Thermogravimetric analysis on the characteristics of oxy-fuel co-combustion of sub-bituminous coal and semi-coke
LI Zhao-yang ,NIU Sheng-li ,HAN Kui-hua ,LI Ying-jie ,WANG Yong-zheng ,LU Chun-mei
(School of Energy and Power Engineering, Shandong University (Shandong 250061,China))
Abstract: The co-combustion of the low-rank coal with coal derived semi-coke is of great significance to solve the urgent problem of excessively produced semi-coke in China.In this research, the oxy-fuel co-combustion characteristics of Zhundong sub-bituminous coal with bituminous coal derived semi-coke are systematically investigated using thermogravimetric analysis.Compared with air combustion, oxy-fuel atmosphere increased the ignition and burnout temperature by 10 and 40 °C, respectively.Increasing the oxygen concentration to 30% strongly compensated for the slight reduction of the combustion parameters under oxy-fuel condition and much better co-combustion performance was obtained.Three iso-conversional methods, namely, Flynn-Wall-Ozawa (FWO), Kissinger-Akahira-Sunose (KAS) and Starink, were applied to estimate the activation energy, which can be divided into two stages during the co-combustion process.The average activation energy of sub-bituminous coal, the blend and semi-coke were 49.31, 50.82 and 59.00 kg/mol, respectively.Further,the pre-exponential factor and thermodynamic parameters of the enthalpy change, Gibbs free energy change and entropy change were calculated.Interaction indices were innovatively used for both kinetic-thermodynamic parameters and DTG values.An obvious interaction can be observed during the co-combustion process.The kinetic and thermodynamic results demonstrated that the 30% semi-coke ratio was beneficial to co-combustion.Meanwhile, X-ray fluorescence (XRF) and ash fusion analyses proved that the slagging tendency of sub-bituminous coal ash reduced by blending of semi-coke.
Key words: oxy-fuel co-combustion;coal semi-coke;thermogravimetric analysis;kinetics;thermodynamics;ash sintering
Cascade utilization of coal is a reliable technology to improve the utilization efficiency and reduce the environmental pollution in recent years[1].This technology extracts high-value oil and gas from lowrank coals through pyrolysis or gasification without direct burning process, and thus realizes the clean and efficient usage.A by-product left by cracking raw coal is called semi-coke, which is commonly used in smelting, calcium carbide and other fields[2,3].However,the supply of the over-produced coal semi-coke has exceeded the demand in recent years and how to deal with the excessive semi-coke becomes an urgent problem[4].
Semi-coke is a carbon-based material with low volatile and high calorific value.In theory, combustion of semi-coke can provide a lot of thermal energy to realize its reasonable application.However, due to the extensively low content of volatile substances,hydrogen and oxygen, semi-coke is widely recognized with the difficulty in ignition, unstable flame and low combustion efficiency.Thus, it is practically hard to be applied as a single fuel in the pulverized coal furnaces[5].Experiments about semi-coke combustion in circulating fluidized bed boilers (CFBB) have been reported, where the high-temperature preheating combustion approach (>1000 °C), fuel-rich conditions and optimization of the positions of secondary and tertiary air ports can apparently reduce NOxgeneration during the semi-coke combustion[6-8].However, the high cost introduced by the auxiliary heating equipment obviously cannot meet the requirements of the power plants.
Among the combustion strategies, the cocombustion appears to be proper in improving the poor combustion performance of semi-coke, which attracts many attentions spontaneously.The combustion of blends is believed to achieve a better combustion behavior than pure substances because of synergetic effect between the two fuels.Researchers have carried out lots of studies on co-combustion, such as the binary blending of coals[9-11], coal and biomass blending[12,13],coal and solid waste blending[14].In some of the semi-coke co-combustion studies, synergetic effect occurs during the oil shale semi-coke and biomass cocombustion process[15-17], while other studies observe no significant interaction[18,19].But it should be noted that huge differences exist between the oil shale semi-coke and coal derived semi-coke.For example, the ash content of oil shale semi-coke is extremely high (more than 80%), and its calorific value is almost one order lower than that of the raw coal[20].On the contrary, the calorific value of the coal semi-coke is higher than that of the raw coal[2].By contrasting with the cocombustion studies of oil shale semi-coke and other fuels, kinetic characteristics investigation into the cocombustion of coal derived semi-coke and low-rank coal is rarely employed.From a small-scale plant test,the co-combustion of semi-coke with coal was proved feasible in pulverized coal injection (PCI) operation[21].Further, industrial-scale turbulent chemical simulations showed the semi-coke and bituminous coal can achieve stable co-combustion under certain conditions[22].
Co-combustion of the high volatile raw coal with the coal semi-coke is a theoretically feasible solution to consume the excessively produced semi-coke, as the addition of semi-coke may strengthen the slagging resistance of the low-rank coals.In previous studies,the ignition and combustion stability of oil shale semicoke could be improved through the high volatile lowrank coal addition[23].Wang et al.[24]used drop tube furnace (DTF) to simulate the co-combustion process in a pulverized coal boiler and concluded that the NOxand unburned carbon ratio generated by the semi-coke combustion were higher.Moreover, the blending ratio significantly determines the NOxformation[4,25].Zhu et al.[26]studied the co-combustion of Shenmu coal with char and found that the ignition performance and combustion stability of the semi-coke were significantly improved.However, the existing experiments did not pay much attention to the byproduct (coal semi-coke) from low-rank coal cascade utilization and may underestimated the great value of coal derived semi-coke by confusing it with oil shale semi-coke.
Meanwhile, the carbon capture and storage (CCS)is a reliable way to reduce greenhouse gas emission caused by the coal combustion[27].In particular, with the peak of carbon emissions by 2030 and carbon neutral by 2060 plan proposed by China, many coal-fired power plants turn to explore the viable CCS methods[28,29].The oxy-fuel combustion technology can achieve a carbon enrichment higher than 90%.Thus, is considered as a promising commercial approach for CCS[30,31].Moreover, the oxy-fuel combustion was chosen as the only CCS technology introduced in coal power industry, which however has not been put into commercial application in China[32].To achieve effective carbon emission reduction, unconventional combustion technologies like oxy-fuel co-combustion must undergo basic experimental verification to provide theoretical support for further large-scale industrial applications.Meanwhile, the notable slagging problem of low-rank coal may also be relieved due to the blending of coal semi-coke.
To demonstrate the feasibility of co-combustion of low-rank coal and coal derived semi-coke (not oil shale semi-coke) in thermal power plants, the thermogravimetric analysis was used to study the kinetic and thermodynamic perspectives for cocombustion of low-rank coal and coal derived semi-coke in this paper.Detailed intentions are to (1) investigate the possibility of substituting oxy-fuel combustion for air combustion to achieve convenient carbon capture; (2) evaluate the specific influences of cocombustion conditions on the co-combustion behavior;(3) innovatively assess the possible interaction during co-combustion processes employing synergistic index for both kinetic and thermodynamic parameters;(4) determine the slagging risk of industrial cocombustion application by X-ray fluorescence (XRF)and ash fusion analyses.
1 Materials and methods
1.1 Sample preparation
To obtain the coal semi-coke under real condition,bituminous coal was put into a tube furnace and heated from room temperature to the medium temperature(600 °C) at the heating rate of 5 °C/min and kept for 60 min before the natural cooling process.The whole heating process was conducted under pure N2atmosphere.The raw coal used in this study is Zhundong sub-bituminous coal, which is a well-known low-rank coal in China[33].Both the two samples were ground and sieved to a particle size of less than 75 μm and dried in air at 110 °C for 24 h to ensure the mass kept constant.Table 1 gives the proximate and ultimate analyses of the coal semi-coke and sub-bituminous coal.Compared with sub-bituminous coal, semi-coke contains extremely low content of volatile matter(8.78%) but relatively high content of ash (32.27%).Moreover, the fixed-carbon content is 58.45%, which is much higher than oil-shale semi-coke and reveals the excellent fuel property of coal derived semi-coke.After cracking, the atomic ratio O/C and H/C of coal semicoke diminished to 0.029 and 0.38, respectively,indicating that the bituminous coal rank could be improved and upgraded as another clean fuel as coal semi-coke.The atomic ratio O/C and H/C of Zhundong sub-bituminous coal are 0.22 and 0.61, respectively.To some extent, the blending of semi-coke with subbituminous coal may compensate for the poor combustion performance of the pure semi-coke.
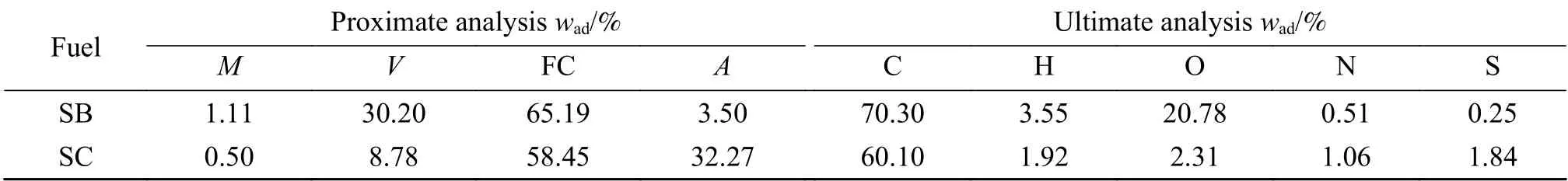
Table 1 Proximate and ultimate analyses of sub-bituminous coal and the bituminous coal derived semi-coke
1.2 TGA experiment
The combustion processes were tested in a thermogravimetric analyzer (NETZSCH STA 2500 Regulus, Germany) with a sensitivity of 10-3mg.Each sample (10±0.5) mg was placed in an Al2O3crucible in the atmosphere composed of O2and CO2to analyze the effect of the oxy-fuel combustion and heated from 30 to 950 °C to observe the whole combustion process.To eliminate the influence of the diffusion effect on combustion, the total flow rate of the carrier gas was controlled at 100 mL/min.In this work, three different heating rates, namely, 20, 25 and 30 °C/min, were chosen to obtain the data for the iso-conversional methods.
1.3 Combustion characteristics
Several combustion parameters were analyzed from the TG-DTG curves.The ignition temperature (Ti)is obtained through extrapolation of the TG curve at the DTG peak temperature.The burnout temperature (Tf)was defined as the temperature at which the conversion degree reached 98%.The comprehensive combustion index (CCI) , as described in Eq.(1), was extensively used to evaluate the combustion characteristics, where a higherCCIvalue means a better combustion performance[34].Besides, the combustion stability index(Rw) described as Eq.(2) was applied as well to investigate the combustion stability[19].
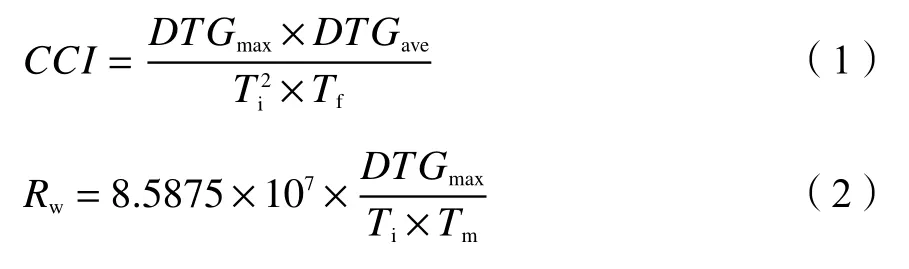
where,DTGmaxandDTGaveare the maximum and average mass loss rates (data fromTitoTf),respectively.Tmrepresents the DTG peak temperature.
1.4 Kinetic parameters calculation
The iso-conversional approaches based on the multiple heating rates are considered as the most reliable methods to calculate activation energy for solid fuel combustion.According to International Confederation for Thermal Analysis and Calorimetry(ICTAC) Kinetics Committee recommendations, the iso-conversion methods are also categorized as differentiation method and integration method.The integration method for kinetics is considered more accurate than the differentiation method[35].So, the integration methods are used to calculate the kinetic parameters.
The kinetic equation for the burning processes is expressed as Eq.(3)[36]:

wheretis time,f(α)is the mechanism function related with the combustion fraction.The mass conversion,α,can be represented as Eq.(4):

wherem0,mtandmfrefer to the mass of a sample at initial stage, timetand the end, respectively.
k(T) is an absolute temperature dependent rate constant that is described by the Arrhenius law in Eq.(5):

whereEa,k0,Rare the activation energy, preexponential factor and universal gas constant,respectively.
Taking Eq.(5) and the heating rate ofβ=dT/dtinto Eq.(3), Eq.(6) is then obtained:

The integration form of Eq.(6) is expressed as Eq.(7)[37]:

Equation (7) can be solved by the approximation methods or numerical methods because it has no exact solution.At the same time, different iso-conversional methods use different approximation approaches to calculate the kinetic parameters.In these isoconversional methods, Flynn-Wall-Ozawa method(FWO) and Kissinger-Akahira-Sunose method (KAS)are the most classic and most used integration methods for solid fuel combustion.In addition, a more accurate method called Starink based on these two methods was also used to calculate and compare the kinetic parameters.
1.4.1 Flynn-Wall-Ozawa method (FWO)
Doyle’s approximation was applied in the integration form of Eq.(7) and then the equation can be written as Eq.(8)[38]:

At every same conversion degree for the different heating rates, different corresponding temperaturesTcan be obtained.Thus, the regression lines of ln(β)versus 1/Tare fitted and the activation energy is calculated from the slope.
1.4.2 Kissinger-Akahira-Sunose method (KAS)
KAS method[39]is described as Eq.(9):
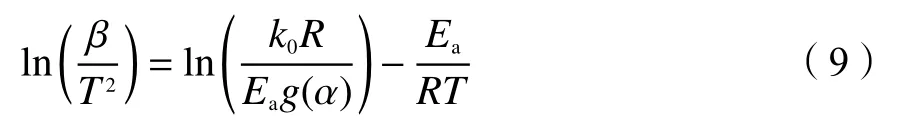
At every heating rate, a chain of temperaturesTare recorded and the regression lines of ln(β/T2) versus 1/Tcan be obtained at each conversion degree.Then,the activation energy is calculated from the slope.
1.4.3 Starink method
Starink method is modified from FWO and KAS and more accurate, as described in Eq.(10)[40]:
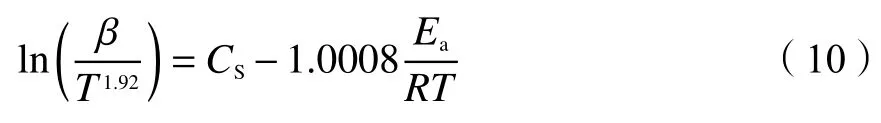
whereCsis a constant.Like KAS method, plot ln(β/T1.92) versus 1/Tat each selectedαand the activation energy is obtained from the slope.
1.4.4 Pre-exponential factor
Based on the activation energy calculated, the preexponential factor for the combustion is suggested by the ICTAC guidelines for model free methods through Eq.(11)[34]:

1.5 Thermodynamic parameters calculation
The present study also calculated three thermodynamic parameters as a supplement of the combustion behavior, which are the enthalpy change(ΔH), Gibbs free energy change (ΔG) and entropy change (ΔS), as described from Eq.(12) to Eq.(14)[40,41]:
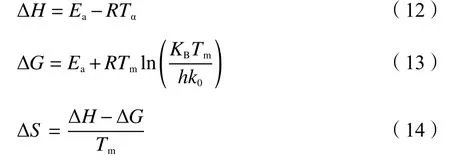
whereTαrepresents the temperature at the correspondingα, KBis the Boltzmann constant of 1.381×10-23J/K, andhis the Plank constant of 6.626×10-34J/s.
1.6 Interaction indices
In the co-combustion process, the combustion behavior differs from both the sub-bituminous coal and semi-coke.In order to illustrate the interaction effect between the coal and semi-coke during the cocombustion process, the theoretical value of the sample blends was calculated from the pure individual sample using the average weight method[42].

whereYcal,YliandYscrefer to the theoretical value of the blend, pure sub-bituminous coal and pure semi-coke,respectively.XliandXscare the mass percentage of subbituminous coal and semi-coke in the blend,respectively.
TheMR(Mean Absolute Error) andRMS(Root Mean Square Error) indices[43], as the mostly widely applied evaluation methods, were conducted to evaluate the interaction effect:

where,andrepresent each experimental value, each theoretical value and the averagetheoretical value.nis the total number of experimental points.A non-zeroMRvalue means interaction effect exists during the co-combustion process andRMSdemonstrates the intensity of the interaction, where a higher value refers to a stronger interaction behavior.
1.7 Ash composition analysis and ash fusion temperature measurement
A major factor restricting the utilization of lowrank coal is the risk of ash slagging caused by the high content of alkali and alkaline earth metals.To obtain ash samples, the semi-coke combustion was conducted in a muffle furnace in air at (815 ± 10) °C.As for subbituminous coal, the temperature was set as (500 ±10) °C to avoid the alkali metals transformation during combustion.Then the major elements of the ash samples were analyzed using an X-ray fluorescence(XRF) spectrometer (SHIMADAZU EDX-7200,Japan).Besides, the four characteristic temperatures of the deformation temperatureTD, softening temperatureTS, hemispheric temperatureTHand flowing temperatureTFof the ash samples were tested by an ash fusion point apparatus (ZDHR-3, China).
2 Results and discussion
2.1 Combustion characteristics in air and oxy-fuel atmospheres
The TG-DTG curves of sub-bituminous coal and semi-coke at the heating rate of 20 K/min under 20%O2/80% CO2and 20% O2/80% N2are shown in Figure 1.According to the TG curves, the semi-coke shows almost no mass loss in the temperature range lower than 480 °C, while the sub-bituminous coal loses nearly 5% of its mass, which corresponds to the bound water and small amount light volatiles released from sub-bituminous coal.During the semi-coke preparation process (600 °C cracking), the free water and bound water as well as the most volatiles were released, while sub-bituminous coal only released free water with the air-dried treatment.The main weight loss for subbituminous coal and semi-coke occurs at 400–600 °C and 480–700 °C, respectively.At the accomplishment of the combustion, the mass remained was about 10%for sub-bituminous coal and nearly 40% for semi-coke,which can be ascribed to the large proportion of ash after the removal of volatiles from semi-coke.Compared with the ash content in Table 1, both of the two fuel residuals were higher than ash weight (subbituminous coal ash 3.5%, semi-coke ash 32.27%),indicating that part of the fixed carbon was not fully burned out.As can be seen from Figure 1(b), oxy-fuel atmosphere decreases the maximum mass loss rate by about 15% when compared with air atmosphere.For example, the DTG peak of sub-bituminous coal is about 9.5%/min in air condition while about 8%/min in oxy-fuel condition.The higher specific heat and density of CO2and lower diffusivity of O2in CO2than N2weaken the transport of O2to the fuel particle surface[43].This results in the suppressed volatiles combustion and reactivity and thus, lower the maximum mass loss rate.
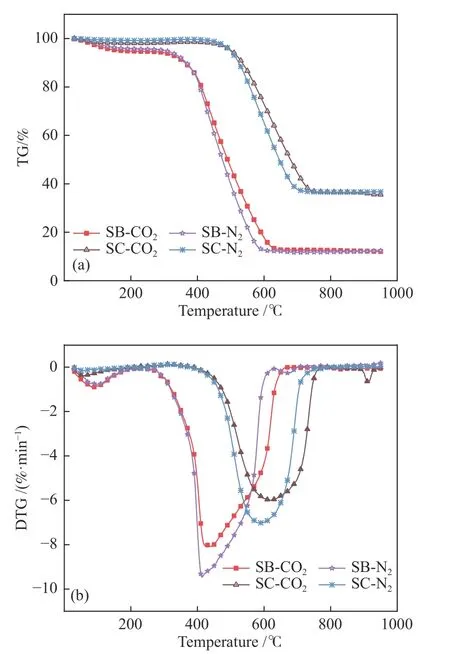
Figure 1 Sub-bituminous coal and semi-coke combustion TG and DTG curves in different atmosphere
Overall, the two fuels displayed highly similar mass loss characteristics in both oxy-fuel and air atmosphere.But the ignition and burnout temperatures of sub-bituminous coal are 364 and 602 °C in air atmosphere and about 374 and 641 °C in oxy-fuel atmosphere.It is indicated that the overall combustion characteristics change little due to the replacement of N2with CO2.However, the ignition and burnout temperatures increase by about 10 and 40 °C,respectively, due to the higher specific heat of CO2thanN2to postpone the combustion process.As CO2suppresses the maximum mass loss rate and the burnout temperature is delayed under the same condition, the peak values in the oxy-fuel atmosphere are smaller than those in the air atmosphere.Notably, a small peak at about 900 °C in the oxy-fuel atmosphere is observed and it may be caused by char-CO2gasification reaction in the high temperature zone[23].Although the ignition temperature and burnout temperature have increased in oxy-fuel atmosphere, the relatively mild combustion environment is conducive to the smooth operation of the fuels in the thermal plant.
2.2 Combustion performances of the blends
The blending ratio is an important parameter to implement the coal co-combustion, where a suitable blending ratio is beneficial to improve the combustion performance and keep stable operation of the thermal plants.Thus, this section focuses on the influence of the blending ratio of semi-coke on co-combustion behavior.
The TG-DTG curves of the pure sub-bituminous coal, pure semi-coke and semi-coke ratios of 10%, 30%,50%, and 70% in the oxy-fuel atmosphere with 20% O2concentration at the heating rate of 20 K/min are shown in Figure 2.In Figure 2, the curves of the blends fall between those of the pure sub-bituminous coal and pure semi-coke sequentially.With the increased proportion of semi-coke in blends, the ash content and the burnout temperature increase correspondingly.On the other hand, DTG curves show significant differences among the blends.As shown in Figure 2(b),the DTG curve of sub-bituminous coal has a small peak(~100 °C) and a large peak (~440 °C).The former peak corresponds to the loss of the free and bound water,and the latter peak corresponds to the combustion of volatiles and fixed-carbon.In biomass and coal cocombustion process, there are generally more than two large peaks, which are caused by the combustion of volatiles and fixed-carbon, respectively[44-46].In this study, the first half of the sub-bituminous coal combustion peak is steep, and the second half is relatively smooth, indicating that the combustion of the fixed-carbon and volatiles do not separate completely but overlap in partial, which is quite different from the biomass combustion.Further, the combustion of the earlier released light volatile matter may promote the ignition of fixed-carbon and therefore realize more continuous and stabler combustion than biomass.The DTG curve of semi-coke presents a large peak and two small peaks.The large peak is almost ascribed to the fixed-carbon combustion as the volatile content in semi-coke is particularly low.The small peak near 100 °C is caused by the release of the bound water and the peak near the end of combustion is for the char-CO2gasification.Besides, the DTG peaks of the sample changes are minor when the blending ratio of semicoke exceeds 30%, which means this blending ratio begins to limit the volatile combustion in low-rank coal and the combustion rate is dominated by coal semicoke.Yao et al.[23]also found similar phenomenon.This may be due to the coating effect of the porous structure of semi-coke that preventing the contact of low-rank coal volatiles with O2.
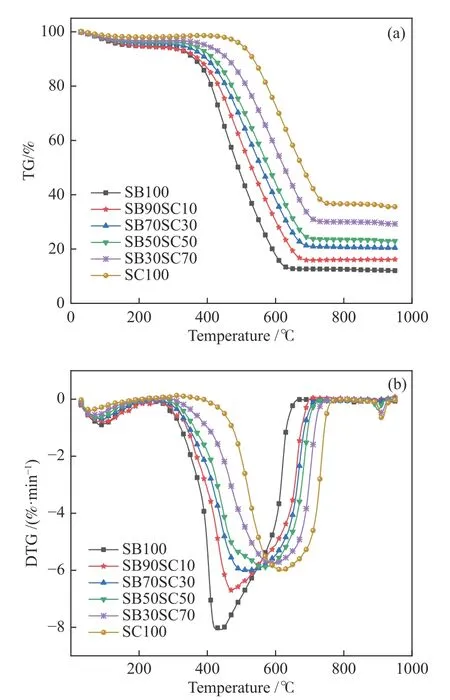
Figure 2 TG and DTG curves of the blends under oxy-fuel atmosphere
The combustion parameters for each sample of the TG-DTG curves are listed in Table 2, which provide a quantitative analysis to further understand the influence of the blending ratio on the oxy-fuel co-combustion characteristics.As the increase of semi-coke ratio,Ti,TmandTfrange from 363.6–510, 439.8–620.6 and 640.6–750.2 °C, respectively.These characteristic temperatures do not increase monotonically with theincrease ratio of coal semi-coke.This finding is in good agreement with previous study by Zheng et al.[2].In this study, the ignition temperatures of sub-bituminous coal and semi-coke are 363.6 and 510 °C, respectively.The low ignition temperature of sub-bituminous coal is consistent with light volatiles combustion, and it is the main reason for the spontaneous combustion of this kind of low-rank coal.On the contrary, the small amount of heavy volatile residuals and relatively high content of fixed-carbon in coal semi-coke result in the highTiand delay the values ofTi,Tm,Tfas well,therefore cut down the reactivity of semi-coke and its blends.As a result, as the blending ratio of the semicoke increases, the characteristic temperatures of the blended fuels all approach pure semi-coke.

Table 2 Combustion characteristic parameters of the blends
According to the trend ofCCI, the value decreases from 5.62×10-7to 2.63×10-7with the blending ratio of semi-coke increase from 0 to 30%, indicating the comprehensive combustion performance of the blended fuel becomes relatively unsatisfactory with a semi-coke blending ratio of 30%.Rwcharacterizes the combustion stability and can be divided into three levels: 4.34×103,2.63×103-3.24×103and 1.63×103-2.16×103.Only the former two levels are relatively suitable for actual combustion.It is inferred that the combustion stability of the pure semi-coke is much lower than that of the pure sub-bituminous coal.With a higher blending ratio of semi-coke, the combustion stability turns worse,where a low combustion stability is not conducive to the smooth operation of the coal-fired boilers.In this study, the suggested proportion of coal semi-coke is 30% to ensure a safe combustion process.
2.3 Effect of O2 concentration
As discussed above, the combustion performance will be slightly reduced when air combustion atmosphere switches to oxy-fuel for the different thermo-physical properties between CO2and N2.To enhance the fuel combustion characteristics, increasing O2concentration is a reliable and feasible method.According to the blending ratio results, the 30% semicoke sample is chosen in this section.The sample under the O2concentrations of 10%, 15%, 20%, 25%and 30%, respectively, were adopted at the heating rate of 20 K/min in oxy-fuel atmosphere.
As shown in Figure 3, the TG curves overlap as the sample heated from room temperature to about 300 °C, where the O2concentration shows little influence on the dehydration and devolatilization process but temperature is the main driving force.In the temperature range of 400–900 °C of the main combustion zone, the TG and DTG curves shifted to the lower temperature sharply with the increased O2concentration and show significant differences in fuel combustion performance.In the main combustion zone,the TG curve gets steep with the increased O2concentration, which also leads to the remarkable changes in DTG curves.The DTG peak at the O2concentration of 10% is only 4%/min and remarkably reaches about 9%/min after increasing the O2concentration to 30%.At the same time, the DTG peak temperature moves from 515 to 465 °C and the peak width reduced by 50% when the O2concentration increases to 30%, which demonstrates the increase of O2concentration causes the intensive combustion of the flammable ingredients and therefore provides higher combustion efficiency of the blended fuel.After the combustion process, the burnout rates are stable at 20% when O2concentration ranges from 10% to 20%,while increasing the O2concentration to 30% will improve the burnout rate to about 15%.The possible reason is that higher O2concentration increase the mass flux rate of O2to the particle surfaces and pores as well as compensates for low flame propagation speed andstability in oxy-fuel atmosphere[43,47].This also provides extra heat feedback to the particle, enhancing devolatilization and combustion[48].

Figure 3 TG and DTG curves of the 30% semi-coke blend under different O2 concentration
Several co-combustion characteristics are listed in Table 3 to validate the influences of O2concentration on the co-combustion performance.Notably,TiandTfsignificantly decrease from 454.1 to 330.9 °C and 869.3 to 609.6 °C with the increased O2concentration.The main reason can be attributed to the large porosity and specific surface area of coal semi-coke to be beneficial to O2diffusion.Moreover, the higher O2concentration can oxidize the fuel at lower temperature.At the same time, the maximum mass loss rate is twice that when O2concentration increases from 10% to 30%.As for the other parameters,CCIandRwincrease from 0.74×10-7to 7.21×10-7and 1.56×103to 4.84×103,respectively, to indicate that the influence of O2concentration on comprehensive combustion performance and burning stability is remarkable.Compared with Table 2, an increased O2concentration can achieve even better combustion performance than pure sub-bituminous coal and offset the instability of co-combustion caused by low reactivity of coal semicoke.Also, the increased O2concentration can make up for the relatively negative effects brought by replacing N2with CO2.Based on the above analyses, an increased O2concentration coupled with oxy-fuel combustion is a favorable choice to achieve proper combustion performance and carbon capture at the same time.
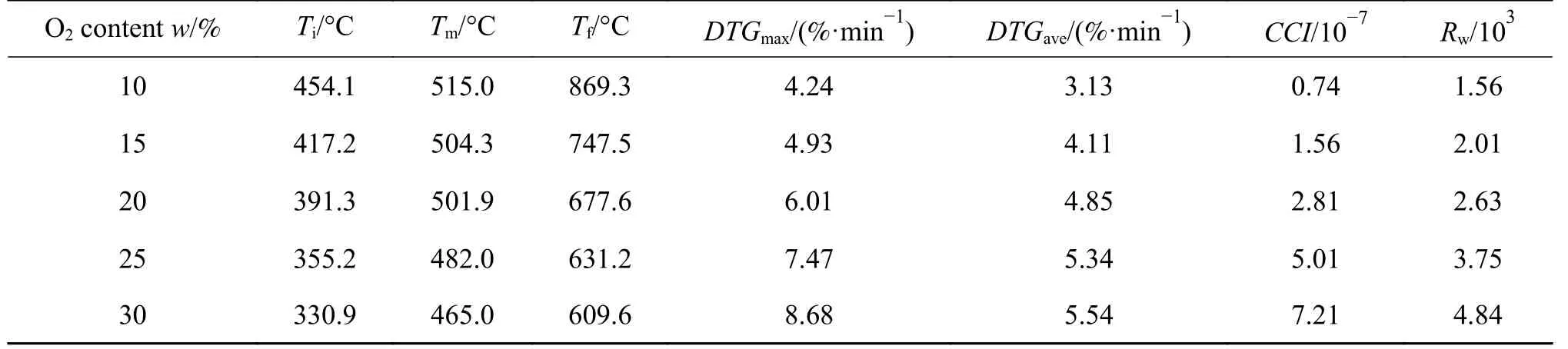
Table 3 Combustion parameters of 30% semi-coke blend in different O2 concentration
2.4 Kinetic analysis
Compared with the model-fitting methods, the isoconversional approaches, also named as the model free method, can calculate activation energy without assuming specific models for the reaction and allow the activation energy to be a function of the conversion degree[49].In this part, the pure sub-bituminous coal,pure semi-coke and the 30% semi-coke blend sample were chosen to evaluate the activation energy in the 30%O2concentration oxy-fuel atmosphere, where the temperature heating rates were set as 20, 25 and 30 K/min.And the iso-conversional methods of FWO,KAS and Starink were applied.Further, the activation energies derived from the Starink method were used to calculate the pre-exponential factork0.
The fitting lines to calculate the activation energy of semi-coke are shown in Figure 4 and theEaresults of the three samples are listed in Table 4.The variations of the slopes confirm thatEais highly correlated with the conversion degreeαthough the contours of the slopes do not show much variation with differentα.Take Starink results of sub-bituminous coal for an example,Eacan divide the combustion into two stages:αfrom 10% to 20% (stage 1) and 20% to 95%(stage 2).At stage 1,Eaincreases dramatically from 35.59 to 115.4 kJ/mol.At stage 2,Eagradually declines from 115.4 to 23.61 kJ/mol.Meanwhile, the regression lines derived from the KAS and Starink methods are similar to each other due to the similarity of the approximate equations used by the two methods.However, the slopes derived from the FWO method look larger than the other two methods.But according to the high value of the correlation coefficient (R), the fitting lines of the three methods all give satisfactory results.
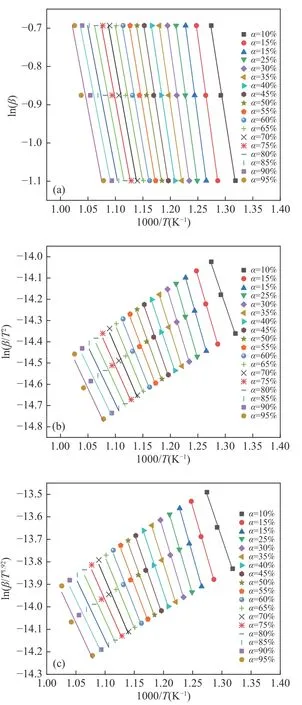
Figure 4 Regression lines of different iso-conversional methods for pure semi-coke activation energy calculation: (a)FWO, (b) KAS, (c) Starink
From Table 4, it is observed that the values ofEacalculated by KAS and Starink are close to each other but about 10 kJ/mol lower than FWO method.This phenomenon can be attributed to the high similarity of the two methods, as shown in Eq.(9) and Eq.(10).In fact, both the FWO and KAS methods include some assumptions and approximations.For instance, in FWO method the analytic solution for the temperature integral is not available, and the Doyle’s approximation is used.The iso-conversional methods require the use of different heating rates to calculate the activation energy for a specific mass conversion.While the heating rates at that mass conversion correspond to different temperatures.Hence, it is more practical to integrate temperature and heating rate, like ln(β/T2) in KAS method.Since the temperature termTis in the denominator, which can alleviate the variation of thelnterm caused by the change of the heating rate.Thus, the calculated slope is smaller than that of the FWO method and further the activation energy is also smaller.It is very noteworthy that sub-bituminous coal,which is easier to ignite (see Table 2), has an activation energy larger than coal semi-coke and their blend in the initial stage.At the mass conversion of 20%, the activation energy of sub-bituminous coal, the blend,and semi-coke are 115.40, 94.21 and 76.46 kJ/mol,respectively.The activation energy refers to the energy required for a molecule to transform from the normal state to the activated state, where the chemical reactions are prone to occur.From the results in Table 4, it is inferred that although sub-bituminous coal is easy to ignite (low ignition temperature), the molecular state of sub-bituminous coal is not so active at the beginning of combustion due to its physicalchemical properties.Thus, the energy performance of sub-bituminous coal at stage 1 is not better than that of semi-coke as expected.

Table 4 Activation energies (Ea) for the samples using different iso-conversional approaches
Meanwhile,Eaof the blend is between the two pure fuels to indicate the blending of semi-coke in this co-combustion stage improves the energy state of the blend.At stage 2,Eaof both sub-bituminous coal and semi-coke decrease while showing a slight increase within the range ofαfrom 80% to 95% as the 30%semi-coke blend sample.Similar phenomenon was found by Zheng et al.[2].The ash generated from subbituminous coal covers the micropores of the semicoke gradually, which results in the increase ofEaat this stage.Notably,Eaof sub-bituminous coal is lower than that of semi-coke and the value of the blend is also between the two pure fuels.As calculated by Starink,the averageEafor sub-bituminous coal, the blend sample and semi-coke are 49.31, 50.82 and 59 kJ/mol,respectively.Thus, sub-bituminous coal improves the combustion performance of semi-coke in a wide combustion range.Moreover, the pre-exponential factork0increases quickly at stage 1 and decreases sharply at stage 2, indicating that a higher rate of molecular collisions and reaction energy is needed in the beginning surface reaction process[40].The variation trends ofk0are similar withEa, which could be explained by the kinetic compensation effect[38].
2.5 Thermodynamic analysis
According to the discussion above, The Starink method is obtained by improving the experimental data on the basis of FWO and KAS methods and its calculation accuracy is higher.Thus, the activation energy derived from Strink method was selected for the calculation of thermodynamic parameters to evaluate the three thermodynamic parameters of enthalpy change ΔH, Gibbs free energy change ΔGand entropy change ΔS, as listed in Table 5.ΔHillustrates the energy difference between the reagent and the activated complex[49].As can be seen in eq.(12), ΔHhas a strong correlation withEa, so it displaysasimilar change tendency withαlikeEadoes.At stage 1 (α< 20%), the ΔHof the blend increases from 53.04 to 88.52 kJ/mol,while decreasing from 88.52 to 35.51 kJ/mol at stage 2(α> 20%).It indicates that more heat energies are required for the initial volatile matters to dissociate the bonds, which agrees with the characteristics ofEa.
The value of ΔGrepresents the total energy required by the system from the initial state to the activated state[50].As listed in Table 5, the ΔGrange of sub-bituminous coal, the blend and semi-coke is 191.19–199.91 kJ/mol, 202.92–208.97 kJ/mol and 256.64–260.56 kJ/mol, respectively.The ΔGfor cocombustion of sub-bituminous coal with semi-coke is close to pure sub-bituminous coal but much smaller than pure semi-coke.As stated in section 3.2, the 30%ratio of semi-coke cannot significantly reduce the combustion performance of the blending fuel.At the same time, the small increase in ΔGwill not have much negative effect on the stable operation of the boiler.
The value of ΔSis a state function of the reaction system, representing the disorder degree of the system.Negative value of ΔSstates that disorder degree of the combustion products is lower than reagents and bigger value of ΔSmeans higher reactivity of the sample[51].As shown in Table 5, the values of ΔSare all negative.At stage 1, the reactivity of the three samples all decline but rise up with increasingαat stage 2, which has a same trend asEachanges.Considering the whole process, the blend gains higher values of the apparent entropy change than semi-coke but lower than subbituminous coal to demonstrate the improved reactivity of the pure semi-coke through blending with subbituminous coal.

Table 5 Thermodynamic parameters of the samples
2.6 Interaction evaluation
Two interaction indices are applied to assess the probability of the interaction existence during the cocombustion process of the blend in this study.TheMRindex represents the difference between the actual value and the theoretical value.For the DTG value, a positiveMRindicates the actual mass loss rate of the blend is higher than expected, which means the existence of the positive interaction.But for kineticthermodynamic parameters, a negativeMRillustrates the actual energy required for combustion is smaller than expected, thus, a positive interaction occurs.The judgement methods forMRof DTG and kineticthermodynamic parameters are exactly opposite.Besides, theRMSindex characterizes the interaction degree.
As shown in Figure 5(a), the DTGMRfactor varies under different semi-coke blending ratios.As the ratio of semi-coke increases,MRdecreases monotonically.When the semi-coke proportion is 30%,MRis positive with a biggestRMSvalue to indicate strong positive interaction occurs during cocombustion.When the semi-coke ratio is more than 30%, theMRbecomes negative with relatively largeRMSvalues, revealing that notable negative interaction occurs and the semi-coke ratio should not increase any more.The possible reason is that the combustion of the sub-bituminous coal volatiles promotes the semi-coke ignition and char combustion, but the high ash content of coal semi-coke can cover up the particle surface and suppress the burning of sub-bituminous coal once the semi-coke ratio exceeds the threshold.In general, the semi-coke blending ratio should be no more than 30%to obtain a good combustion performance under current conditions in this study.
Figure 5(b) shows the kinetic-thermodynamic interaction behaviors of the blend of 30% semi-coke,which is another vital tool to determine interaction from the energy perspective.TheMRvalues of the four parameters are all negative, indicating that the subbituminous coal and semi-coke affects each other and achieve mutual promotion during the co-combustion.Moreover, the values ofRMSindicate that the blended fuel is easier to activate, which is consistent with the above activation energy analyses.In general, the subbituminous coal and semi-coke in this study show obvious interaction during the co-combustion process and similar discussions can be found in previous studies[52,53].While some other studies show weak interaction during the co-combustion of semi-coke and coals/biomass[19,54], where the variations in different research conclusions can be attributed to the differences in the structure and physical-chemical properties of the fuels used.

Figure 5 Interaction indices for: (a) DTG of the blends, (b)kinetic-thermodynamic parameters of the 30% semi-coke blend
2.7 Ash analysis
The ash compositions and four characteristic ash sintering and fusion temperatures are presented in Figure 6(a) and Figure 6(b), respectively.Besides the small amounts of alkali and alkali earth metals, the semi-coke ash is mainly composed of clay minerals with the content of SiO2and Al2O3more than 70%.Chen et al.[55]found that the SiO2in ash could form stable network and the Al2O3could support the skeleton structure of ash to increase the melting point.On the other hand, the contents of the alkali metals in the sub-bituminous coal ash are 43.3%, 6.3% and 10.6% for CaO, Na2O and MgO, respectively.Li et al.[56]studied the ash fusion characteristics during Zhundong sub-bituminous coal combustion and found that calcium in the compound could form eutectics with aluminum and iron that cause an easier fusion of subbituminous coal ash.

Figure 6 Ash analysis of semi-coke and sub-bituminous coal:(a) XRF, (b) ash fusion temperature
Moreover, Figure 6(b) shows four characteristic temperatures of sub-bituminous coal ash which are all lower than that of semi-coke ash.Due to the presence of alkali and alkaline earth metals, sub-bituminous coal ash deforms at about 1130 °C while semi-coke ash deforms only at 1350 °C.During the co-combustion process, SiO2in semi-coke may react with CaO in subbituminous coal to form the calcium compounds and increase the fusion temperature of the blends.The analyses suggest that blending sub-bituminous coal with semi-coke can relieve the risk of slagging and contribute to the cascade utilization of low-rank coals.
In addition to the influence on slagging, the mineral composition also interferes with the cocombustion process of coal and semi-coke.As shown in Figure 6(a), the combustion of sub-bituminous coal produced abundant calcium oxide and other metal oxides.These metal oxides are natural catalysts for coal combustion.For example, the Na+, K+, Ca2+, Mn2+and Fe3+can evidently reduce the ignition point of coal and promote the reactivity of coal[57].In the oxidation reaction, these metal cations enter the molecular structure of coal and semi-coke by ion exchange and further increase the reactivity of volatile substances.The Ca2+has high fluidity and chemical activity, and the formed CaO has better oxidation catalytic performance on coal combustion as well.Zhao et al.[58]also demonstrated that the alkali and alkaline-earth metal (AAEM) species as important parts of mineral matter could be catalytically active during the coal/char combustion.The Zhundong sub-bituminous coal obtains higher combustion activity due to the high content of AAEM.These minerals not only facilitate the combustion of the low-rank coal itself, but also improve the reactivity of the semi-coke.This phenomenon is consistent with the above discussion in section 2.2.
3 Conclusions
The co-combustion performance of Zhundong sub-bituminous coal and bituminous coal derived semicoke is systematically studied by kinetic and thermodynamic analyses.The main conclusions are:
Compared with air atmosphere, the oxy-fuel atmosphere achieved similar co-combustion behaviors but slightly increase the ignition and burnout temperature by about 10 and 40 °C.An increased O2concentration to 30% enhanced the co-combustion performances greatly.
The kinetic parameters were compared by different iso-conversional methods.The averageEacalculated by FWO method is about 10 kJ/mol higher than both KAS and Starink method.The averageEaof 30% semi-coke blend sample (50.82 kJ/mol) is close to sub-bituminous coal (49.31 kJ/mol) but much lower than coal semi-coke (59.00 kJ/mol).
Co-combustion with sub-bituminous coal improved the reactivity of semi-coke.In addition,obvious synergistic effected exists within the blending ratio of semi-coke less than 30%.
The blending of the semi-coke reduced the risk of slagging.This conclusion can be extended to the cocombustion application of coal semi-coke and other low-rank coals.

