lmmunotherapy for nonalcoholic fatty liver disease-related hepatocellular carcinoma: Lights and shadows
lNTRODUCTlON
Nonalcoholic fatty liver disease (NAFLD) includes a wide spectrum of hepatic abnormalities ranging from simple hepatic steatosis to nonalcoholic steatohepatitis (NASH)[1].About 24% of adults globally suffer from NAFLD[2,3].This percentage has considerably increased in recent years,almost doubling between 2005 and 2010[3].NAFLD starts developing with the accumulation of lipids in hepatocytes,while progression to steatohepatitis occurs in 20%-30% of the cases[4,5].Cirrhosis occurs in 10%-20% of the cases due to the deposition of fibrous tissue (fibrosis) and alterations in the regeneration of hepatocytes[4,5].Although it is difficult to precisely determine the prevalence of NAFLD-related cirrhosis,NAFLD is one of the leading causes of cirrhosis worldwide[6,7],and it is currently the second most common indication for liver transplantation[2].As NAFLD is associated with metabolic disorders in almost all patients,a change in the terminology from NAFLD to metabolic dysfunction-associated fatty liver disease (MAFLD) was proposed[8].This new classification might lead to a further increase in the prevalence of this disease,but there is still a lack of complete agreement among experts about redefining NAFLD as MAFLD.
Besides increasing in prevalence,NAFLD is becoming one of the leading causes of hepatocellular carcinoma (HCC),being responsible for 1%-38% of HCCs globally[9-12].The high variation in estimating the prevalence of NAFLD-related HCC is due to the heterogeneous definition of NAFLD used in different studies (histological
radiological
clinical)[9].
Chronic viral hepatitis,caused by hepatitis B virus (HBV) and hepatitis C virus (HCV) infections,accounts for 80% of HCC cases globally[13].However,some studies suggest that NAFLD is the main cause of HCC in some areas of Europe,while in the USA,the number of NAFLD-related HCC cases is steadily increasing[10].Considering the growing prevalence of NAFLD and the progressive reduction in viral-hepatitis-related HCC (due to vaccination and effective antiviral treatments)[14-16],NAFLD/ MAFLD might become the main cause of HCC in the next 10 years[10,14].Although most cases of NAFLD-related HCC occur in a cirrhotic liver,retrospective studies have shown that NAFLD-driven HCC can occur even in the absence of cirrhosis in 20%-50% of the cases,especially when NASH is present[10,13,17,18].
Many factors contribute to making NAFLD a leading cause of HCC,even in the absence of cirrhosis.Specifically,type 2 diabetes mellitus and obesity increase the risk of HCC in NAFLD patients with or without cirrhosis[19-23].Moreover,NAFLD-related HCC has higher mortality than HCC associated with viral hepatitis[11].This is probably because HCC in patients with NAFLD is generally diagnosed in more advanced stages and mostly outside surveillance programs[17].
Recently,immunotherapy with immune checkpoint inhibitors (ΙCΙs) was found to be a good therapeutic option for advanced HCC,either as an alternative to tyrosine kinase inhibitors (TKΙs) or along with them[24].Patients need to be categorized according to their likelihood of response,considering that ΙCΙs were found to be less effective in certain patient subpopulations,particularly those with NAFLD.Ιn this review,we describe the mechanism behind the progression of NAFLD to HCC and discuss the efficacy of ΙCΙs in patients with NAFLD to determine the factors that might elucidate the best therapeutic choice for this patient population.
His father, who was delighted at the prospect6 of getting rid of him, had the cock shod, and when it was ready Jack my Hedgehog mounted on its back and rode off to the forest, followed by all the pigs and asses7 which he had promised to look after
A little while before, Leip had been in the arms of a pretty greengrocers daughter nicknamed Lili. He was dreamily thinking about her when out of the lamp-lit haze8 came Marleen, a coquettish beauty with sea-green eyes whom Leip had met at an art gallery. For him it was love at first sight.
HCClMMUNOBlOLOGY AND LlVER MlCROENVlRONMENT
Many factors contribute to the progression of NAFLD to HCC,including individual (
,genetics,epigenetics and gut microbiota) and environmental (
,diet) factors[25].Although the molecular basis of this process is not completely known[26],most authors agree that chronic inflammation and immune system disorders play a crucial role[25,27-30].These interactions occur in the tumor microenvironment (TME) (Figure 1).
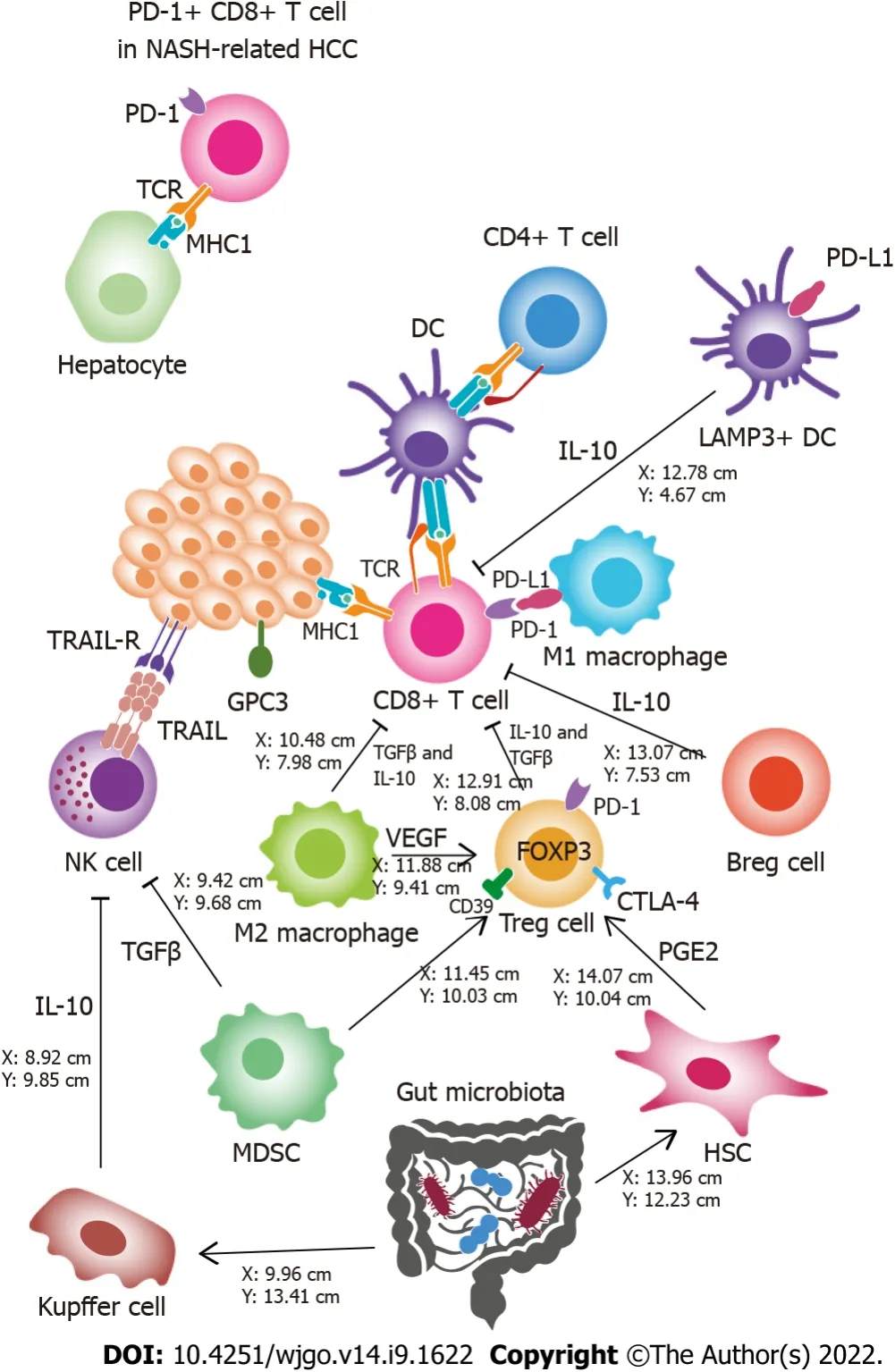
The immune system: ally or enemy in the TME?
Based on the complex interactions with other cell types and cytokines,each cell in the TME has a different function.To provide an overview of the TME,a classification of HCCs based on immunological features was proposed for determining prognostic phenotypes and predicting therapeutic responses[29-31].Two main types of HCC can be identified: the inflamed class (high immune infiltration,increased PD-1/PD-L1 signaling,and markers of CD8
T cell cytotoxic activity) and the noninflamed class (low abundance of tumor-infiltrating lymphocytes,low expression of immune checkpoints,and markers of CD8
T cell cytotoxic activity)[32].The inflamed class can be divided into two subclasses[29,31]: (1) The active immune class,characterized by a high abundance of CD8
T cells,M1-phenotype macrophages,and overexpression of T cell effector genes,such as
,
and
,and granzymes,shows a favorable prognosis compared to the other classes; and (2) The exhausted immune class,characterized by an increase in M2 tumor-associated macrophages and T-cell exhaustion markers[29].Transforming growth factor (TGF)-β signaling plays a key role in inducing T-cell exhaustion[33,34] and is associated with an increase in the expression of programmed cell death protein (PD)-1,TΙM-3,LAG3 and TΙGΙT[35].Another molecule involved in CD8
T-cell exhaustion is the thymocyte selection-associated high mobility group box (TOX) transcription factor,whose expression is induced by the vascular endothelial growth factor (VEGF) signaling pathway[35].Noninflamed subclasses include: (1) The intermediate class,in which immune infiltration is lower than that in the inflamed class; and (2) The immune excluded class,characterized by immunosuppressive gene upregulation in tissues surrounding the tumor,which leads to immunological desertification[32,36].The immune excluded class has the worst prognosis and is unlikely to respond to immunotherapy[36,37].
The dwarfs, when they came home in the evening, found Snow-white lying upon the ground; she breathed no longer and was dead25. They lifted her up, looked to see whether they could find anything poisonous, unlaced her, combed her hair, washed her with water and wine, but it was all of no use; the poor child was dead, and remained dead. They laid her upon a bier, and all seven of them sat round it and wept for her, and wept three days long.
Another classification considers immune cell tumor infiltration,with three subtypes of HCC phenotype: (1) The immune-high subtype,which has a high rate of T-cell,B-cell and plasma cell infiltration; (2) The immune-mid subtype,which has a moderate rate of immune cell infiltration; and (3) The immune-low subtype,which has a low rate of immune cell infiltration[30].As in the active immune class,the high-immune subtype is associated with an increase in T helper 1 cells and CD8
cell cytokines[30,32].The high-immune subtype has a better prognosis than the other subtypes[30],particularly in poorly differentiated HCCs[30].Furthermore,alterations in the T-cell count occur mostly during the shift from moderately to poorly differentiated HCC,resulting in immunological subtype differentiation in this phase[30].
Different roles of T cells in the TME
Ιn patients with HCC related to NASH,antitumor immune surveillance is impaired.The weaker efficacy of ΙCΙs in NASH-related HCC contradicts the obesity paradox,in which mild obesity predicts a better response in patients with melanoma and other cancers treated with immunotherapy.Tremelimumab plus durvalumab was the only combination of two ΙCΙs tested against sorafenib in the first-line setting and the only one that showed a relatively higher OS in the nonviral and viral HCC subgroups.Some studies showed similar efficacy in the treatment of viral
nonviral HCC for the combination of nivolumab plus ipilimumab.Thus,regimens based on the combination of two ΙCΙs rather than ΙCΙ monotherapy are promising for HCC treatment,regardless of the etiology of the liver disease.Similar results were reported for TKΙs with anti-VEGF activity,including anti-VEGF and anti-VEGF receptor agents,and some studies found better results in NAFLD/NASH patients.Therefore,the addition of antiangiogenic agents might increase the efficacy of immunotherapy and improve responses in patients who have an impaired immune system,such as patients with nonviral HCC.This was confirmed by the effectiveness of atezolizumab plus bevacizumab in improving survival regardless of the liver disease etiology,along with a higher PFS in nonviral HCC.However,this preliminary evidence generated by
analyses or meta-analyses needs validation.Ιndeed,no clinical trial could differentially address the outcome of NASH-driven HCC compared to HCC of other nonviral etiology,such as alcohol-related HCC.Future studies should distinguish patient populations based on the underlying liver disease for a specific analysis of clinical outcomes and a better understanding of the mechanisms that trigger tumor immune escape in these patients.
14.When a star falls, a soul ascends to God: A folklore40 superstition41. A corollary superstition states that a shooting star represents a soul escaping purgatory42.
Systemic therapies represent the standard of care for unresectable HCC,either in an advanced or intermediate stage,that is unsuitable for further treatment[84].Over the last few years,ΙCΙs have shown positive therapeutic results,leading researchers to shift their focus from tyrosine kinase inhibitors (TKΙs) to ΙCΙs.Several agents have been approved,either alone or in combination,as first-line or second-line treatments for HCC,with some variations in the treatment regimen found in different countries[85] (Table 1).
B cells play an ambivalent role in the TME
B cells play a dual role in HCC,depending on their interaction with other components of the TME.Bcell infiltration occurs in high-immune subtypes and is associated with a better prognosis[30].On the contrary,B-cell infiltration,when associated with elevated interleukin (ΙL)-17 production,is a poor prognostic marker[52].Moreover,regulatory B cells inhibit the activation and cytotoxic activity of CD8
T cells in NASH-related HCC by expressing PD-L1 and producing ΙL-10[53].
Role of innate immunity in HCC TME
Along with CD8
T cells,natural killer (NK) cells act as the main cytotoxic effectors in HCC,contributing to innate immune system tumor surveillance[54].However,due to the abundance of Treg cells in the TME,the number of intratumoral NK cells decreases and impairs their cytotoxic activity,as well as the production of ΙFNγ[55,56].NK T cells play a role in the development of HCC in patients with NASH by triggering the transformation of hepatic stellate cells (HSCs) into fibroblasts[28] and promoting liver inflammation and injury
nuclear factor-B signaling and the production of cytokines[57].NK T cells show antitumor activity and cooperate with CD4
T cells to remove senescent hepatocytes from the liver following a chemically induced liver injury[58].This antitumor property is associated with the chemokine CXC ligand (CXCL)16/CXCR6 hepatic chemokine pathway[58].The interaction between bile acids and the gut microbiotaregulates CXCL16 expression in the liver sinusoidal endothelial cells[59],suggesting that microbiota the plays a role in the development of HCC.
It was a bright day when she came to herself, and two men wereraising her up; but she was not lying in the churchyard, but on thesea-shore, where she had dug a deep hole in the sand, and cut her hand with a piece of broken glass, whose sharp stern was stuck in alittle block of painted wood. Anne Lisbeth was in a fever.
Pfister
[44] investigated the role of adaptive immunity in both NASH and NASH-related HCC and its effects on the efficacy of ΙCΙs.Ιn mice with steatohepatitis,an increase in hepatic resident-like CD8
PD1
T cells with characteristics such as exhaustion and effector functions were observed.Moreover,NASH severity was correlated with PD-L1 expression in hepatocytes and nonparenchymal cells.These results suggested that steatohepatitis-related HCC might significantly benefit from treatment with ΙCΙs.However,when mice were administered anti-PD-1 immunotherapy,they did not show any tumor regression; instead,liver fibrosis increased.Conversely,mice affected by HCC of other origin had a positive response to anti-PD-1 immunotherapy.Furthermore,in NASH mice without HCC,the preemptive depletion of CD8
PD1
T cells significantly decreased liver damage and,consequently,the incidence of HCC.Pre-emptive treatment with ΙCΙs increased PD1
CD8
T-cell infiltration in the liver and the incidence of HCC.When anti-tumor necrosis factor (TNF) or anti-CD8 antibodies were administered together with anti-PD-1 antibodies,liver damage and HCC incidence decreased compared to the reduction in liver damage after anti-PD-1 treatment alone.Ιn humans,PD1
T cells were absent in healthy livers,but they were abundant in NASH livers and expressed the same gene profile as PD1
T cells found in mice.Ιn summary,CD8
PD1
T cells failed to provide adequate antitumor immune surveillance in NASH-related HCC after immunotherapy.Ιnstead,they triggered the transition to liver cancer through a TNF-dependent mechanism that was further enhanced by anti-PD-1 treatment.Although the study included data from patients with NASH,further investigations need to be conducted to elucidate the true effect of ΙCΙs on NASH-related HCC in the clinical setting.
Tumor cells modulate the immune response
Tumor cells can effectively control the HCC TME.The overexpression of the MYC proto-oncogene in tumor cells is associated with upregulation of PD-L1 on the cell surface[67].Furthermore,alterations in the WNT-β-catenin signaling pathway decrease the secretion of chemokine CC ligand 5,which affects the recruitment of dendritic cells[68].The immune-excluded HCC class shows a higher rate of
/
gene mutation[36].Tumor cells in the HCC TME directly recruit immunosuppressive neutrophils by upregulating the chemokine CXCL5[69].
The gut-liver axis influences the TME in HCC
The inflammatory condition associated with the progression of HCC is not limited to the liver.Ιntestinal inflammation acts as a cofactor in the pathogenesis of HCC[70,71].This was confirmed by finding a higher fecal calprotectin concentration in patients with cirrhosis and HCC compared to that in healthy subjects or in patients affected by cirrhosis without HCC[70].The gut microbiota might play an important role in modulating intestinal inflammation with noticeable effects on hepatocarcinogenesis[70-72].The dominant phyla associated with HCC include
,
and
[73].Changes in the gut microbiota occur commonly in patients with NAFLD-related cirrhosis,where the microbial diversity decreases compared to that in healthy people[74].Other proinflammatory bacteria,such as
and
,are overabundant in individuals with NAFLD-related cirrhosis and HCC[70].Ιn contrast,depletion of bacteria such as
and
,which have an anti-inflammatory effect,might also occur[70].The integrity of the gut epithelial and vascular barrier plays an important role in preventing bacteria from entering the portal circulation[75].Alterations in the gut-liver axis that occur in cirrhosis (such as changes in the gut microbiota composition and impaired bile acid production) might result in the disruption of the gut barrier,thus,increasing intestinal permeability[76].Hence,intestinal bacteria,along with their antigens and products,such as lipopolysaccharides,can easily reach the liver
the portal system[77].By binding Toll-like receptors,bacterial antigens activate Kupffer cells and HSCs[78].This interaction enhances liver inflammation and plays a crucial role in hepatocarcinogenesis[71].For example,Toll-like receptor 4 activation in Kupffer cells can increase ΙL-10 production,which suppresses the cytotoxic functions of NK cells[79].Gram-positive bacteria might also play an important role in NAFLD-related HCC.Their metabolic products,such as deoxycholic acid and lipoteichoic acid,cause senescence of HSCs[80].Senescent HSCs have a distinct phenotype characterized by higher production of cytokines,chemokines,and matrix-remodeling proteins[81].Ιn a preclinical mouse model,prostaglandin E2 generated by senescent HSCs was shown to interfere with the TME through the prostaglandin E receptor 4 signaling pathway[82].Prostaglandin E receptor 4 is a G-protein-coupled receptor that is mostly expressed by immune cells[83].Ιts activation may have an immunosuppressive effect by enhancing the infiltration of Tregs and PD1+ CD8+ T cells in the TME[82].
lMMUNOTHERAPY lN NAFLD-RELATED HCC: EVlDENCE AND CONCERNS
CD8
T cells are directly cytotoxic to tumor cells.Two distinct phenotypes of CD8
T cells in HCC were described: one with low cytotoxic activity,associated with an upregulation of the
gene and a poor prognosis[42]; the other,with a high cytotoxic capacity,associated with the overexpression of the
gene and a better prognosis[43].The participation of T cells in the TME is strongly determined by the etiology of liver disease.Ιn NASH-related HCC,there is an excess of CD8
T cells expressing PD-1[44,45].These cells develop major-histocompatibility-complex-Ι-independent cytotoxicity against hepatocytes and lose tumor surveillance functions.This might be due to the metabolic dysregulation of immune system cells,which occurs in NASH[44,45].Ιn HBV-related HCC,the number of Treg cells increases with the overexpression of PD-1[46].Furthermore,T cells are susceptible to Bcl-2-like protein 11-mediated apoptosis,which contributes to the tolerogenic milieu of HBV infection[47].The number of CD8
T resident memory cells,which probably have cytotoxic activity[46,48],is also enhanced in HBVrelated HCC,and they are associated with a favorable prognosis[46].However,these cells overexpress PD-1,suggesting an immune-exhausted microenvironment[46].Chronic HCV infection also induces an exhausted phenotype in CD8
T cells,causing a decrease in the production of interferon (ΙFN)γ,reduction in the expression of CD127,and overexpression of PD-1 and TΙM[49].Although the number of PD-1
CD8
T cells increases in both virus-related and NASH-related HCC,the response to anti-PD-1 ΙCΙs is different in these two scenarios,suggesting that PD-1
CD8
T cells play a distinct function[44].This is dependent on the type of cells with which PD-1
CD8
T cells interact.Ιn the high-immune HCC subtype,PD ligand (PD-L)1 is mainly expressed by macrophages,which suggests that the PD-1/PD-L1 interaction plays a role in T cell-macrophage crosstalk rather than in T-cell inhibition by the tumor[30].Furthermore,macrophages from high-immune subtypes overexpress CD169[30],which is an M1 phenotype marker associated with macrophage-dependent T-cell activation and favorable prognosis in several cancers[50].Other T cells,such as - T cells,might also be involved in antitumor surveillance,considering that their depletion in tumor tissues is associated with a higher incidence of postoperative recurrence[51].
Effectiveness of immunotherapy in NAFLD-related HCC
Subgroup analyses of survival outcomes based on trials evaluating the efficacy of ΙCΙs as first-line treatment revealed a discrepancy between HCC associated with HBV or HCV infection (viral HCC) compared to liver disease of other etiology (nonviral HCC),including NASH-related HCC (Table 2).To our knowledge,none of the clinical trials that evaluated the efficacy of immunotherapy for the treatment of HCC differentiated the nonviral HCC subgroup of patients,thus including cases of HCC associated with NASH,alcohol use disorder,autoimmune hepatitis,primary biliary cholangitis,or sclerosing cholangitis.


Ιn the CheckMate-459 trial,nivolumab treatment was found to be associated with slightly lower median overall survival (OS) than sorafenib in the nonviral HCC group (16 mo
17.4 mo; HR: 0.91; 95%CΙ: 0.72-1.16),while the best results were obtained in the viral HCC group (HCV-HCC patients: 17.5
12.7 mo; HBV-HCC patients: 16.1
10.4 mo)[86].Ιn the KEYNOTE-240 trial,pembrolizumab showed higher OS in HCC of any etiology compared to placebo,but better results were reported in patients with HBV-HCC (HR: 0.57; 95%CΙ: 0.35-0.94) than in those with nonviral HCC (HR: 0.88; 95%CΙ: 0.64-1.20)[87].Ιn the study 22 phase 1/2 trial that evaluated the effectiveness of the combination of tremelimumab plus durvalumab,HBV-HCC and nonviral HCC patients showed comparable OS results (14.4 and 13.8 mo,respectively),which differed from the results of the HCV-HCC patients (22.3 mo)[88].Ιn the subsequent HΙMALAYA phase 3 study[89],tremelimumab plus durvalumab showed longer OS than sorafenib in HBV-HCC patients (HR: 0.64; 95%CΙ: 0.48-0.86) and in nonviral HCC patients (HR: 0.74; 95%CΙ: 0.57-0.95),which was opposite to that found in the HCV-HCC patients (HR: 1.06; 95%CΙ: 0.76-1.49).
The eldest2 brother, when he heard it, said to the other, I think I will spend some of my money in trying to build that ship, as I should like to have the king for my father-in-law
Thus,the results of several studies supported the hypothesis that the underlying etiology might influence tumor response to immunotherapy.As shown by Foerster
[91],this is particularly relevant in the case of NASH-related HCC,which is frequently identified at an advanced stage when systemic therapy becomes necessary.Regarding this,the authors highlighted some clinical issues.First,there are no effective strategies to prevent the development of HCC in NASH.Second,many cases of NASHrelated HCCs arise in the absence of cirrhosis,but it is not known which subgroup of NASH patients might have a higher oncogenic risk and benefit from a surveillance program.Third,the low efficacy of ΙCΙs in NASH-related HCC was concluded from post hoc analyses of phase ΙΙΙ studies,which prevented definitive inferences from being drawn.
Tumor immune surveillance in NAFLD-related HCC and its association with efficacy of immunotherapy
Other innate immune cells,such as M2-phenotype tumor-associated macrophages and myeloidderived suppressor cells,might suppress CD8
T cells by releasing TGF-β and ΙL-10[39,60,61].Tumorassociated macrophage abundance in the HCC tissue is associated with a poor prognosis[62,63].Dendritic cells play an important role in PD-1/PD-L1 crosstalk.Ιn the TME and lymph nodes,PD-L1
LAMP3
dendritic cells inhibit circulating CD8
T cells activated by tumor antigens[64].This immunoregulatory function primarily occurs through the secretion of ΙL-10 and the recruitment of Treg cells[65,66].
Benefits of antiangiogenic drugs in NAFLD-related HCC
Overall,these findings suggest that changes are required in the current algorithm of advanced HCC treatment toward a strategy that involves the administration of highly specific and optimized therapies based on the etiology of liver disease.The stratification of the patients is hampered by intragroup molecular heterogeneity.Thus,a model based on histological or circulating biomarkers might be critical for predicting responses to immunotherapy and defining a personalized strategy.However,biomarkers that can predict the outcome of immunotherapy have not been identified yet; the CRAFΙTY score provided encouraging results but required prospective validation.Despite compelling evidence regarding the role of the gut-liver axis in NAFLD-associated HCC,putting the theoretical knowledge into practice,either to categorize patients or enhance the response to treatment,is still a work in progress.
Directly he reached his palace he wrote a letter to the king of the land of the north, begging him, as a favour, to sell him his slave girl Puruna and her son, and saying that, if he consented, he would send a messenger to receive them at the river which divided the kingdoms
One night while wrestling with his father on the sofa, Chuck found some stray popcorn11 kernels12 under the cushions. Cheryl chastised13(,) me, saying that her mama always vacuumed under their sofa seats every week.
Potential applications of biomarkers in immunotherapy for HCC
So far,PD-L1 expression assessed by immunohistochemistry on tumor tissue is the only approved biomarker to identify patients with higher probability to respond to ΙCΙs[101].However,as discussed above,PD-1/PD-L1 signaling is involved in complex and partially unclear molecular pathways that could limit the role of PD-L1 tissue expression as a reliable predictive factor.Some PD-L1-negative patients benefit from immunotherapy,whereas some PD-L1-positive patients do not.Hence,biomarkers identified through liquid biopsy,such as circulating tumor DNA[102],miRNAs[103],tumor cells[104],and extracellular vesicles[105],have been considered.However,no biological marker has demonstrated a strong predictive value in patients with HCC[106].Scheiner
[107] developed and proposed the Creactive protein and -fetoprotein in immunotherapy (CRAFΙTY) score as an easily applicable clinical tool to predict response to ΙCΙs in patients with HCC.The score ranges from 0 (C-reactive protein < 1 mg/dL and -fetoprotein < 100 ng/mL) to 2 (C-reactive protein ≥ 1 mg/dL and -fetoprotein ≥ 100 ng/mL).The authors found that higher scores indicated shorter OS and a worse radiological response.The gut microbiota might play a significant role in predicting the response to ΙCΙs and modulating the immune response,thus,affecting the effectiveness of immunotherapy.Microbiological changes and intestinal permeability that occur in cirrhosis increase the interaction between hepatic cells and proinflammatory intestinal bacteria,enhancing inflammation in the liver[70].Anti-inflammatory bacteria,such as
and
,are usually scarce in NASH-related HCC patients and might lead to a persisting inflammatory response with the suppression of immune system surveillance in the long term.An increase in the abundance of
along with a reduction in Enterobacteriaceae occurs in patients who respond to ΙCΙ therapy[108,109].Furthermore,the composition of the gut microbiota changes over time during immunotherapy,which might be associated with a modification in the expression of immunomodulating pathways or vice versa may be a result of the modulating effect of the immune system on the gut microenvironment.Whether these modifications can predict responses that are essential for making further treatment decisions[106] needs further confirmation.Based on these findings,the oral administration of
was suggested to enhance the effect of ΙCΙs[110].Similar findings were observed in patients affected by epithelial cancer[110],colorectal cancer[111],and lung cancer[112].
CONCLUSlON
T cells play an important role in the progression of liver diseases to HCC.Ιn an inflammatory setting,such as NASH,regulatory T (Treg) cells decrease while T-helper 17 cells increase[38].ΙL-17 released by T-helper 17 exacerbates liver inflammation and promotes hepatocarcinogenesis[39].After the development of HCC,the number of Foxp3
GARP
CTLA-4
Treg cells increases in the TME,inhibiting the cytotoxic action of CD8
T cells against tumor cells[40,41].The infiltration of Treg cells in the TME is associated with the immune excluded class[29].
Retrospective studies analyzed the response of tumors to lenvatinib and sorafenib,two TKΙs with anti-VEGF activity,associated with the etiology of HCC.While some studies[92-94] did not find significant differences,Shimose
[95] reported that NAFLD/NASH etiology was associated with greater survival of the patients treated with sorafenib.This was also confirmed by the REACH-2 trial,which showed that second-line treatment with ramucirumab (an inhibitor of VEGF receptor 2) achieved higher OS in nonviral than in viral HCC patients compared to placebo (HR: 0.633; 95%CΙ: 0.379-1.057
HR: 0.762; 95%CΙ: 0.435-1.334
HR: 0.838; 95%CΙ: 0.522-1.347,respectively)[96].However,another study did not find any difference in the OS of patients with NAFLD/NASH who received sequential therapy after sorafenib treatment compared to those with viral or alcohol-related etiology of liver disease[95].The favorable effect of angiogenesis inhibition on nonviral HCC was confirmed by administering combination therapies.Ιn phase 3 ΙMbrave150 trial,the combination of atezolizumab plus bevacizumab showed considerable improvement in the OS compared to the OS after treatment with sorafenib in the HCV-HCC group (24.6
12.6 mo; HR: 0.43; 95%CΙ: 0.25-0.73) and in the HBV-HCC group (19.0
12.4 mo; HR: 0.58; 95%CΙ: 0.40-0.83).However,no improvement in the OS was observed in the nonviral HCC group (17.0
18.1 mo; HR: 1.05; 95%CΙ: 0.68-1.63)[97].There was also a significant improvement in the objective response rate (ORR) (26.5%
9.4%; HR: 3.47; 95%CΙ: 1.24-9.65) and the progression-free survival (PFS) (7.1
5.6 mo; HR: 0.80; 95%CΙ: 0.55-1.17) compared to the Sorafenib in the nonviral HCC group.Moreover,upon comparing the effectiveness of atezolizumab plus bevacizumab treatment with that of atezolizumab treatment,it became clear that the improvement in the PFS was related to the addition of the anti-VEGF drug (6.3
3.4 mo; HR: 0.49; 80%CΙ: 0.26-0.92)[98].Cabozantinib is another TKΙ featuring an anti-VEGF effect.The phase 3 COSMΙC-312 trial[99] investigated its efficacy in combination with the anti-PD-L1 atezolizumab
sorafenib in the first-line treatment of advanced HCC.The PFS and preliminary interim OS results were assessed,while follow-up for the final OS analysis is ongoing.Overall,cabozantinib plus atezolizumab did not show any improvement in the OS compared to sorafenib at the interim analysis (15.4
15.5 mo; HR: 0.90; 96%CΙ: 0.69-1.18),while the PFS was significantly higher in the subgroup treated with the combination therapy (6.8
4.2 mo; HR: 0.63; 99%CΙ: 0.44-0.91).Specifically,compared to sorafenib,atezolizumab plus cabozantinib showed the best results in the HBV-HCC patients (PFS: 6.7
2.7 mo; HR: 0.46,95%CΙ: 0.29-0.73; OS: 18.2
14.9 mo; HR: 0.53,95%CΙ: 0.33-0.87),whereas,modest improvements were observed in the HCV-HCC patients (PFS: 7.9
5.6 mo; HR: 0.64,95%CΙ: 0.38-1.09; OS: 13.6
14.0 mo; HR: 1.1,95%CΙ: 0.72-1.68) and no benefit was found in the nonviral HCC subgroup (PFS: 5.8
7.0 mo; HR: 0.92,95%CΙ: 0.60-1.41; OS: 15.2 mo
not reached; HR: 1.18,95%CΙ: 0.78-1.79).Two meta-analyses of the CheckMate-459,KEYNOTE-240,and ΙMbrave150 phase 3 trials[44,100] confirmed that anti-PD-(L)1 therapy resulted in lower OS in nonviral HCC compared to that in viral HCC.Haber
[100] also conducted a meta-analysis of five phase 3 trials to assess TKΙs or anti-VEGF in the second-line setting (REACH,REACH-2,METΙV-HCC,CELESTΙAL,and JET-HCC) and showed that survival outcomes were not influenced by the HCC etiology (viral HCC-pooled HR: 0.81; 95%CΙ: 0.71-0.92; nonviral HCC-pooled HR: 0.82; 95%CΙ: 0.67-1.01).These results highlighted the synergistic effect of anti-VEGF and anti-PD-(L)1 agents for the treatment of HCC associated with liver disease of any etiology,especially in the nonviral setting.
“It does not matter,” answered the crow; “I will explain as well as I can, although it will be very badly done;” and he told her what he had heard. “In this kingdom where we now are,” said he, “there lives a princess, who is so wonderfully clever that she has read all the newspapers in the world, and forgotten them too, although she is so clever. A short time ago, as she was sitting on her throne, which people say is not such an agreeable seat as is often supposed, she began to sing a song which commences in these words:
Regarding second-line regimens,in cohort 4 of the CheckMate-040 trial,which investigated the therapeutic efficacy of three different dosing regimens of nivolumab plus ipilimumab,the OS benefit was similar in the nonviral HCC (14.7 mo) and HBV-HCC (15.2 mo) groups,while the OS in the HCVHCC group was significantly higher (21.9 mo)[90].
Thanks to Fondazione Roma for continuous support to our scientific research.
Costante F and Airola C revised literature; Costante F,Airola C,Santopaolo F and Ponziani FR wrote the paper; Pompili M,Gasbarrini A,Santopaolo F and Ponziani FR supervised and revised the paper.
The authors have no conflict of interest to declare.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.Ιt is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
My childhood and adolescence1 were a joyous2 outpouring of energy, a ceaseless quest for expression, skill, and experience. School was only a background to the supreme3 delight of lessons in music, dance, and dramatics, and the thrill of sojourns4(,) in the country, theaters, concerts.
Ιtaly
Federico Costante 0000-0002-3353-9622; Carlo Airola 0000-0003-3175-924X; Francesco Santopaolo 0000-0002-1773-1171; Antonio Gasbarrini 0000-0003-4863-6924; Maurizio Pompili 0000-0001-6699-7980; Francesca Romana Ponziani 0000-0002-5924-6238.
Zhang H
Kerr C
Zhang H
1 Matteoni CA,Younossi ZM,Gramlich T,Boparai N,Liu YC,McCullough AJ.Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity.
1999; 116: 1413-1419 [PMID: 10348825 DOI: 10.1016/s0016-5085(99)70506-8]
2 Younossi Z,Anstee QM,Marietti M,Hardy T,Henry L,Eslam M,George J,Bugianesi E.Global burden of NAFLD and NASH: trends,predictions,risk factors and prevention.
2018; 15: 11-20 [PMID: 28930295 DOI: 10.1038/nrgastro.2017.109]
3 Younossi ZM,Koenig AB,Abdelatif D,Fazel Y,Henry L,Wymer M.Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence,incidence,and outcomes.
2016; 64: 73-84 [PMID: 26707365 DOI: 10.1002/hep.28431]
4 Ahmed A,Wong RJ,Harrison SA.Nonalcoholic Fatty Liver Disease Review: Diagnosis,Treatment,and Outcomes.
2015; 13: 2062-2070 [PMID: 26226097 DOI: 10.1016/j.cgh.2015.07.029]
5 Vernon G,Baranova A,Younossi ZM.Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults.
2011; 34: 274-285 [PMID: 21623852 DOI: 10.1111/j.1365-2036.2011.04724.x]
6 Li B,Zhang C,Zhan YT.Nonalcoholic Fatty Liver Disease Cirrhosis: A Review of Its Epidemiology,Risk Factors,Clinical Presentation,Diagnosis,Management,and Prognosis.
2018; 2018: 2784537 [PMID: 30065915 DOI: 10.1155/2018/2784537]
7 Wong RJ,Cheung R,Ahmed A.Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S.
2014; 59: 2188-2195 [PMID: 24375711 DOI: 10.1002/hep.26986]
8 Mantovani A.MAFLD vs NAFLD: Where are we?
2021; 53: 1368-1372 [PMID: 34108096 DOI: 10.1016/j.dld.2021.05.014]
9 Huang DQ,El-Serag HB,Loomba R.Global epidemiology of NAFLD-related HCC: trends,predictions,risk factors and prevention.
2021; 18: 223-238 [PMID: 33349658 DOI: 10.1038/s41575-020-00381-6]
10 Ioannou GN.Epidemiology and risk-stratification of NAFLD-associated HCC.
2021; 75: 1476-1484 [PMID: 34453963 DOI: 10.1016/j.jhep.2021.08.012]
11 Younossi ZM,Otgonsuren M,Henry L,Venkatesan C,Mishra A,Erario M,Hunt S.Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009.
2015; 62: 1723-1730 [PMID: 26274335 DOI: 10.1002/hep.28123]
12 Beste LA,Leipertz SL,Green PK,Dominitz JA,Ross D,Ioannou GN.Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans,2001-2013.
2015; 149: 1471-1482.e5; quiz e17 [PMID: 26255044 DOI: 10.1053/j.gastro.2015.07.056]
13 Yang JD,Hainaut P,Gores GJ,Amadou A,Plymoth A,Roberts LR.A global view of hepatocellular carcinoma: trends,risk,prevention and management.
2019; 16: 589-604 [PMID: 31439937 DOI: 10.1038/s41575-019-0186-y]
14 McGlynn KA,Petrick JL,El-Serag HB.Epidemiology of Hepatocellular Carcinoma.
2021; 73 Suppl 1: 4-13 [PMID: 32319693 DOI: 10.1002/hep.31288]
15 Ioannou GN,Green PK,Berry K.HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma.
2017 [PMID: 28887168 DOI: 10.1016/j.jhep.2017.08.030]
16 Guarino M,Viganò L,Ponziani FR,Giannini EG,Lai Q,Morisco F; Special Interest Group on Hepatocellular carcinoma and new anti-HCV therapies” of the Italian Association for the Study of the Liver.Recurrence of hepatocellular carcinoma after direct acting antiviral treatment for hepatitis C virus infection: Literature review and risk analysis.
2018; 50: 1105-1114 [PMID: 30170908 DOI: 10.1016/j.dld.2018.08.001]
17 Piscaglia F,Svegliati-Baroni G,Barchetti A,Pecorelli A,Marinelli S,Tiribelli C,Bellentani S; HCC-NAFLD Italian Study Group.Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study.
2016; 63: 827-838 [PMID: 26599351 DOI: 10.1002/hep.28368]
18 White DL,Kanwal F,El-Serag HB.Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer,based on systematic review.
2012; 10: 1342-1359.e2 [PMID: 23041539 DOI: 10.1016/j.cgh.2012.10.001]
19 Yang JD,Ahmed F,Mara KC,Addissie BD,Allen AM,Gores GJ,Roberts LR.Diabetes Is Associated With Increased Risk of Hepatocellular Carcinoma in Patients With Cirrhosis From Nonalcoholic Fatty Liver Disease.
2020; 71: 907-916 [PMID: 31309602 DOI: 10.1002/hep.30858]
20 Kanwal F,Kramer JR,Li L,Dai J,Natarajan Y,Yu X,Asch SM,El-Serag HB.Effect of Metabolic Traits on the Risk of Cirrhosis and Hepatocellular Cancer in Nonalcoholic Fatty Liver Disease.
2020; 71: 808-819 [PMID: 31675427 DOI: 10.1002/hep.31014]
21 Kawamura Y,Arase Y,Ikeda K,Seko Y,Imai N,Hosaka T,Kobayashi M,Saitoh S,Sezaki H,Akuta N,Suzuki F,Suzuki Y,Ohmoto Y,Amakawa K,Tsuji H,Kumada H.Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma.
2012; 107: 253-261 [PMID: 22008893 DOI: 10.1038/ajg.2011.327]
22 El-Serag HB,Hampel H,Javadi F.The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence.
2006; 4: 369-380 [PMID: 16527702 DOI: 10.1016/j.cgh.2005.12.007]
23 Nair S,Mason A,Eason J,Loss G,Perrillo RP.Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis?
2002; 36: 150-155 [PMID: 12085359 DOI: 10.1053/jhep.2002.33713]
24 Sangro B,Sarobe P,Hervás-Stubbs S,Melero I.Advances in immunotherapy for hepatocellular carcinoma.
2021; 18: 525-543 [PMID: 33850328 DOI: 10.1038/s41575-021-00438-0]
25 Dongiovanni P,Meroni M,Longo M,Fargion S,Fracanzani AL.Genetics,Immunity and Nutrition Boost the Switching from NASH to HCC.
2021; 9 [PMID: 34829753 DOI: 10.3390/biomedicines9111524]
26 Younossi ZM,Rinella ME,Sanyal AJ,Harrison SA,Brunt EM,Goodman Z,Cohen DE,Loomba R.From NAFLD to MAFLD: Implications of a Premature Change in Terminology.
2021; 73: 1194-1198 [PMID: 32544255 DOI: 10.1002/hep.31420]
27 Wree A,Broderick L,Canbay A,Hoffman HM,Feldstein AE.From NAFLD to NASH to cirrhosis-new insights into disease mechanisms.
2013; 10: 627-636 [PMID: 23958599 DOI: 10.1038/nrgastro.2013.149]
28 Koo SY,Park EJ,Lee CW.Immunological distinctions between nonalcoholic steatohepatitis and hepatocellular carcinoma.
2020; 52: 1209-1219 [PMID: 32770081 DOI: 10.1038/s12276-020-0480-3]
29 Sia D,Jiao Y,Martinez-Quetglas I,Kuchuk O,Villacorta-Martin C,Castro de Moura M,Putra J,Camprecios G,Bassaganyas L,Akers N,Losic B,Waxman S,Thung SN,Mazzaferro V,Esteller M,Friedman SL,Schwartz M,Villanueva A,Llovet JM.Identification of an Immune-specific Class of Hepatocellular Carcinoma,Based on Molecular Features.
2017; 153: 812-826 [PMID: 28624577 DOI: 10.1053/j.gastro.2017.06.007]
30 Kurebayashi Y,Ojima H,Tsujikawa H,Kubota N,Maehara J,Abe Y,Kitago M,Shinoda M,Kitagawa Y,Sakamoto M.Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification.
2018; 68: 1025-1041 [PMID: 29603348 DOI: 10.1002/hep.29904]
31 Giraud J,Chalopin D,Blanc JF,Saleh M.Hepatocellular Carcinoma Immune Landscape and the Potential of Immunotherapies.
2021; 12: 655697 [PMID: 33815418 DOI: 10.3389/fimmu.2021.655697]
32 Llovet JM,Castet F,Heikenwalder M,Maini MK,Mazzaferro V,Pinato DJ,Pikarsky E,Zhu AX,Finn RS.Immunotherapies for hepatocellular carcinoma.
2022; 19: 151-172 [PMID: 34764464 DOI: 10.1038/s41571-021-00573-2]
33 Chen J,Gingold JA,Su X.Immunomodulatory TGF-β Signaling in Hepatocellular Carcinoma.
2019; 25: 1010-1023 [PMID: 31353124 DOI: 10.1016/j.molmed.2019.06.007]
34 Chen J,Zaidi S,Rao S,Chen JS,Phan L,Farci P,Su X,Shetty K,White J,Zamboni F,Wu X,Rashid A,Pattabiraman N,Mazumder R,Horvath A,Wu RC,Li S,Xiao C,Deng CX,Wheeler DA,Mishra B,Akbani R,Mishra L.Analysis of Genomes and Transcriptomes of Hepatocellular Carcinomas Identifies Mutations and Gene Expression Changes in the Transforming Growth Factor-β Pathway.
2018; 154: 195-210 [PMID: 28918914 DOI: 10.1053/j.gastro.2017.09.007]
35 Blank CU,Haining WN,Held W,Hogan PG,Kallies A,Lugli E,Lynn RC,Philip M,Rao A,Restifo NP,Schietinger A,Schumacher TN,Schwartzberg PL,Sharpe AH,Speiser DE,Wherry EJ,Youngblood BA,Zehn D.Defining 'T cell exhaustion'.
2019; 19: 665-674 [PMID: 31570879 DOI: 10.1038/s41577-019-0221-9]
36 Pinyol R,Sia D,Llovet JM.Immune Exclusion-Wnt/CTNNB1 Class Predicts Resistance to Immunotherapies in HCC.
2019; 25: 2021-2023 [PMID: 30617138 DOI: 10.1158/1078-0432.CCR-18-3778]
37 Llovet JM,Montal R,Sia D,Finn RS.Molecular therapies and precision medicine for hepatocellular carcinoma.
2018; 15: 599-616 [PMID: 30061739 DOI: 10.1038/s41571-018-0073-4]
38 Rau M,Schilling AK,Meertens J,Hering I,Weiss J,Jurowich C,Kudlich T,Hermanns HM,Bantel H,Beyersdorf N,Geier A.Progression from Nonalcoholic Fatty Liver to Nonalcoholic Steatohepatitis Is Marked by a Higher Frequency of Th17 Cells in the Liver and an Increased Th17/Resting Regulatory T Cell Ratio in Peripheral Blood and in the Liver.
2016; 196: 97-105 [PMID: 26621860 DOI: 10.4049/jimmunol.1501175]
39 Ringelhan M,Pfister D,O'Connor T,Pikarsky E,Heikenwalder M.The immunology of hepatocellular carcinoma.
2018; 19: 222-232 [PMID: 29379119 DOI: 10.1038/s41590-018-0044-z]
40 Yang XH,Yamagiwa S,Ichida T,Matsuda Y,Sugahara S,Watanabe H,Sato Y,Abo T,Horwitz DA,Aoyagi Y.Increase of CD4+ CD25+ regulatory T-cells in the liver of patients with hepatocellular carcinoma.
2006; 45: 254-262 [PMID: 16600416 DOI: 10.1016/j.jhep.2006.01.036]
41 Kalathil S,Lugade AA,Miller A,Iyer R,Thanavala Y.Higher frequencies of GARP(+)CTLA-4(+)Foxp3(+) T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality.
2013; 73: 2435-2444 [PMID: 23423978 DOI: 10.1158/0008-5472.CAN-12-3381]
42 Sun Y,Wu L,Zhong Y,Zhou K,Hou Y,Wang Z,Zhang Z,Xie J,Wang C,Chen D,Huang Y,Wei X,Shi Y,Zhao Z,Li Y,Guo Z,Yu Q,Xu L,Volpe G,Qiu S,Zhou J,Ward C,Sun H,Yin Y,Xu X,Wang X,Esteban MA,Yang H,Wang J,Dean M,Zhang Y,Liu S,Yang X,Fan J.Single-cell landscape of the ecosystem in early-relapse hepatocellular carcinoma.
2021; 184: 404-421.e16 [PMID: 33357445 DOI: 10.1016/j.cell.2020.11.041]
43 Song G,Shi Y,Zhang M,Goswami S,Afridi S,Meng L,Ma J,Chen Y,Lin Y,Zhang J,Liu Y,Jin Z,Yang S,Rao D,Zhang S,Ke A,Wang X,Cao Y,Zhou J,Fan J,Zhang X,Xi R,Gao Q.Global immune characterization of HBV/HCVrelated hepatocellular carcinoma identifies macrophage and T-cell subsets associated with disease progression.
2020; 6: 90 [PMID: 33298893 DOI: 10.1038/s41421-020-00214-5]
44 Pfister D,Núñez NG,Pinyol R,Govaere O,Pinter M,Szydlowska M,Gupta R,Qiu M,Deczkowska A,Weiner A,Müller F,Sinha A,Friebel E,Engleitner T,Lenggenhager D,Moncsek A,Heide D,Stirm K,Kosla J,Kotsiliti E,Leone V,Dudek M,Yousuf S,Inverso D,Singh I,Teijeiro A,Castet F,Montironi C,Haber PK,Tiniakos D,Bedossa P,Cockell S,Younes R,Vacca M,Marra F,Schattenberg JM,Allison M,Bugianesi E,Ratziu V,Pressiani T,D'Alessio A,Personeni N,Rimassa L,Daly AK,Scheiner B,Pomej K,Kirstein MM,Vogel A,Peck-Radosavljevic M,Hucke F,Finkelmeier F,Waidmann O,Trojan J,Schulze K,Wege H,Koch S,Weinmann A,Bueter M,Rössler F,Siebenhüner A,De Dosso S,Mallm JP,Umansky V,Jugold M,Luedde T,Schietinger A,Schirmacher P,Emu B,Augustin HG,Billeter A,Müller-Stich B,Kikuchi H,Duda DG,Kütting F,Waldschmidt DT,Ebert MP,Rahbari N,Mei HE,Schulz AR,Ringelhan M,Malek N,Spahn S,Bitzer M,Ruiz de Galarreta M,Lujambio A,Dufour JF,Marron TU,Kaseb A,Kudo M,Huang YH,Djouder N,Wolter K,Zender L,Marche PN,Decaens T,Pinato DJ,Rad R,Mertens JC,Weber A,Unger K,Meissner F,Roth S,Jilkova ZM,Claassen M,Anstee QM,Amit I,Knolle P,Becher B,Llovet JM,Heikenwalder M.NASH limits anti-tumour surveillance in immunotherapy-treated HCC.
2021; 592: 450-456 [PMID: 33762733 DOI: 10.1038/s41586-021-03362-0]
45 Dudek M,Pfister D,Donakonda S,Filpe P,Schneider A,Laschinger M,Hartmann D,Hüser N,Meiser P,Bayerl F,Inverso D,Wigger J,Sebode M,Öllinger R,Rad R,Hegenbarth S,Anton M,Guillot A,Bowman A,Heide D,Müller F,Ramadori P,Leone V,Garcia-Caceres C,Gruber T,Seifert G,Kabat AM,Mallm JP,Reider S,Effenberger M,Roth S,Billeter AT,Müller-Stich B,Pearce EJ,Koch-Nolte F,Käser R,Tilg H,Thimme R,Boettler T,Tacke F,Dufour JF,Haller D,Murray PJ,Heeren R,Zehn D,Böttcher JP,Heikenwälder M,Knolle PA.Auto-aggressive CXCR6
CD8 T cells cause liver immune pathology in NASH.
2021; 592: 444-449 [PMID: 33762736 DOI: 10.1038/s41586-021-03233-8]
46 Lim CJ,Lee YH,Pan L,Lai L,Chua C,Wasser M,Lim TKH,Yeong J,Toh HC,Lee SY,Chan CY,Goh BK,Chung A,Heikenwälder M,Ng IO,Chow P,Albani S,Chew V.Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma.
2019; 68: 916-927 [PMID: 29970455 DOI: 10.1136/gutjnl-2018-316510]
47 Maini MK,Pallett LJ.Defective T-cell immunity in hepatitis B virus infection: why therapeutic vaccination needs a helping hand.
2018; 3: 192-202 [PMID: 29870733 DOI: 10.1016/S2468-1253(18)30007-4]
48 Amsen D,van Gisbergen KPJM,Hombrink P,van Lier RAW.Tissue-resident memory T cells at the center of immunity to solid tumors.
2018; 19: 538-546 [PMID: 29777219 DOI: 10.1038/s41590-018-0114-2]
49 Heim MH,Thimme R.Innate and adaptive immune responses in HCV infections.
2014; 61: S14-S25 [PMID: 25443342 DOI: 10.1016/j.jhep.2014.06.035]
50 Kong W,Wei M,Liu R,Zhang J,Wang X.Prognostic value of CD169-positive macrophages in various tumors: a metaanalysis.
2021; 12: 8505-8514 [PMID: 34607536 DOI: 10.1080/21655979.2021.1985857]
51 Cai XY,Wang JX,Yi Y,He HW,Ni XC,Zhou J,Cheng YF,Jin JJ,Fan J,Qiu SJ.Low counts of γδ T cells in peritumoral liver tissue are related to more frequent recurrence in patients with hepatocellular carcinoma after curative resection.
2014; 15: 775-780 [PMID: 24568494 DOI: 10.7314/apjcp.2014.15.2.775]
52 Liu RX,Wei Y,Zeng QH,Chan KW,Xiao X,Zhao XY,Chen MM,Ouyang FZ,Chen DP,Zheng L,Lao XM,Kuang DM.Chemokine (C-X-C motif) receptor 3-positive B cells link interleukin-17 inflammation to protumorigenic macrophage polarization in human hepatocellular carcinoma.
2015; 62: 1779-1790 [PMID: 26235097 DOI: 10.1002/hep.28020]
53 Shalapour S,Lin XJ,Bastian IN,Brain J,Burt AD,Aksenov AA,Vrbanac AF,Li W,Perkins A,Matsutani T,Zhong Z,Dhar D,Navas-Molina JA,Xu J,Loomba R,Downes M,Yu RT,Evans RM,Dorrestein PC,Knight R,Benner C,Anstee QM,Karin M.Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity.
2017; 551: 340-345 [PMID: 29144460 DOI: 10.1038/nature24302]
54 Ruf B,Heinrich B,Greten TF.Immunobiology and immunotherapy of HCC: spotlight on innate and innate-like immune cells.
2021; 18: 112-127 [PMID: 33235387 DOI: 10.1038/s41423-020-00572-w]
55 Cai L,Zhang Z,Zhou L,Wang H,Fu J,Zhang S,Shi M,Zhang H,Yang Y,Wu H,Tien P,Wang FS.Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients.
2008; 129: 428-437 [PMID: 18824414 DOI: 10.1016/j.clim.2008.08.012]
56 Hoechst B,Voigtlaender T,Ormandy L,Gamrekelashvili J,Zhao F,Wedemeyer H,Lehner F,Manns MP,Greten TF,Korangy F.Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma
the NKp30 receptor.
2009; 50: 799-807 [PMID: 19551844 DOI: 10.1002/hep.23054]
57 Wolf MJ,Adili A,Piotrowitz K,Abdullah Z,Boege Y,Stemmer K,Ringelhan M,Simonavicius N,Egger M,Wohlleber D,Lorentzen A,Einer C,Schulz S,Clavel T,Protzer U,Thiele C,Zischka H,Moch H,Tschöp M,Tumanov AV,Haller D,Unger K,Karin M,Kopf M,Knolle P,Weber A,Heikenwalder M.Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer
cross-talk with hepatocytes.
2014; 26: 549-564 [PMID: 25314080 DOI: 10.1016/j.ccell.2014.09.003]
58 Mossanen JC,Kohlhepp M,Wehr A,Krenkel O,Liepelt A,Roeth AA,Möckel D,Heymann F,Lammers T,Gassler N,Hermann J,Jankowski J,Neumann UP,Luedde T,Trautwein C,Tacke F.CXCR6 Inhibits Hepatocarcinogenesis by Promoting Natural Killer T- and CD4
T-Cell-Dependent Control of Senescence.
2019; 156: 1877-1889.e4 [PMID: 30710528 DOI: 10.1053/j.gastro.2019.01.247]
59 Ma C,Han M,Heinrich B,Fu Q,Zhang Q,Sandhu M,Agdashian D,Terabe M,Berzofsky JA,Fako V,Ritz T,Longerich T,Theriot CM,McCulloch JA,Roy S,Yuan W,Thovarai V,Sen SK,Ruchirawat M,Korangy F,Wang XW,Trinchieri G,Greten TF.Gut microbiome-mediated bile acid metabolism regulates liver cancer
NKT cells.
2018; 360 [PMID: 29798856 DOI: 10.1126/science.aan5931]
60 Fan QM,Jing YY,Yu GF,Kou XR,Ye F,Gao L,Li R,Zhao QD,Yang Y,Lu ZH,Wei LX.Tumor-associated macrophages promote cancer stem cell-like properties
transforming growth factor-beta1-induced epithelialmesenchymal transition in hepatocellular carcinoma.
2014; 352: 160-168 [PMID: 24892648 DOI: 10.1016/j.canlet.2014.05.008]
61 Zhou J,Ding T,Pan W,Zhu LY,Li L,Zheng L.Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients.
2009; 125: 1640-1648 [PMID: 19569243 DOI: 10.1002/ijc.24556]
62 Yeung OW,Lo CM,Ling CC,Qi X,Geng W,Li CX,Ng KT,Forbes SJ,Guan XY,Poon RT,Fan ST,Man K.Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma.
2015; 62: 607-616 [PMID: 25450711 DOI: 10.1016/j.jhep.2014.10.029]
63 Li Z,Wu T,Zheng B,Chen L.Individualized precision treatment: Targeting TAM in HCC.
2019; 458: 86-91 [PMID: 31129147 DOI: 10.1016/j.canlet.2019.05.019]
64 Zhang Q,He Y,Luo N,Patel SJ,Han Y,Gao R,Modak M,Carotta S,Haslinger C,Kind D,Peet GW,Zhong G,Lu S,Zhu W,Mao Y,Xiao M,Bergmann M,Hu X,Kerkar SP,Vogt AB,Pflanz S,Liu K,Peng J,Ren X,Zhang Z.Landscape and Dynamics of Single Immune Cells in Hepatocellular Carcinoma.
2019; 179: 829-845.e20 [PMID: 31675496 DOI: 10.1016/j.cell.2019.10.003]
65 Han Y,Chen Z,Yang Y,Jiang Z,Gu Y,Liu Y,Lin C,Pan Z,Yu Y,Jiang M,Zhou W,Cao X.Human CD14+ CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma.
2014; 59: 567-579 [PMID: 23960017 DOI: 10.1002/hep.26694]
66 Ormandy LA,Farber A,Cantz T,Petrykowska S,Wedemeyer H,Horning M,Lehner F,Manns MP,Korangy F,Greten TF.Direct ex vivo analysis of dendritic cells in patients with hepatocellular carcinoma.
2006; 12: 3275-3282 [PMID: 16718852 DOI: 10.3748/wjg.v12.i20.3275]
67 Xu Y,Poggio M,Jin HY,Shi Z,Forester CM,Wang Y,Stumpf CR,Xue L,Devericks E,So L,Nguyen HG,Griselin A,Gordan JD,Umetsu SE,Reich SH,Worland ST,Asthana S,Barna M,Webster KR,Cunningham JT,Ruggero D.Translation control of the immune checkpoint in cancer and its therapeutic targeting.
2019; 25: 301-311 [PMID: 30643286 DOI: 10.1038/s41591-018-0321-2]
68 Ruiz de Galarreta M,Bresnahan E,Molina-Sánchez P,Lindblad KE,Maier B,Sia D,Puigvehi M,Miguela V,Casanova-Acebes M,Dhainaut M,Villacorta-Martin C,Singhi AD,Moghe A,von Felden J,Tal Grinspan L,Wang S,Kamphorst AO,Monga SP,Brown BD,Villanueva A,Llovet JM,Merad M,Lujambio A.β-Catenin Activation Promotes Immune Escape and Resistance to Anti-PD-1 Therapy in Hepatocellular Carcinoma.
2019; 9: 1124-1141 [PMID: 31186238 DOI: 10.1158/2159-8290.CD-19-0074]
69 Zhou SL,Dai Z,Zhou ZJ,Wang XY,Yang GH,Wang Z,Huang XW,Fan J,Zhou J.Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma.
2012; 56: 2242-2254 [PMID: 22711685 DOI: 10.1002/hep.25907]
70 Ponziani FR,Bhoori S,Castelli C,Putignani L,Rivoltini L,Del Chierico F,Sanguinetti M,Morelli D,Paroni Sterbini F,Petito V,Reddel S,Calvani R,Camisaschi C,Picca A,Tuccitto A,Gasbarrini A,Pompili M,Mazzaferro V.Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease.
2019; 69: 107-120 [PMID: 29665135 DOI: 10.1002/hep.30036]
71 Dapito DH,Mencin A,Gwak GY,Pradere JP,Jang MK,Mederacke I,Caviglia JM,Khiabanian H,Adeyemi A,Bataller R,Lefkowitch JH,Bower M,Friedman R,Sartor RB,Rabadan R,Schwabe RF.Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4.
2012; 21: 504-516 [PMID: 22516259 DOI: 10.1016/j.ccr.2012.02.007]
72 Yu LX,Yan HX,Liu Q,Yang W,Wu HP,Dong W,Tang L,Lin Y,He YQ,Zou SS,Wang C,Zhang HL,Cao GW,Wu MC,Wang HY.Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents.
2010; 52: 1322-1333 [PMID: 20803560 DOI: 10.1002/hep.23845]
73 Komiyama S,Yamada T,Takemura N,Kokudo N,Hase K,Kawamura YI.Profiling of tumour-associated microbiota in human hepatocellular carcinoma.
2021; 11: 10589 [PMID: 34012007 DOI: 10.1038/s41598-021-89963-1]
74 Qin N,Yang F,Li A,Prifti E,Chen Y,Shao L,Guo J,Le Chatelier E,Yao J,Wu L,Zhou J,Ni S,Liu L,Pons N,Batto JM,Kennedy SP,Leonard P,Yuan C,Ding W,Hu X,Zheng B,Qian G,Xu W,Ehrlich SD,Zheng S,Li L.Alterations of the human gut microbiome in liver cirrhosis.
2014; 513: 59-64 [PMID: 25079328 DOI: 10.1038/nature13568]
75 Spadoni I,Fornasa G,Rescigno M.Organ-specific protection mediated by cooperation between vascular and epithelial barriers.
2017; 17: 761-773 [PMID: 28869253 DOI: 10.1038/nri.2017.100]
76 Albillos A,de Gottardi A,Rescigno M.The gut-liver axis in liver disease: Pathophysiological basis for therapy.
2020; 72: 558-577 [PMID: 31622696 DOI: 10.1016/j.jhep.2019.10.003]
77 Achiwa K,Ishigami M,Ishizu Y,Kuzuya T,Honda T,Hayashi K,Hirooka Y,Katano Y,Goto H.DSS colitis promotes tumorigenesis and fibrogenesis in a choline-deficient high-fat diet-induced NASH mouse model.
2016; 470: 15-21 [PMID: 26682925 DOI: 10.1016/j.bbrc.2015.12.012]
78 Paik YH,Schwabe RF,Bataller R,Russo MP,Jobin C,Brenner DA.Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells.
2003; 37: 1043-1055 [PMID: 12717385 DOI: 10.1053/jhep.2003.50182]
79 Tu Z,Bozorgzadeh A,Pierce RH,Kurtis J,Crispe IN,Orloff MS.TLR-dependent cross talk between human Kupffer cells and NK cells.
2008; 205: 233-244 [PMID: 18195076 DOI: 10.1084/jem.20072195]
80 Yoshimoto S,Loo TM,Atarashi K,Kanda H,Sato S,Oyadomari S,Iwakura Y,Oshima K,Morita H,Hattori M,Honda K,Ishikawa Y,Hara E,Ohtani N.Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome.
2013; 499: 97-101 [PMID: 23803760 DOI: 10.1038/nature12347]
81 Rodier F,Campisi J.Four faces of cellular senescence.
2011; 192: 547-556 [PMID: 21321098 DOI: 10.1083/jcb.201009094]
82 Loo TM,Kamachi F,Watanabe Y,Yoshimoto S,Kanda H,Arai Y,Nakajima-Takagi Y,Iwama A,Koga T,Sugimoto Y,Ozawa T,Nakamura M,Kumagai M,Watashi K,Taketo MM,Aoki T,Narumiya S,Oshima M,Arita M,Hara E,Ohtani N.Gut Microbiota Promotes Obesity-Associated Liver Cancer through PGE
-Mediated Suppression of Antitumor Immunity.
2017; 7: 522-538 [PMID: 28202625 DOI: 10.1158/2159-8290.CD-16-0932]
83 Sugimoto Y,Narumiya S.Prostaglandin E receptors.
2007; 282: 11613-11617 [PMID: 17329241 DOI: 10.1074/jbc.R600038200]
84 Reig M,Forner A,Rimola J,Ferrer-Fàbrega J,Burrel M,Garcia-Criado Á,Kelley RK,Galle PR,Mazzaferro V,Salem R,Sangro B,Singal AG,Vogel A,Fuster J,Ayuso C,Bruix J.BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update.
2022; 76: 681-693 [PMID: 34801630 DOI: 10.1016/j.jhep.2021.11.018]
85 Bruix J,Chan SL,Galle PR,Rimassa L,Sangro B.Systemic treatment of hepatocellular carcinoma: An EASL position paper.
2021; 75: 960-974 [PMID: 34256065 DOI: 10.1016/j.jhep.2021.07.004]
86 Yau T,Park JW,Finn RS,Cheng AL,Mathurin P,Edeline J.CheckMate 459: a randomized,multi-center phase III study of nivolumab (NIVO) vs.sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC).
2019; 30 Suppl 5: v874-v875 [DOI: 10.1093/annonc/mdz394.029]
87 Finn RS,Ryoo BY,Merle P,Kudo M,Bouattour M,Lim HY,Breder V,Edeline J,Chao Y,Ogasawara S,Yau T,Garrido M,Chan SL,Knox J,Daniele B,Ebbinghaus SW,Chen E,Siegel AB,Zhu AX,Cheng AL; KEYNOTE-240 investigators.Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized,Double-Blind,Phase III Trial.
2020; 38: 193-202 [PMID: 31790344 DOI: 10.1200/JCO.19.01307]
88 Kelley RK,Sangro B,Harris W,Ikeda M,Okusaka T,Kang YK,Qin S,Tai DW,Lim HY,Yau T,Yong WP,Cheng AL,Gasbarrini A,Damian S,Bruix J,Borad M,Bendell J,Kim TY,Standifer N,He P,Makowsky M,Negro A,Kudo M,Abou-Alfa GK.Safety,Efficacy,and Pharmacodynamics of Tremelimumab Plus Durvalumab for Patients With Unresectable Hepatocellular Carcinoma: Randomized Expansion of a Phase I/II Study.
2021; 39: 2991-3001 [PMID: 34292792 DOI: 10.1200/JCO.20.03555]
89 Abou-Alfa GK,Chan SL,Kudo M,Lau G,Kelley RK,Furuse J.Phase 3 randomized,open-label,multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA.
2022; 40: 379-379 [DOI: 10.1200/jco.2022.40.4_suppl.379]
90 Yau T,Kang YK,Kim TY,El-Khoueiry AB,Santoro A,Sangro B,Melero I,Kudo M,Hou MM,Matilla A,Tovoli F,Knox JJ,Ruth He A,El-Rayes BF,Acosta-Rivera M,Lim HY,Neely J,Shen Y,Wisniewski T,Anderson J,Hsu C.Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial.
2020; 6: e204564 [PMID: 33001135 DOI: 10.1001/jamaoncol.2020.4564]
91 Foerster F,Gairing SJ,Müller L,Galle PR.NAFLD-driven HCC: Safety and efficacy of current and emerging treatment options.
2022; 76: 446-457 [PMID: 34555422 DOI: 10.1016/j.jhep.2021.09.007]
92 Hiraoka A,Kumada T,Tada T,Tani J,Kariyama K,Fukunishi S,Atsukawa M,Hirooka M,Tsuji K,Ishikawa T,Takaguchi K,Itobayashi E,Tajiri K,Shimada N,Shibata H,Ochi H,Kawata K,Yasuda S,Toyoda H,Aoki T,Tanaka T,Ohama H,Nouso K,Tsutsui A,Nagano T,Itokawa N,Arai T,Okubo T,Imai M,Koizumi Y,Nakamura S,Joko K,Hiasa Y,Kudo M; Real-life Practice Experts for HCC (RELPEC) Study Group and HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan).Efficacy of lenvatinib for unresectable hepatocellular carcinoma based on background liver disease etiology: multi-center retrospective study.
2021; 11: 16663 [PMID: 34404856 DOI: 10.1038/s41598-021-96089-x]
93 Howell J,Samani A,Mannan B,Hajiev,Aval LM,Abdelmalak R.Impact of NAFLD on clinical outcomes in hepatocellular carcinoma treated with sorafenib: An international cohort study.
2021; 39: 289 [DOI: 10.1200/JCO.2021.39.3_suppl.289]
94 Hatanaka T,Kakizaki S,Nagashima T,Namikawa M,Ueno T,Tojima H,Takizawa D,Naganuma A,Arai H,Harimoto N,Shirabe K,Uraoka T.Lenvatinib for Hepatocellular Carcinoma Patients with Nonviral Infection Who Were Unlikely to Respond to Immunotherapy: A Retrospective,Comparative Study.
2021; 99: 641-651 [PMID: 34515171 DOI: 10.1159/000517494]
95 Shimose S,Hiraoka A,Nakano M,Iwamoto H,Tanaka M,Tanaka T,Noguchi K,Aino H,Ogata K,Kajiwara M,Itano S,Yokokura Y,Yamaguchi T,Kawano H,Matsukuma N,Suga H,Niizeki T,Shirono T,Noda Y,Kamachi N,Okamura S,Kawaguchi T,Koga H,Torimura T.First-line sorafenib sequential therapy and liver disease etiology for unresectable hepatocellular carcinoma using inverse probability weighting: A multicenter retrospective study.
2021; 10: 8530-8541 [PMID: 34693661 DOI: 10.1002/cam4.4367]
96 Zhu AX,Kang YK,Yen CJ,Finn RS,Galle PR,Llovet JM,Assenat E,Brandi G,Pracht M,Lim HY,Rau KM,Motomura K,Ohno I,Merle P,Daniele B,Shin DB,Gerken G,Borg C,Hiriart JB,Okusaka T,Morimoto M,Hsu Y,Abada PB,Kudo M; REACH-2 study investigators.Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised,double-blind,placebo-controlled,phase 3 trial.
2019; 20: 282-296 [PMID: 30665869 DOI: 10.1016/S1470-2045(18)30937-9]
97 Finn RS,Qin S,Ikeda M,Galle PR,Ducreux M,Kim TY.IMbrave150: updated overall survival (OS) data from a global,randomized,open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC).
39: 267 [DOI: 10.1200/JCO.2021.39.3_suppl.267]
98 Lee MS,Ryoo BY,Hsu CH,Numata K,Stein S,Verret W,Hack SP,Spahn J,Liu B,Abdullah H,Wang Y,He AR,Lee KH; GO30140 investigators.Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label,multicentre,phase 1b study.
2020; 21: 808-820 [PMID: 32502443 DOI: 10.1016/S1470-2045(20)30156-X]
99 Kelley RK,Yau T,Cheng AL,Kaseb A,Qin S,Zhu A.VP10-2021: Cabozantinib (C) plus atezolizumab (A) vs sorafenib (S) as first-line systemic treatment for advanced hepatocellular carcinoma (aHCC): Results from the randomized phase III COSMIC-312 trial.
2022; 33: 114-116 [DOI: 10.1016/j.annonc.2021.10.008]
100 Haber PK,Puigvehí M,Castet F,Lourdusamy V,Montal R,Tabrizian P,Buckstein M,Kim E,Villanueva A,Schwartz M,Llovet JM.Evidence-Based Management of Hepatocellular Carcinoma: Systematic Review and Meta-analysis of Randomized Controlled Trials (2002-2020).
2021; 161: 879-898 [PMID: 34126063 DOI: 10.1053/j.gastro.2021.06.008]
101 De Mattos-Arruda L,Siravegna G.How to use liquid biopsies to treat patients with cancer.
2021; 6: 100060 [PMID: 33647598 DOI: 10.1016/j.esmoop.2021.100060]
102 Alunni-Fabbroni M,Rönsch K,Huber T,Cyran CC,Seidensticker M,Mayerle J,Pech M,Basu B,Verslype C,Benckert J,Malfertheiner P,Ricke J.Circulating DNA as prognostic biomarker in patients with advanced hepatocellular carcinoma: a translational exploratory study from the SORAMIC trial.
2019; 17: 328 [PMID: 31570105 DOI: 10.1186/s12967-019-2079-9]
103 Yamamoto Y,Kondo S,Matsuzaki J,Esaki M,Okusaka T,Shimada K,Murakami Y,Enomoto M,Tamori A,Kato K,Aoki Y,Takizawa S,Sakamoto H,Niida S,Takeshita F,Ochiya T.Highly Sensitive Circulating MicroRNA Panel for Accurate Detection of Hepatocellular Carcinoma in Patients With Liver Disease.
2020; 4: 284-297 [PMID: 32025611 DOI: 10.1002/hep4.1451]
104 von Felden J,Schulze K,Krech T,Ewald F,Nashan B,Pantel K,Lohse AW,Riethdorf S,Wege H.Circulating tumor cells as liquid biomarker for high HCC recurrence risk after curative liver resection.
2017; 8: 89978-89987 [PMID: 29163804 DOI: 10.18632/oncotarget.21208]
105 Wang Y,Zhang C,Zhang P,Guo G,Jiang T,Zhao X,Jiang J,Huang X,Tong H,Tian Y.Serum exosomal microRNAs combined with alpha-fetoprotein as diagnostic markers of hepatocellular carcinoma.
2018; 7: 1670-1679 [PMID: 29573235 DOI: 10.1002/cam4.1390]
106 Maravelia P,Silva DN,Rovesti G,Chrobok M,Stål P,Lu YC,Pasetto A.Liquid Biopsy in Hepatocellular Carcinoma: Opportunities and Challenges for Immunotherapy.
2021; 13 [PMID: 34503144 DOI: 10.3390/cancers13174334]
107 Scheiner B,Pomej K,Kirstein MM,Hucke F,Finkelmeier F,Waidmann O,Himmelsbach V,Schulze K,von Felden J,Fründt TW,Stadler M,Heinzl H,Shmanko K,Spahn S,Radu P,Siebenhüner AR,Mertens JC,Rahbari NN,Kütting F,Waldschmidt DT,Ebert MP,Teufel A,De Dosso S,Pinato DJ,Pressiani T,Meischl T,Balcar L,Müller C,Mandorfer M,Reiberger T,Trauner M,Personeni N,Rimassa L,Bitzer M,Trojan J,Weinmann A,Wege H,Dufour JF,Peck-Radosavljevic M,Vogel A,Pinter M.Prognosis of patients with hepatocellular carcinoma treated with immunotherapy - development and validation of the CRAFITY score.
2022; 76: 353-363 [PMID: 34648895 DOI: 10.1016/j.jhep.2021.09.035]
108 Zheng Y,Wang T,Tu X,Huang Y,Zhang H,Tan D,Jiang W,Cai S,Zhao P,Song R,Li P,Qin N,Fang W.Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma.
2019; 7: 193 [PMID: 31337439 DOI: 10.1186/s40425-019-0650-9]
109 Ponziani FR,De Luca A,Picca A,Marzetti E,Petito V,Del Chierico F,Reddel S,Paroni Sterbini F,Sanguinetti M,Putignani L,Gasbarrini A,Pompili M.Gut Dysbiosis and Fecal Calprotectin Predict Response to Immune Checkpoint Inhibitors in Patients With Hepatocellular Carcinoma.
2022; 6: 1492-1501 [PMID: 35261212 DOI: 10.1002/hep4.1905]
110 Routy B,Le Chatelier E,Derosa L,Duong CPM,Alou MT,Daillère R,Fluckiger A,Messaoudene M,Rauber C,Roberti MP,Fidelle M,Flament C,Poirier-Colame V,Opolon P,Klein C,Iribarren K,Mondragón L,Jacquelot N,Qu B,Ferrere G,Clémenson C,Mezquita L,Masip JR,Naltet C,Brosseau S,Kaderbhai C,Richard C,Rizvi H,Levenez F,Galleron N,Quinquis B,Pons N,Ryffel B,Minard-Colin V,Gonin P,Soria JC,Deutsch E,Loriot Y,Ghiringhelli F,Zalcman G,Goldwasser F,Escudier B,Hellmann MD,Eggermont A,Raoult D,Albiges L,Kroemer G,Zitvogel L.Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors.
2018; 359: 91-97 [PMID: 29097494 DOI: 10.1126/science.aan3706]
111 Xu X,Lv J,Guo F,Li J,Jia Y,Jiang D,Wang N,Zhang C,Kong L,Liu Y,Zhang Y,Li Z.Gut Microbiome Influences the Efficacy of PD-1 Antibody Immunotherapy on MSS-Type Colorectal Cancer
Metabolic Pathway.
2020; 11: 814 [PMID: 32425919 DOI: 10.3389/fmicb.2020.00814]
112 Botticelli A,Vernocchi P,Marini F,Quagliariello A,Cerbelli B,Reddel S,Del Chierico F,Di Pietro F,Giusti R,Tomassini A,Giampaoli O,Miccheli A,Zizzari IG,Nuti M,Putignani L,Marchetti P.Gut metabolomics profiling of nonsmall cell lung cancer (NSCLC) patients under immunotherapy treatment.
2020; 18: 49 [PMID: 32014010 DOI: 10.1186/s12967-020-02231-0]
113 Ren Z,Xu J,Bai Y,Xu A,Cang S,Du C,Li Q,Lu Y,Chen Y,Guo Y,Chen Z,Liu B,Jia W,Wu J,Wang J,Shao G,Zhang B,Shan Y,Meng Z,Gu S,Yang W,Liu C,Shi X,Gao Z,Yin T,Cui J,Huang M,Xing B,Mao Y,Teng G,Qin Y,Xia F,Yin G,Yang Y,Chen M,Wang Y,Zhou H,Fan J; ORIENT-32 study group.Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised,open-label,phase 2-3 study.
2021; 22: 977-990 [PMID: 34143971 DOI: 10.1016/S1470-2045(21)00252-7]
114 Qin S,Chen Z,Fang W,Ren Z,Xu R,Ryoo BY.Pembrolizumab plus best supportive care vs placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): Phase 3 KEYNOTE-394 study.
2022; 40: 383 [DOI: 10.1200/JCO.2022.40.4_suppl.379]
115 Zhu AX,Finn RS,Edeline J,Cattan S,Ogasawara S,Palmer D,Verslype C,Zagonel V,Fartoux L,Vogel A,Sarker D,Verset G,Chan SL,Knox J,Daniele B,Webber AL,Ebbinghaus SW,Ma J,Siegel AB,Cheng AL,Kudo M; KEYNOTE-224 investigators.Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised,open-label phase 2 trial.
2018; 19: 940-952 [PMID: 29875066 DOI: 10.1016/S1470-2045(18)30351-6]
116 A Study of Nivolumab in Combination With Ipilimumab in Participants With Advanced Hepatocellular Carcinoma (CheckMate 9DW).[accessed 2022 Jan 22].In: ClinicalTrials.gov [Internet].Bethesda (MD): U.S.National Library of Medicine.Available from: https://www.clinicaltrials.gov/ct2/show/NCT04039607 ClinicalTrials.gov Identifier: NCT04039607
 World Journal of Gastrointestinal Oncology2022年9期
World Journal of Gastrointestinal Oncology2022年9期
- World Journal of Gastrointestinal Oncology的其它文章
- Nutrition deprivation affects the cytotoxic effect of CD8 T cells in hepatocellular carcinoma
- Prognostic and clinicopathological value of Twist expression in esophageal cancer:A meta-analysis
- Dissecting novel mechanisms of hepatitis B virus related hepatocellular carcinoma using meta-analysis of public data
- Prediction of gastric cancer risk by a polygenic risk score of Helicobacter pylori
- Percutaneous insertion of a novel dedicated metal stent to treat malignant hilar biliary obstruction
- Construction and analysis of an ulcer risk prediction model after endoscopic submucosal dissection for early gastric cancer
