intercalation leads to more lithium ion adsorption sites. Under the high current density of 0.4 A g-1, the half-cell capacity retention rate of ZnCl2-OHC anode can still reach 47.6%,the discharge specific capacity is still 87.7 mAh·g-1after 1 000 cycles, and the Coulombic efficiency is close to 100%. At the same time, with the increase of the scan rate, the percentage of capacitance retention of ZnCl2-OHC will increase to 78.4% at a high scan rate of 1 mV·s-1.
Acknowledgements
The authors acknowledge the financial support from the National Natural Science Foundation of China (51777140), Science and Technology Support Project of Ministry of Science and Technology(2015BAG06B00), and Fundamental Research Funds for the Central Universities at Tongji University(22120180519).
- 新型炭材料的其它文章
- Progress and prospects of graphene for in-plane micro-supercapacitors
- Self-healing polymer binders for the Si and Si/carbon anodes of lithium-ion batteries
- Recent advances in carbon materials for flexible zinc ion batteries
- Carbon-based flexible electrodes for electrochemical potassium storage
- Recent progress on freestanding carbon electrodes for flexible supercapacitors
- Three-dimensional printed carbon-based microbatteries:progress on technologies, materials and applications

