Hierarchical and self-supporting honeycomb LaNi5 alloy on nickel foam for overall water splitting in alkaline media
Ynze Wu,Yln Liu,Kui Liu,Lin Wng,Lei Zhng,Dego Wng,Zhifng Chi,,Weiqun Shi,
a Engineering Laboratory of Advanced Energy Materials,Ningbo Institute of Materials Technology and Engineering,Chinese Academy of Sciences,Ningbo,315201,Zhejiang,China
b Laboratory of Nuclear Energy Chemistry and Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety,Institute of High Energy Physics,Chinese Academy of Sciences,Beijing,100049,China
c University of Chinese Academy of Sciences,Beijing,100049,China
Abstract Ni-based metallic foams possessing large specific surfaces and open cell structures are of specific interest as catalysts or catalyst carriers for electrolysis of water.Traditional fabrication of Nickel foam limits the element modification choices to several inert transition metals only on polymer foam precursor and subsequent preparation of foam-based catalysts in aqueous solution or organic electrolyte.To expand the modification horizon,molten salt with wide electrochemical window and fast ion diffusion can achieve the reduction of highly active elements.Herein,we reported is a general and facile method to deposit directly of highly reactive element La and prepare hierarchical honeycomb LaNi5 alloy on Ni foam(ho-LaNi5/NF).This self-supporting electrode presents excellent electrical coupling and conductivity between the Ni foam and LaNi5,which provides a 3D self-supported heterostructure with outstanding electrocatalytic activity and excellent durability for the hydrogen evolution reaction(HER)and oxygen evolution reaction(OER).It exhibits excellent overpotential(1.86 V)comparable to commercial coupled IrO2//Pt/C(1.85 V)at a high current density of 100 mA cm-2.This work may pave the way for fabricating novel 3D self-supported honeycomb alloy that can be applied as electrode for usage of clean energy.
Keywords: Honeycomb alloy;Hierarchical structures;Molten salt;Hydrogen evolution reaction;Oxygen evolution reaction
1.Introduction
As industrial society develops rapidly,resource shortages and environmental pollution are becoming major concerns for human society[1,2].It is of great importance to develop clean and renewable energy,such as solar energy,wind energy,nuclear energy,biomass energy,tidal energy,etc.[3].These new energies,however,lack high energy utilization,because they are either inefficient,intermittent,dangerous or regional.On the other hand,hydrogen energy is regarded as the most promising clean energy in the 21st century due to its highest calorific value and almost zero pollution [4-7].To obtain hydrogen efficiently,electrocatalytic water splitting stands out due to its high energy utilization rate and simple operation[8].Its major challenge is to reduce the overpotential during hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) processes.Commercial catalysts with ultra-low overpotential are usually noble metals,eg.Pt,Ir,and Ru,but they are expensive,scarce,and unstable [9,10].It is significantly important to develop non-precious metal catalysts with optimized performance.
Ni-based catalyst is one of the promising non-precious metal catalysts,which owns earth abundance,high stability,and excellent electrocatalytic performance,therefore it has great advantages in HER and OER [10-13].Moreover,Nibased metallic foams as catalysts and catalyst carriers are of specific interest due to their large specific surfaces and open cell structures [14-19].Their fabrication is usually conducted by electrodeposition and hydrothermal synthesis in aqueous solution or organic electrolyte [20,21],which limits the modification choices to only several transition metal elements(such as Fe,Co,Cu,Cr,Zn,Mo,etc.)in the low activity media and low operation temperatures.
Meanwhile,rare earth (RE) elements with unique properties of empty or unfilled vacant d orbitals have been applied in the field of catalysis.But their cations or species with high activity and low electronegativity are basically difficult to be reduced to crystalline metal or alloy in the above aqueous solution or organic electrolyte.In contrast,high temperature molten salt possessing large decomposition potential,high conductivity and heat capacity [22,23],is a superb media to confer privilege for electrochemical preparation of more active refractory metals and relative alloys,such as the lanthanide and actinides [24] etc.,so that they are expected to be combined with Ni-based metallic foams.
Herein,we report a facile and general method to directly deposit high reactive element La on Ni foam and prepare a special honeycomb LaNi5alloy (ho-LaNi5/NF) in high temperature molten salt.It has fully exposed active area of honeycomb submicron pores and the 3D self-supporting network structure,which promotes the rapid transfer of electrons and gas evolution at the three-phase interface in the HER and OER processes.
2.Experimental
2.1.Materials synthesis
Firstly,a piece of Ni foam (30 mm × 10 mm x 1.5 mm)and a piece of Ni plate (30 mm × 10 mm x 0.5 mm) were ultrasonicated with acetone,3 mol L-1HCl aqueous solution and deionized water for 5 min,respectively.Then,the Ni foam and Ni plate were quickly dried after rinsing with anhydrous alcohol.An electrochemical analyzer (Autolab PGSTAT302N,Metrohm) was employed to perform all electrochemical tests.The preparation of ho-LaNi5/NF was applied by potentiostatic electrolysis on a piece of Ni foam at-1.68 V for 2 h in LiCl-KCl-LaCl3(2 wt%)melts at 923 K.The experimental operations were carried out in a glove box in which the concentrations of oxygen and moisture were controlled to be less than 0.5 ppm.Graphite rod and Ag/AgCl fabricated by an alundum tube were used as the counter electrode and reference electrode,respectively.The preparation of LaNi5/NP was applied by potentiostatic electrolysis on a piece of Ni plate under the same conditions as ho-LaNi5/NP.Pt/C electrode was prepared by loading Pt/C ink(22 mg 20 wt% commercial Pt/C and 20 μL 5 wt% Nafion solution in mixture of 500 μL deionized water and 480 μL isopropanol)onto a piece of Ni foam (1 × 1 cm) and drying at room temperature ambience.IrO2electrode was prepared by the same way as Pt/C electrode.
2.2.Materials characterization
Scanning electron microscopy-Energy dispersive spectrometer (SEM-EDS,Thermo Scientific,Quanta 250) was employed to analyze the surface morphology and element composition of the np-LaNi5/NF.X-ray diffraction (XRD,Bruker,D8 Advance)was applied to identify the formation of LaNi5phase.X-ray photoelectron spectroscopy(XPS,Thermo Scientific,ESCALAB 250Xi)was used to detect the chemical composition of the ho-LaNi5/NF.
2.3.Electrochemical characterization
Electrochemical measurements were performed with a electrochemical analyzer (Autolab PGSTAT302N,Metrohm)in a standard three-electrode system in 1 mol L-1KOH electrolyte.Before the HER or OER measurement,the electrolyte was bubbled with Ar or O2flow for 30 min,respectively.A gas flow was maintained over the electrolyte during the measurement to ensure continuous gas saturation.A saturated Hg/HgO electrode was used as the reference electrode,a graphite rod and a platinum plate were here used as the counter electrode for HER and OER,respectively.All potentials measured were calibrated to RHE using the following Equation:E(RHE)=E(Hg/HgO)+0.0591×pH+0.098.An as-prepared ho-LaNi5/NF,an as-prepared LaNi5/NP,bare Ni foam,bare Ni plate,Pt/C/Ni foam and IrO2/Ni foam with geometric area of 1 × 1 cm2were used as the working electrode,respectively.Polarization curves for evaluation of HER and OER performance were recorded by linear sweep voltammetry (LSV) with a scan rate of 5 mV s-1.All the polarization curves presented in this work were corrected for ohmic losses.The electrochemical impedance spectroscopies(EIS) were carried out at the static potential of -136 mV vs.RHE (HER) and 271 mV vs.RHE (OER) from frequency range of 100 kHz to 0.01 Hz with an AC amplitude of 5 mV.The Electrochemical active surface area (ECSA) of each catalyst was estimated from their electrochemical capacitances using the cyclic voltammetry method at different scan rates(10,20,30,40,50,60,70,80,90 and 100 mV s-1) in the potential range from 0.7 V to 0.8 V vs.RHE.
3.Results and discussion
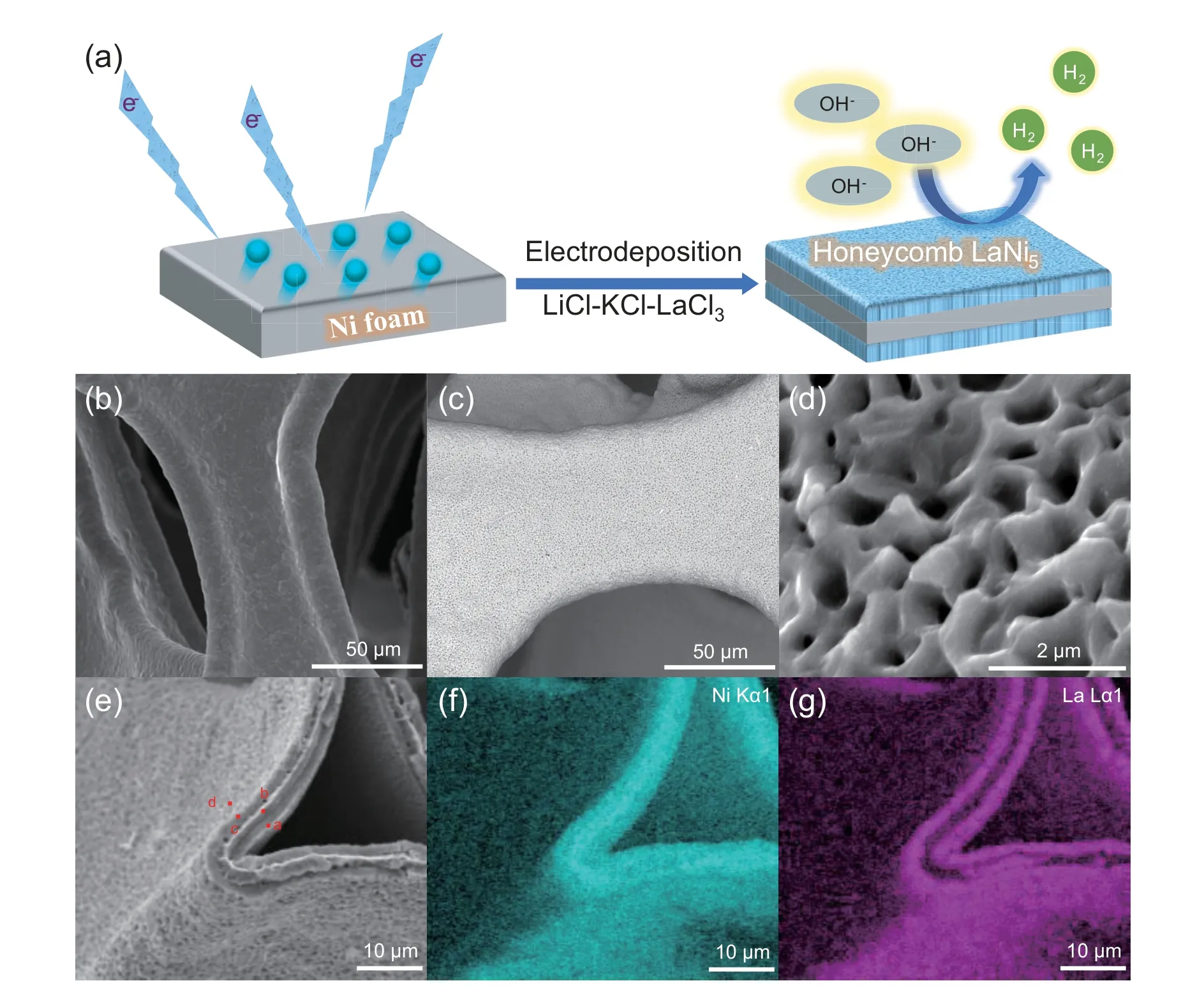
Fig.1.(a) Schematic illustration of the preparation procedure for ho-LaNi5/NF;(b) SEM images of the surface of the pristine Ni foam;(c,d) the surface of ho-LaNi5/NF;(e) the cross section of ho-LaNi5/NF;(f,g) EDS of the selected area on ho-LaNi5/NF.
A piece of commercial Ni foam was used as the cathode in a high temperature cell.Honeycomb LaNi5alloy was directly deposited on the surface of Ni foam in LiCl-KCl-LaCl3molten salt by potentiostatic electrolysis at -1.68 V (vs.Ag/AgCl) for 2 h,as shown in Fig.1a and S1.After the deposition,residual salt was removed by ultrasonic washing,and then the bright silvery Ni foam turned black alloy (Inset,Fig.S2).XRD characterization reveals distinctive diffraction peaks cor111responding to the crystalline planes of intermetallic compound LaNi5(Fig.S2).The mass loading of LaNi5on Ni foam is about 40 wt% determined by ICP-OES (0.80 mg La and 5.4 mg Ni in 6.3 mg ho-LaNi5/NF).The SEM image for the surface of the pristine Ni foam is presented in Fig.1b and S3a,the smooth and dense structure on the skeleton of Ni foam is clearly observed.After alloying with lanthanum into LaNi5,the initial compact surface on the skeleton of Ni foam with dense structure changed to rough honeycomb with porous structure (ho-LaNi5/NF) as shown in Fig.1c-d and S3b.The cross section of ho-LaNi5/NF shows an obvious three-layer structure as shown in Fig.1e-g.The EDS confirms the composition and distribution of alloy and metal Ni by the elemental weight ratio of a,b and c points (Table S1).The LaNi5alloy layer with submicron-holes or channels are uniformly located on the surface of the hollow skeleton,while the metal nickel is wrapped between two layers of alloy to form a stable self-supporting heterostructure with three layers.
As shown above,the honeycomb LaNi5alloy was obtained by electrolysis on a Ni foam electrode.At the same time,electrolysis on a smooth Ni plate electrode under the same condition was also conducted for comparison.Notably,porous honeycomb LaNi5alloy can only be deposited on the Ni foam electrode but not on the Ni plate electrode.Commercial Ni foam fabricated from polymer precursor compulsively has endured the reduction process of Ni2+in inorganic salt where the operation temperature must be lower than the melting point of Nickel to guarantee the formation of integrated cellular structure [25].Thus,commercial Ni foam is naturally heterogeneous and significantly porous [26],which is constructed by numerous reduced Ni particles with distinct grain boundaries,also resulting in worse mechanical strength than complete nickel sheet.Consequently,the following diffusion of La atoms into the Ni skeleton will induce a high porosity of LaNi5alloy layer after the deposition of La atoms on the Ni foam.However,Ni plate electrode is usually fabricated by well melted metal (Fig.S4a) and provides a homogenous phase allowing a stable diffusion and formation of welldefined compact LaNi5layer without porous honeycomb structure[27],as shown in Fig.S4b.The hierarchical structure of the active material with high porosity supported on metal substrate will provide large specific surface area,fast electrolyte/ion diffusion rate,and high stability [28-30].With the unique 3D and submicron-pores structure of ho-LaNi5/NF,it may promote the rapid transfer of electrons and gas evolution at the three-phase interface in HER.
The electrocatalytic activity of the ho-LaNi5/NF,along with those of LaNi5/NP (abbreviated from LaNi5/Ni plate),Ni foam,Ni plate and commercial Pt/C toward the HER was accessed in 1 mol L-1KOH using a standard three-electrode system.As expected,Pt/C delivers the lowest overpotential of 45 mV at -10 mA cm-2(Fig.2a).The 3D ho-LaNi5/NF displays an excellent activity with a required overpotential of 136 mV at -10 mA cm-2and 231 mV at -100 mA cm-2,which is close to the commercial Pt/C (218 mV at-100 mA cm-2) and surpasses it rapidly when the cathode potential is more negative than -235 mV.Meanwhile the LaNi5/Ni plate,Ni foam and Ni plate show poor activity with a required overpotential of 186 mV,199 mV,274 mV at-10 mA cm-2and 281 mV,408 mV,459 mV at-100 mA cm-2(Fig.2a),respectively.The unique honeycomb structure of LaNi5alloy exposes more reactive sites than bare Ni foam,resulting in more excellent overpotential in HER.The Faraday efficiency was measured by drainage method,which is agree well with the theoretical yields,suggesting more than 99%.The relevant experimental equipment and data are shown in the Fig.S7.In addition,Fig.2b presents the cor111responding Tafel plots of these cathodes to understand the kinetics of HER processes to reveal the involved possible rate-determining steps.The Tafel slope of fabricated ho-LaNi5/NF electrode was calculated to be 84 mV dec-1,which is much smaller than that of LaNi5/Ni plate (135 mV dec-1),Ni foam (183 mV dec-1)and Ni plate (130 mV dec-1).According to the Tafel slope of ho-LaNi5/NF,the HER occurred on it was controlled by a Volmer-Heyrovsky mechanism,which includes the fast discharge reaction (Volmer) and the dominant chemical desorption reaction (Heyrovsky).The results of overpotential and Tafel slope of ho-LaNi5/NF demonstrate that it possesses much more excellent electrocatalytic activities than those of previously reported alloy-based catalysts and NF-based catalysts (Fig.2c and Table S2),such as Ni0.9Fe0.1,Ni0.6Mo0.4,Fe0.8Mo0.2,Ni1Cu1,Ni94Y6,Fe1.0Co1.1Ni1.4,NiFeMoP,MXene/NF,NiCo2S4/NF,NiFe-LDH/NF,Ni3S2/NF,CeOx-LDH/NF,etc.To further investigate the outstanding HER activity of ho-LaNi5/NF,we carried out electrochemical impedance spectroscopy (EIS) analyses for these cathodes.The collected Nyquist plots at an overpotential of 136 mV in the frequency range of 105-0.01 Hz are shown in Fig.2d.Curve fitting of the Nyquist plots gives the charge-transfer resistances (Rct) correlated with the electrocatalytic kinetics.The ho-LaNi5/NF presents the smallest Rct value (3.97 Ω) compared with LaNi5/Ni plate (10.38 Ω),Ni foam (35.97 Ω) and Ni plate (111.12 Ω),indicating its fastest electron transfer rate and the best HER activity,which is consistent with the result obtained from polarization measurements.
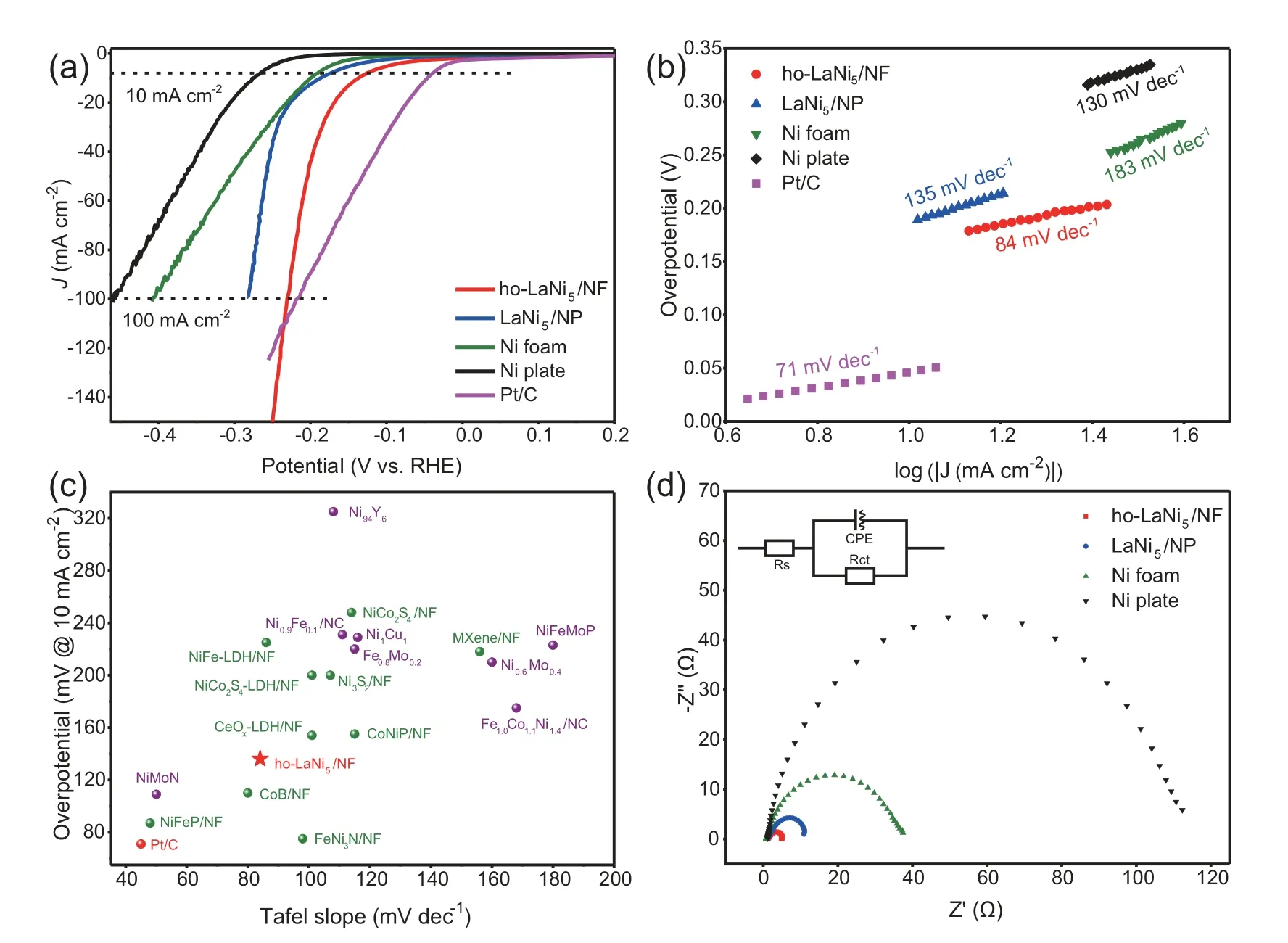
Fig.2.(a)HER polarization curves of ho-LaNi5/NF,LaNi5/NP,Ni foam,Ni plate and commercial Pt/C;(b)Tafel plots;(c)Comparison of overpotential and Tafel slope for various alloy or NF-based catalysts for HER;(d) EIS Nyquist plots.
The outstanding catalytic performance of the ho-LaNi5/NF electrode might be ascribed to the following three aspects.Firstly,according to the Brewer-Engel theory,whenever metals of the left half of the transition series(such as La)that have empty or unfilled vacant d orbitals are alloyed with metals of the right half of the transition series(such as Ni)that do not have internally paired d electrons available for bonding in the pure metal,there arises change in the electronic structure of the active element Ni by the electronic interaction with La [31,32].This change in the electronic structure of Ni caused by alloying with La results in a stronger water dissociation energy than the one without La [33-35] and an optimized adsorption property of H*in ho-LaNi5/NF,thereby promoting the HER.Secondly,the 3D self-supported porous structure of ho-LaNi5/NF possesses a large surface area,which extends the effective active surface and enhances the mass transport of hydrogen.Thirdly,the in-situ deposition of honeycomb LaNi5alloy on Ni foam provides perfect assembly between the alloy and the matrix,which not only enables good mechanical stability but also gives excellent electrical coupling to the Ni current collector,leading to the decrease of charge-transfer resistance Rct as disclosed by the EIS analysis[36,37].
Moreover,the OER activities of ho-LaNi5/NF,LaNi5/NP,Ni foam,Ni plate and commercial IrO2were accessed.The 3D ho-LaNi5/NF displays an excellent activity with a required overpotential of 271 mV at 10 mA cm-2and 347 mV at 100 mA cm-2,which is very close to the commercial IrO2(332 mV at 100 mA cm-2) and surpasses it rapidly when the anode potential is more positive than 1.59 V;while the LaNi5/Ni plate,Ni foam and Ni plate show poor activity with a required overpotential of 360 mV,324 mV,341 mV at 10 mA cm-2and 446 mV,414 mV,424 mV at 100 mA cm-2(Fig.3a),respectively.In addition,Fig.3b presents the cor111responding Tafel plots of these electrodes and the Tafel slope of fabricated ho-LaNi5/NF electrode was calculated to be 82 mV dec-1,which is much smaller than those of LaNi5/Ni plate (139 mV dec-1),Ni foam (87 mV dec-1) and Ni plate(109 mV dec-1).The results of overpotential and Tafel slope about ho-LaNi5/NF are much more favorable than that of previously reported alloy-based catalysts and NF-based catalysts (Fig.3c and Table S3),such as Ru3Ni,Co1Ni1,FeCoNi,MnFeCoNi,RuO2/NF,Co3O4/NF,MoCo/NF,CoB/NF,Ni12P5/NF,CoNiB/NF,NiMoCo/NF,etc.
As of note,ho-LaNi5/NF exhibits the best OER activity,whileLaNi5/NP exhibits the worst OER activity,since it has the maximum overpotential (360 mV at 10 mA cm-2) and Tafel slope(139 mV dec-1).From the EIS analyses in Fig.3d,the ho-LaNi5/NF also has the minimum Rct value (6.19 Ω)while the LaNi5/NP has the maximum Rct (124.25 Ω);it tells us the huge difference in charge transfer capacity between ho-LaNi5/NF and LaNi5/NP,indicating that the LaNi5alloy itself possesses the worst OER activity.Nevertheless,the as-synthesized ho-LaNi5/NF has numerous submicron holes and channels due to its unique honeycomb structure unlike LaNi5/NP,making it easier for Ni in the deeper base of the Ni foam than those in the surface to dissolve and oxidize to high oxidized Ni,hence an excellent OER performance.This was also confirmed by the most obvious oxidation peak of ho-LaNi5/NF near 1.4 V [38] in Fig.3a.
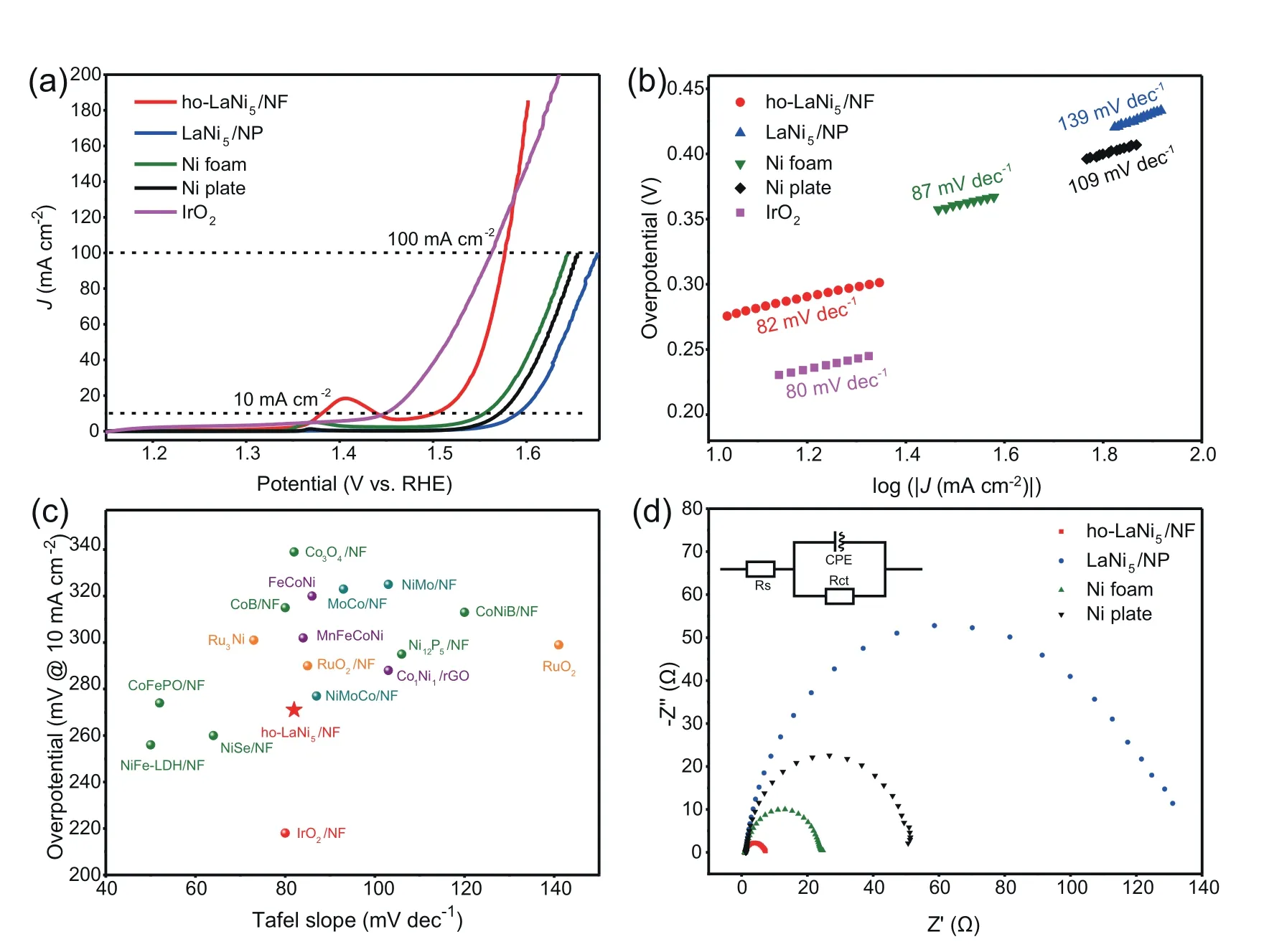
Fig.3.(a)OER polarization curves of ho-LaNi5/NF,LaNi5/NP,Ni foam,Ni plate and commercial Pt/C;(b)Tafel plots;(c)Comparison of overpotential and Tafel slope for various alloy or NF-based catalysts for OER;(d) EIS Nyquist plots.
XPS was conducted to further confirm the occurrence and chemical states of Ni3+in the ho-LaNi5/NF after OER.Since the surface change after a single OER by LSV was out of XPS detection,we selected ho-LaNi5/NF after 10 h of chronopotentiometry at 10 mA cm-2to conduct XPS research on the surface and the etching depth of 30 nm.As shown in Fig.4b,the Ni 2P3/2under the surface(854.8 eV)shifts towards the high-energy direction compared with the Ni 2P3/2on the surface(855.4 eV),and the same law is shown in Ni 2p1/2(from 872.6 eV under the surface to 873.1 eV on the surface),indicating that the Ni on the surface has a higher oxidation state(III) [39-42].In addition,at 851.6 eV [43],the surface Ni0is much less than that under the surface,which means that most of the surface Ni element is composed of Ni3+after OER.The La 3d5/2doublet splitting decreases from 3.8 eV(under surface)to 3.4 eV(on surface)as shown in Fig.4c,meaning the emergence of the La(OH)3and more complete hydroxylation on surface[44,45].Compared with O2-at 528.9 eV (from the lattice oxygen of La2O3) under the surface in O 1s spectrum [46],O2-(528.9 eV) on the surface is greatly reduced,and OH-at 532.3 eV appears [47,48],indicating that La2O3on the surface was transformed into LaOH3,which is consistent with the La 3d spectrum.The O2-at 530.8 eV[41,42]is attributed to lattice oxygen of NiO,and it is known from Fig.4b-d that Ni3+and OH-appear on the surface.Therefore,we can speculate that the existence form of Ni on the surface is NiOOH,which is an excellent OER catalyst after self-reconstruction in the alkaline electrolyte [48,49].Due to the numerous submicron channels on the surface of the honeycomb alloy,a large amount of Ni element was dissolved out in situ and reconstructed as NiOOH on the surface.The schematic diagram of active sites and detailed summary about the active sites evolution are given in section S8 of the Supporting Information.
The steady-state polarization curves of ho-LaNi5/NF show excellent activity close to that of commercial catalysts at±100 mA cm-2for HER and OER,as shown in Fig.5a.Encouraged by both excellent HER and OER activities,the asprepared ho-LaNi5/NF and other comparative samples were employed as both the cathode and anode for overall water spitting in a two-electrode system.As shown in Fig.5b,an extremely low cell voltage of ho-LaNi5/NF as a bifunctional catalyst for overall water splitting is 1.68 V at 10 mA cm-2,which surpasses many reported electrocatalysts (Table S4).Furthermore,ho-LaNi5/NF required a cell voltage of 1.86 V to deliver a current density of 100 mA cm-2,which is compared favorably with the cell voltage of 1.85 V from the coupled noble metal catalysts.To better understand the intrinsic activity of ho-LaNi5/NF catalyst,the electrochemically active surface area(ECSA) was determined through the measurements of electrochemical double-layer capacitance (Cdl) by cyclic voltammetry method as shown in Fig.5c(Fig.S5).The ho-LaNi5/NF has a much larger Cdl value of 16.2 mF cm-2than that of LaNi5/NP (3.2 mF cm-2),Ni foam (1.2 mF cm-2) and Ni plate(0.02 mF cm-2).The significantly enhanced ECSA indicates the catalyst possesses more active sites,it may explain a better HER/OER catalytic activity.Besides the catalytic performance,the long-term operation should be also considered in practice application.The stability of the ho-LaNi5/NF catalyst was assessed by chronopotentiometry at fixed cathodic current density of-10 mA cm-2and-100 mA cm-2in HER and fixed anodic current density of 10 mA cm-2and 100 mA cm-2in OER(Fig.5d),both of which display remarkable stability with negligible degradation.After long-term stability tests,the ho-LaNi5/NF still maintains a stable sandwich honeycomb alloy without the alloy layer falling off,no matter after HER(Fig.S6a)or OER(Fig.S6b).The composition of ho-LaNi5/NF after stability tests of HER and OER remails the same as before,and there is no phase change,as shown in XRD of Fig.S6c.All of the above confirm the super stability of ho-LaNi5/NF,which might be attributed to the strong coupling between the alloy and the substrate during the in-situ growth process.
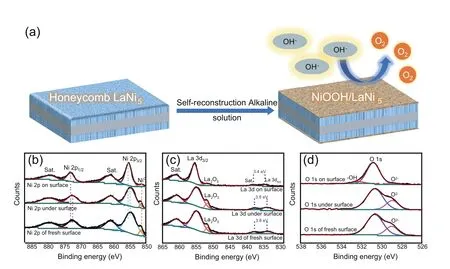
Fig.4.(a) Schematic illustration of the self-reconstruction procedure for ho-LaNi5/NF;(b,c,d) Ni 2p,La 3d and O 1s XPS spectra under and on surface of ho-LaNi5/NF.
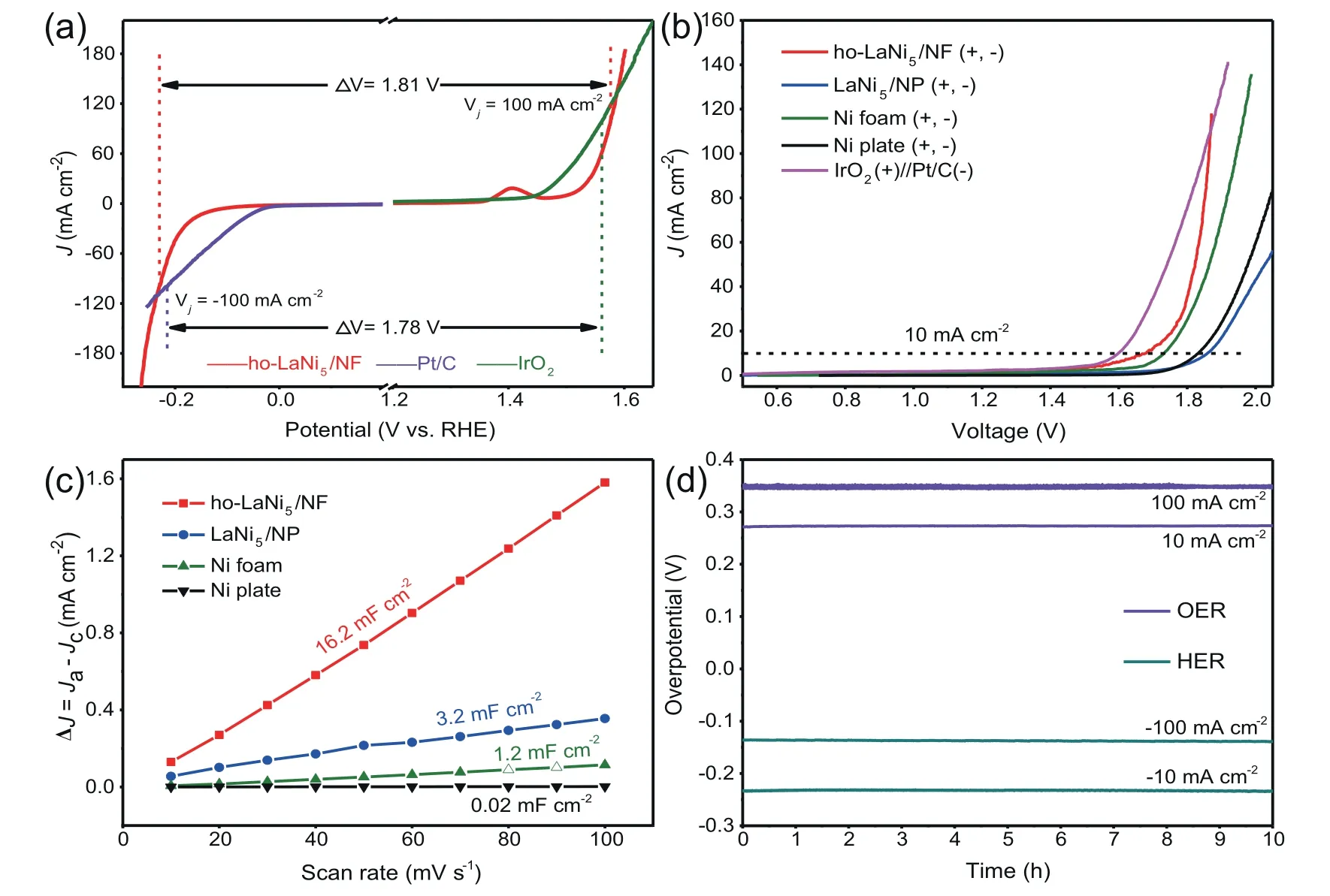
Fig.5.(a)Steady-state polarization curves of ho-LaNi5/NF and commercial catalysts for comparison under HER and OER;(b)The polarization curves of a twoelectrode electrolyzer with ho-LaNi5/NF as both the anode and cathode at a scan rate of 5 mV s-1.As comparison,the performance of electrolyzers with LaNi5/NP(+,-),bare Ni foam(+,-),bare Ni plate(+,-)and IrO2(+)//Pt/C(-)is also tested under identical conditions;(c)Liner fitting of the capacitive currents versus CV scan rates;(d) Chronopotentiometric curves of ho-LaNi5/NF at fixed current density of ±10 mA cm-2 and ±100 mA cm-2 in OER and HER.
4.Conclusions
In conclusion,a general and convenient approach was successfully developed to prepare a typical 3D self-supported ho-LaNi5/NF electrode for overall water splitting by direct electrodeposition on Ni foam in high temperature LiCl-KCl molten salt.The honeycomb LaNi5alloy on Ni foam can be directly utilized as efficient cathode for the HER and anode for OER with excellent electrocatalytic activity and durability in alkaline media,surpassing most analogous alloy materials and NF-based materialsintheirpropertiesforthewatersplitting.Inaddition,our work provides a new concept to prepare novel versatile alloy foam with more flexible choice in elementary composition for broad applications in new energy and new materials.Moreover,as the method developed in this work can be readily scaled up,it maypavethewayforfabricatingmoreefficientfoammetal-based materials for large-scale commercial hydrogen production.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
All authors commented on the final manuscript.This work was supported by the National Science Fund for Distinguished Young Scholars(No.21925603).We also thank for support of the Major Program of the National Natural Science Foundation of China (No.21790373).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gee.2021.09.005.
 Green Energy & Environment2022年4期
Green Energy & Environment2022年4期
- Green Energy & Environment的其它文章
- GUIDE FOR AUTHORS
- Increasing the greenness of an organic acid through deep eutectic solvation and further polymerisation
- Ni2P/MoS2 interfacial structures loading on N-doped carbon matrix for highly efficient hydrogen evolution
- One-pot green mass production of hierarchically porous carbon via a recyclable salt-templating strategy
- The feasibility study of the indium oxide supported silver catalyst for selective hydrogenation of CO2 to methanol
- Enhanced CO2 electroreduction to ethylene via strong metal-support interaction
