Improved methanol synthesis performance of Cu/ZnO/Al2O3 catalyst by controlling its precursor structure
Fn Zhng,Xioying Xu,Zhengpu Qiu,Bo Feng,Yun Liu,Aihu Xing,Mohong Fn
a National Institute of Clean-and-Low-Carbon Energy,Beijing,102211,China
b Departments of Chemical and Petroleum Engineering,University of Wyoming,Laramie,WY,82071,USA
Abstract Methanol,a versatile chemical,fuel additive and potential H2 carrier,has attracted great attention.Despite of the wide industrialization,improvement of Cu-based methanol-synthesis catalysts is highly anticipated.Accordingly,a series of Cu/ZnO/Al2O3 with designed precursor structures were prepared,and its structure-function relationship was investigated to make progress on this area.Results showed the catalyst derived from highly zinc-substituted malachite demonstrated the best catalytic performance in this work.It was found that the well-behaved catalyst possessed relatively high Cu specific surface area and exposed Cu concentration,and the well Cu/ZnO synergy.CuZn alloy was found by In-situ XRD tests,and its effect on the catalyst's thermostability was discussed.Fractional precipitation,which facilitated the Cu2+ substitution by Zn2+ in malachite lattice,could be an efficient preparation method of the Cu/ZnO/Al2O3 catalyst.
Keywords: Zinc malachite;Cu2+ substitution;Hydrotalcite-like compounds;Fractional precipitation;In-situ XRD
1.Introduction
Methanol,a versatile chemical and potential carrier for clean fuels,has attracted increasing attention in the world[1,2].Industrial methanol is typically produced by hydrogenation of carbon oxides over Cu/ZnO/Al2O3catalysts at the reaction conditions of 200-320°C and 5-10 MPa [3,4].Precipitation is considered as an effective method to prepare the Cu/ZnO/Al2O3catalyst,and was widely studied by researchers[5,6].With carbonates used as precipitant,the Cu-containing precursors comprised a phase mixture of malachite(Cu2(OH)2CO3),zinc malachite ((Cu,Zn)2(OH)2CO3),and hydrotalcite-like compounds (Htlc) with the general formula of ((Cu,Zn)1-xAlx)(OH)2(CO3)x/2·mH2O [6-8].The Cu-containing precursor structure has great influence in the structure and performance of the final catalyst [7-9].Zinc malachite and Htlc used as the catalyst precursors are widely discussed in literatures [10-12].
Zinc malachite was derived from malachite with partial Cu2+ions substituted by Zn2+in its lattice during precipitation [5,13].The substitution reduces the average Jahn-Teller distortions of the MO6octahedra in the crystal structure,leading to a detectable shift of 20-1 and 21-1 reflections to low d-spacings by X-ray diffraction [14].It increases Cu dispersion and promotes its interaction with Zn during reaction.Thus,some researchers are focused on the optimization of coprecipitation process,in order to promote the Zn incorporation into malachite phase.Baltes et al.systematically investigated the co-precipitation process of Cu/ZnO/Al2O3catalysts,and suggested that catalysts derived from zinc malachite precipitated in the pH range of 6-8 at 70°C demonstrates the best catalytic performance[9].Behrens et al.reported that the best catalysts can be obtained by constant pH co-precipitation with Na2CO3solution at pH 6 or 7 and at elevated temperatures around 60-70°C [15].However,when scaling up the catalyst production in an industrial unit,the mentioned low pH is liable to the release of CO2because of the hysteretic control,by the following equilibrium,
The general structure of Htlc composes of brucite-like(OH)2-layers and charge compensating interlayer anions [17,18].The contained metal ions share the octahedrally coordinated sites in the layers,and dispersed at an atomic level [19,20].Htlc-derived Metallic oxides are supposed to possess high dispersion of the metal species,enhanced metal-oxide interaction,and high stability against sintering [18,19,21].Cu-based Htlc for methanol synthesis from CO2have been investigated and the catalysts derived from Htlc performed substantial stability and good catalytic performance[22-24].However,the unfavorable effect of Htlc precursors on the methanol synthesis catalyst was also reported[18,19].Kuhl et al.[25]found that the Htlc-derived Cu particles are highly embedded in the amorphous Htlc structure,leading to the low Cu surface area and absolute activity of the catalyst.
It is reported that fractional precipitation was used to prepare the methanol synthesis catalysts,by which Al3+is precipitated independently of Cu2+and Zn2+[12,26].With this method,the formation of Htlc in precursors can be controlled by adjusting the co-precipitation contents of Al3+with Cu2+and Zn2+.Pure phase Cu/Zn/Al Htlc were regarded to be synthesized by co-precipitation method when Al content was raised to ≥30 mol% [18,19].It can be speculated that Cu0.47Zn0.23Al0.3catalysts can be obtained from precursors including zinc malachite,Htlc,or the mixture of zinc malachite and Htlc by using different precipitation methods.
In the present work,Cu0.47Zn0.23Al0.3catalysts originated from the above-mentioned three different kinds of precursor components were designed by controlling the precipitation methods.The efficient precursors and their structure-function relationship for Cu-based methanol-synthesis catalyst were examined according to the catalyst characterization and evaluation results.
2.Experimental
2.1.Catalyst preparations
The element composition and preparation methods of the catalyst samples were listed in Table 1.The preparation methods were interpreted in detail elsewhere [27].Generally speaking,Cu(NO3)2·3H2O,Zn(NO3)2·6H2O,and Al(NO3)3·9H2O were used as metal sources,and were dissolved in demineralized water with 1 mol/l metal ion.Then the metal ion solution was precipitated in a balloon flask at 80°C at a constant flow rate with 1 mol/l Na2CO3solution.The pH of the suspension was maintained at 7.0 and 8.5 by controlling the flow rate of Na2CO3solution.
As shown in Table 1,for sample HT,the metal ion mixture with mole ratio of Cu2+:Zn2+:Al3+as 46.7:23.3:30 were co-precipitated with 1 mol/l.It was presumed that the obtained Cu-Zn precursor would be pure phase Htlc.For sample ZM-1,all of Al3+was precipitated within step I in a flask and the sediment was aged for 30 min,after that the solution containing Cu2+and Zn2+were precipitated within step II in the same flask.The obtained Cu-Zn precursor was regarded as zinc malachite.For sample ZM-2,part of Al3+was precipitated within step I in a flask and aged for 30 min,and then the metal ion mixture with mole ratio of Cu2+: Zn2+: Al3+as 6 : 3 : 1 was precipitated within step II in the same flask.The Cu-Zn precursors should be the mixture of Htlc and zinc malachite.

Table 1 The atomic ratio and preparation methods of the catalyst samples.
The obtained precipitates were aged for 2 h,and then were washed with demineralized water to get rid of Na+.Subsequently,the precipitates were dried at 110°C for 12 h,and the precursor samples were obtained.Finally,they were calcined at 350°C in a muffle furnace for 4 h (2°C min-1),and the calcined catalyst samples were obtained.Additionally,several zinc malachite samples with different zinc content were prepared by using the described co-precipitation method for comparison.
2.2.Catalyst characterization
Several characterization methods used in this work were described in detail elsewhere [28].Generally speaking,X-ray diffraction (XRD) analyses were performed on precursor samples and calcined samples in a Bruker D8 Advance X-ray diffraction system using the Cu Kαline (1.5406 Å),with 2θ ranging from 10°to 80°.In situ XRD analyses were performed by a Rigaku X-ray diffractometer in 66.7%H2-N2atmosphere(30 mL min-1)with temperature rising from 110°C to 640°C(2°C min-1),with 2θ ranging from 10°to 80°with 0.020°steps.Element composition of the calcined samples were measured by X-ray fluorescence spectroscopy (XRF),using a Rigaku ZSX PrimusIIX-Ray spectrometer.X-ray photoelectron spectroscopy(XPS)was carried out on Thermo Scientific K-alpha spectrometer system.The obtained binding energies were calibrated by using the C 1s peak at 284.6 eV.
Thermogravimetric mass spectrometry analyses (TG-MS)were conducted on the powered samples by using a Netzsch STA 449F3 Jupiter thermogravimetric differential thermal analyzer with a MS403C Aeolos mass spectrometer.The precursor samples (~50 mg) were treated from 110°C to 750°C at 10°C min-1in synthetic air.Specific surface area and pore volume of the samples were measured by Micromeritics Tristar 3000 equipment.Sample degassing was carried out at 300°C for 4 h prior to adsorption.H2temperatureprogrammed reduction(TPR)experiments were conducted by Micromeritics Autochem II 2920 auto adsorption apparatus.100 mg of sample was firstly degassed and reduced in 10 vol%H2/Ar with a flow rate of 50 mL min-1.The temperature was ramped linearly from 50°C to 300°C at 2°C min-1,and then ramped from 300°C to 900°C at 10°C min-1[29].The H2consumption was detected by a thermal conductivity detector(TCD) during the run.Scanning electron microscope (SEM)images of calcined samples were recorded by JSM-5900LV type series instrument with a Schottky field emission gun.Transmission electron microscopy (TEM) images were taken with JEOL 2011 Electron Microscope operating at 200 kV with simultaneous energy dispersive X-ray spectroscopy(EDS).
The dispersion of Cu(DCu)and the exposed Cu surface area(SCu)were obtained by N2O adsorption,and the measurement was described in detail elsewhere [28].Generally,the amount of H2consumption in the first reduction step was denoted as X.After exposure in 10%N2O/Ar atmosphere,the sample was reduced again,and the consumed H2amount was denoted as Y.Dcuand SCuwere determined by the following equations:
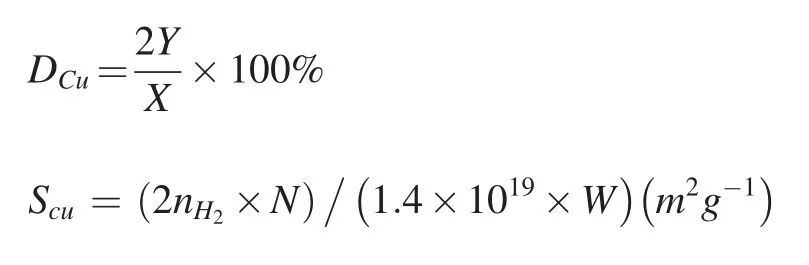
here nH2is the molar number of H2consumed in second reduction;N is Avogadro's constant (6.02 × 1023atoms mol-1);1.4 × 1019is the number of copper atoms per square meter;and W is the weight of reduced catalyst [28].
2.3.Catalyst evaluation
The evaluation experiment was interpreted in detail elsewhere [27].In general,a catalyst sample (100 mg,40-60 mesh)diluted with quartz sand was placed in a fix-bed reactor and was reduced in 5 vol%H2/N2at 230°C under atmospheric pressure.The initial activity was measured under the conditions of 230°C,5 MPa,and GHSV=10,000 h-1with feed gas composed of 80% H2,13% CO,2% CO2and 5% N2(Air Liquide).After that,the catalyst sample was treated at 320°C for 24 h,and finally was tested at 230°C for another 24 h.The gas products were quantitatively analyzed with an online gas chromatograph.The CO conversion of each test was calculated by an internal normalization method.
3.Results and interpretation
3.1.Physiochemical properties of the catalyst samples
For understanding the physiochemical properties,the precursor and calcined samples,including HT,ZM-1 and ZM-2,were characterized by XRD,TG-MS,XRF,BET,N2O chemisorption,XPS,and TPR,with their results demonstrated as follows.
As shown in Fig.1a,the pattern of HT exhibits strong peaks for Htlc.Additionally,some small peaks for zinc malachite were also observed,which is different from Behrens’ results[18].The possible reason may be the use of different precipitants [30].Prepared by fractional precipitation,only the peaks belonging to zinc malachite were found in the pattern of ZM-1.The pattern of ZM-2 shows obvious peaks for both zinc malachite and Htlc,as part of Al3+co-precipitating with Cu2+and Zn2+in the second step.The difference is that the diffraction peaks for Htlc are much smaller than them in the pattern of HT.
To distinguish the formed zinc malachite in different precursor samples,the patterns from 23.5°to 34°were scaled up and exhibited in Fig.1b.The XRD result of the malachite sample was also listed for comparison.As aforementioned,zinc malachite can be obtained from substituting part of the Cu2+ions by Zn2+in malachite lattice,which led to a shift of 20-1 and 21-1 reflections to higher angles [12,31,32].As shown in Fig.1b,compared with 20-1 and 21-1 reflections of malachite,the counterparts of HT,ZM-1 and ZM-2 shifted to higher angles to different degrees.It can be speculated that the Zn content in zinc malachite should increase as follows:HT <ZM-2 <ZM-1.Zinc malachite is responsible for the well dispersion of Cu and ZnO,which facilitates their synergistic action during reaction [33].

Fig.1.XRD patterns of the precursor (a,b) and calcined samples (c,d).
Fig.1c and d show the XRD patterns of the calcined and reduced samples.Distinct diffraction peaks for CuO were observed in all the XRD results of calcined samples.After reduction,strong peaks for Cu and small peaks for ZnO were exhibited in the patterns.It is interesting that the CuO and Cu peaks of HT performed sharp tip while broad base.The possible reason is that CuO or Cu in HT was derived from two disparate precursors,zinc malachite and Htlc.CuO or Cu from zinc malachite with low zinc content was relatively well crystallized and contributed the sharp tip of the peaks,while the other from Htlc was well dispersed and caused the wide base.CuO grains derived from Htlc was supposed to be relatively small and distributed homogenously in the structure[18,25].As shown in Fig.1d,ZnO can be hardly observed in sample HT.The reason was ascribed to the Htlc structure,which was beneficial to the dispersion of metal oxides [19].
To further examine the precursor composition,pyrolysis process of the precursor samples was investigated by TG experiment,with the evolved H2O and CO2detected by mass spectrometry.As shown in Fig.2b,the DTG peaks appeared at different temperatures,with the release of H2O and/or CO2.It is reported that for Htlc decomposition,weight loss at~140°C was caused by the escape of physically adsorbed and interlayer water molecules,while the peak at~600°C were attributed to the decomposition of high-temperature carbonates (HT-CO3) [24,25].As for zinc malachite,the pyrolysis process is partially depended on its zinc content [34].With low zinc content,zinc malachite was likely to decompose in one step,as the result of malachite shown in Fig.2a.As Zn/Cu mole ratio increased,zinc malachite tended to decompose in two steps [34,35],:

Fig.2.The TG and DTG-MS profiles of the precursor samples (10°C min-1,in synthetic air).
Accordingly,as shown in Fig.2b,the DTG peaks of HT at~150°C and~620°C was ascribed to Htlc;the peaks at~250°C and~510°C belonged to zinc malachite with relatively high zinc content;the peaks of ZM-2 at~140°C and~590°C was also resulted from Htlc;and the peaks at 360°C was attributed to zinc malachite with low zinc content,which decomposed in one step.The Htlc peaks of ZM-2 shifted to low temperatures compared with their counterparts of HT,which may be caused by its low crystallinity.
Elemental analysis results and N2physisorption results of the calcined samples were listed in Table 2.The element composition of each sample determined by XRF was generally in agreement with the experiment design,suggesting a full precipitation of the metal ions.Compared with HT mainly derived from Htlc,ZM-1 possessed much higher specific surface areas,pore volumes and average pore sizes.The formation of Htlc in precursor could be adverse to gas diffusion during the catalytic reaction.The Cu dispersion results of ZM-1and ZM-2 were also listed in Table 2.Originated from zinc malachite with more zinc content,ZM-1 demonstrated relatively higher Cu specific surface area.It indicated that the Cu substitution by Zn2+in the malachite lattice could promote the Cu specific surface area,which usually had a positive relationship with the catalyst activity [16].
Surface composition of the calcined samples was analyzed by XPS.As shown in Table 3,the surface Cu concentration of HT was distinctly less than its counterparts of ZM-1 and ZM-2.The reason is that the metal oxides derived from Htlc contains relatively high concentration of Al3+while low concentration of Cu2+,compared with zinc malachite.As the active element,low Cu concentration on the catalyst surface would inhibit the active sites formed by Cu from absorbing the reactive gas during reaction.
Reduction behavior of the calcined samples was investigated by H2-TPR measurements,with results shown in Fig.3.Several sharp peaks appearing before 300°C was ascribed to CuO reduction.A broad peak from~320°C to~700°C was attributed to ZnO reduction in this work,which triggered by the hydrogen spillover from Cu particles [36].Only the ZnO adjacent to Cu particles could be reduced,thus its reduction proceeded slowly,as the Cu particle size increased in a gradual way.The reduction of a small part of ZnO benefited to the migration of Zn species to the Cu surface,which was advantageous to the strong metal support interaction between Cu and ZnOxand the catalyst activity[37].To further examine the reduction behavior,the profiles of CuO reduction were magnified,and shown in Fig.3b.It is reported that zinc malachite could offer well-dispersed CuO after calcination,which can be reduced at relatively low temperatures [12,38];while CuO coming from malachite would have higher reduction temperature[38,39].On the other hand,Htlc-derived CuO was supposed to be embedded in an amorphous oxide matrix,leading to relatively higher reduction temperatures[18,25].To distinguish CuO from different precursors,the TPR profiles of HT,ZM-1 and ZM-2 were dismantled into several Gaussiantype peaks.The obtained peak positions and concentrations were presented in Table 4.Peak α was assigned to the reduction of well-dispersed CuO from zinc malachite;peak β was related to the reduction of bulk-like CuO from zinc malachite with less zinc,or Htlc-derived CuO.For the profile of HT,peak γ and λ were ascribed to the reduction of CuO from Htlc [25].Through avoiding co-precipitating Cu2+and Zn2+with Al3+by fractional precipitation,ZM-1 possessed the largest amount of well-dispersed CuO from zinc malachite in this work.
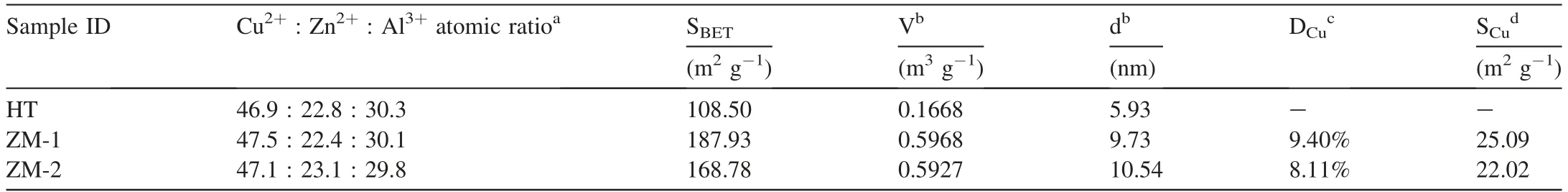
Table 2 Physicochemical properties of the calcined samples.

Table 3 XPS parameters of the calcined catalyst samples.
3.2.SEM/TEM results of the calcined samples
SEM images of the calcined samples are demonstrated in Fig.4.Mainly platelets around 200 nm were found in the image of HT,which was cor111responding to the typical plateletlike morphology of Htlc [19,40].ZM-1 and ZM-2 comprised mainly a large amount of small fiber structures,along with a few irregular agglomerates.The fiber-like morphology was ascribed to the formation of zinc malachite [9,12,31].The agglomerates may be originated from the aggregation of aluminum oxide.The catalyst samples remain their precursor morphology because of the so-called chemical memory [31].The SEM results exhibited the typical morphologies of zinc malachite and Htlc,which were in agreement with the abovementioned XRD and DTG results.

Fig.3.TPR profiles of the calcined catalyst samples (2°C min-1 from room temperature to 300°C;10°C min-1 from 300°C to 900°C).
To further understand those morphologies,TEM investigations of the samples were performed on HT and ZM-1,with element analyses listed in Table 5.As shown in Fig.5a,two different types of microstructures were captured in sample HT,cor111responding to the Htlc-derived metal oxides (Area 1)and malachite-derived metal oxides (Area 2),respectively[9,18].The element composition of Htlc-derived phase (Area 1) shown in Table 5 were generally in agreement with the surface element results of HT determined by XPS.The low Cu/Zn ratio suggests that the formation of Htlc has occupied excessive Zn2+during precipitation.Accordingly,the malachite-derived phase showed higher Cu/Zn ratio,which implied low Cu2+substitution by Zn2+in the co-produced malachite lattice.
Fig.5b shows the high magnification image of Htlc-derived phase.Its surface was covered with some pores,which was caused by the elimination of H2O and CO2during the calcination.The pore structure could not be observed in Area 1 of Fig.5(a),which may be attributed to its low magnification.The marked area in Fig.5b was further amplified and was shown in Fig.5c.It seems that the CuO particles were in intimate contact with the surrounding Zn-Al oxides and sealed in the matrix [19,25].This may be the reason that HT performed low specific surface area and low surface Cu concentration.
Fig.6 shows the TEM image of the fiber-like morphology in ZM-1,which is supposed to be derived from zin malachite.It is interesting to find that the fibers were actually composed of many small grains.The element analyses suggest that the fiber phase (area 3) mainly comprised of Cu and Zn with an atomic Cu/Zn ratio close to 4:1.During the HT preparation,a high proportion of Zn2+was consumed in the formation of Htlc,which had an atomic Cu/Zn ratio of approximately 1.4 :1,as shown in Table 5.A small part of Zn2+remained and participated in the formation of zinc malachite.On the other hand,the formation of Htlc was controlled by fractional precipitation during the preparation of ZM-1.Thus the zinc malachite of ZM-1 has higher Cu substitution by Zn2+than its counterpart in HT.
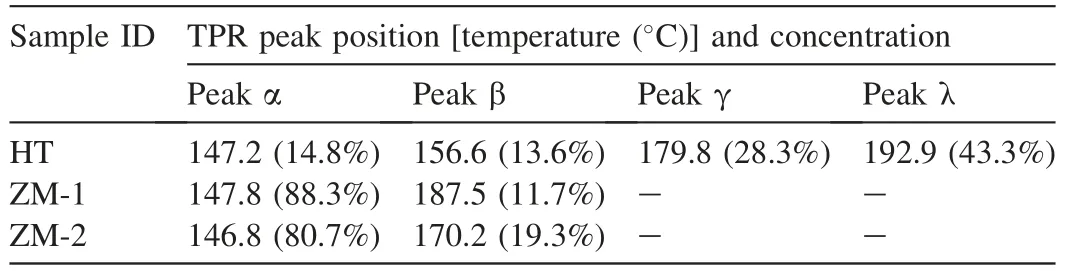
Table 4 Deconvolution results of the TPR profiles over calcined catalyst samples.
To further examine these grains,a small part of the fibers(area 3),was amplified to higher magnification (scale bar of 5 nm),as shown in Fig.6b.The micrographs demonstrated that the grains were mainly nano-structured CuO grains ranging from 4 to 6 nm,with well-dispersed Zn species adjacent to them.The small CuO grains besieged with Zn species are an ideal combination mode.The intimate contact of ZnO and CuO benefits to the synergy of Cu and ZnO at the interface during reaction [33,41].It is also suggested that the existence of ZnO could prevent the growth of Cu grains [42].
3.3.Catalytic performance
In methanol synthesis,the main reactions are the CO hydrogenation and CO2hydrogenation,as shown below:
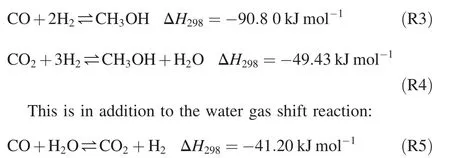
Firstly,the catalyst samples were evaluated at 230°C for 24 h(Stage I).Then they were thermally treated at 320°C for 24 h.After that the temperature dropped to 230°C and lasted for another 24 h(Stage II).The evaluation results of HT,ZM-1 and ZM-2 are listed in Table 6.As the similar methanol selectivity,CO conversion was considered as an important catalytic activity indicator of the catalyst.Fig.7 shows the CO conversion results of the catalyst samples as a function of evaluation time.
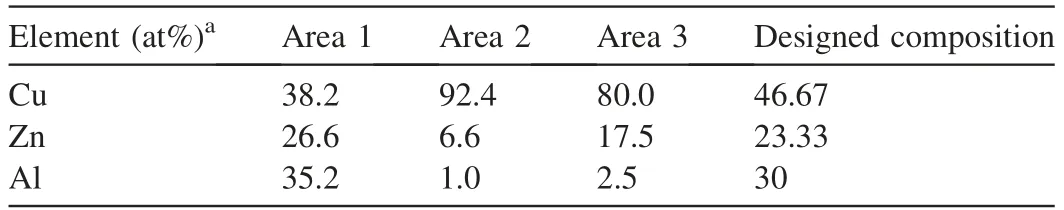
Table 5 Relative concentrations of the elements in the fragments.
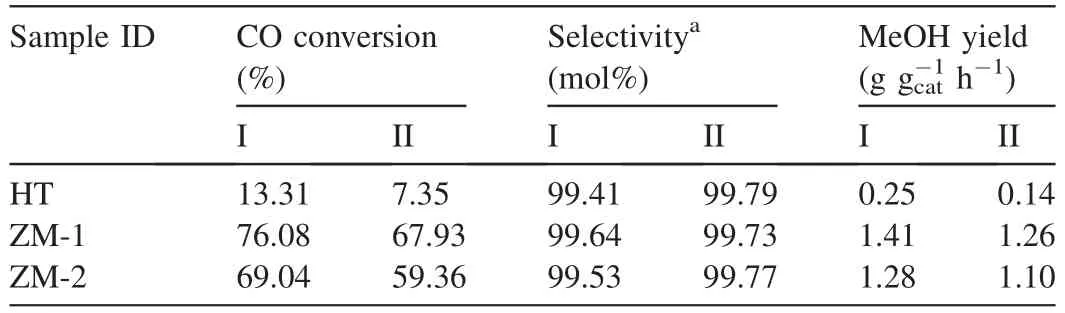
Table 6 The average CO conversion and activity loss before and after heat treatment.
The results show that HT demonstrated low CO conversion during the whole evaluation test.ZM-1 exhibited the highest CO conversion,followed by sample ZM-2.After heat treatment,the CO conversions of catalyst samples decreased to different degree.As shown in Fig.7,the CO conversion of ZM-1 and ZM-2 dropped by 10.7%and 14.0%,respectively.It shows that ZM-1 performed the best catalyst activity and thermostability in this work.

Fig.4.SEM images of the calcined catalyst samples.
Characterization results suggested that HT was mainly derived from Htlc precursor,which led to low specific surface area and low Cu surface concentration of the calcined sample.The co-produced zinc malachite had low Cu2+substitution by Zn2+in the lattice.They should be responsible for the poor catalyst activity.This was further proved by the characterization and evaluation results of ZM-2.With a few amount of Htlc precursor,the specific surface area,Cu surface concentration of ZM-2 and the Zn content in zinc malachite are all slightly inferior compared with their counterparts of ZM-1 mainly derived from zinc malachite.Correspondingly,the CO conversions of ZM-2 are a litter lower than them of ZM-1 both before and after heat treating.
It is reported that ZnO in Cu/ZnO/Al2O3catalysts has two main functions:1)As nanoparticles it acts as a physical spacer between the Cu particles,stabilizing the porous microstructure;and 2)as a thin layer at the surface of the Cu particles it is an essential ingredient for the active site [43-45].Low substitution of Cu2+by Zn2+in the malachite lattice is detrimental to the catalyst activity and stability,as shown in Fig.8.This may be the main reason that ZM-2 performed lower activity and thermal stability compared with ZM-1.It indicated that zinc malachite with high zinc content could be the efficient catalyst precursor of Cu/ZnO/Al2O3catalyst,while the formation of Htlc should be avoided during catalyst preparation.Fractional precipitation,which could efficiently control the precursor formation,is an effective method for Cu/ZnO/Al2O3catalyst preparation.
3.4.In-situ XRD results
Thermostability is an important characteristic for the methanol synthesis catalyst.Sintering of copper in the Cu/ZnO/Al2O3catalysts has been identified as a major deactivation mechanism in the process[46,47].In-situ XRD tests were conducted on ZM-1 from 110°C to 640°C in reduction atmosphere,to monitor the change of Cu and Zn species at relatively high temperatures.
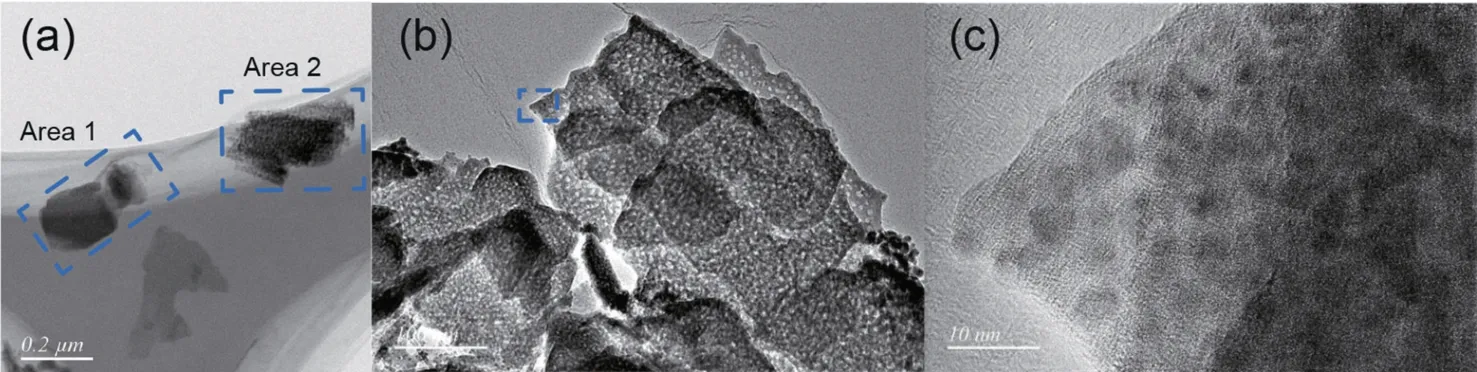
Fig.5.TEM images of the calcined sample HT (a.200 nm;b.100 nm;c.10 nm).

Fig.6.TEM images of the calcined sample ZM-1.
As shown in Fig.9,the diffraction peaks of Cu species shifted to small angle with the increase of temperature.XRD analyses suggested that the peaks at low temperatures pointed to pure Cu,while the peaks at relative high temperatures pointed to CuZn alloy.It indicated that the peak shift should be caused by the incorporation of Zn into Cu grains.Fig.9b shows the evolution of Zn species in ZM-1 during the test.It is reported that ZnO reduction was observed at the interface of ZnO layer and Cu grains in the reaction atmosphere because of Hydrogen spillover from Cu [41,48].TPR profiles of the calcined sample showed a broad peak from~300°C to~700°C,which was also ascribed to ZnO reduction in this work.Since its great dependency on Cu,ZnO reduction proceeded slowly with the growth of Cu particles.The diffraction peaks for ZnO shown in Fig.9b were weakened at relatively high temperatures,which may be also ascribed to ZnO reduction.At~590°C,ZnO peaks disappeared while the peaks of ZnAlO4were observed.After that these peaks of ZnAlO4were intensified with temperature.Fig.10 shows the in-situ XRD results of Cu/ZnO samples derived from malachite with different zinc contents at 320°C in reduction atmosphere.It can be seen that Zn species derived from malachite could efficiently prohibit the growth of Cu particles at the beginning.However,with the exposing time extended,CuZn alloy was observed,and the Cu grains grew inevitably because of the loss of ZnO protection.

Fig.7.The evaluation results for the catalyst samples with different precursor components.(230°C,5 MPa,and GHSV=10,000 h-1,80%H2,13%CO,2%CO2 and 5% N2).
ZnO is an important barrier for preventing the Cu particles from sintering.The TPR and XRD results indicated that the adjacent ZnO could be reduced and incorporated into Cu lattice in reduction atmosphere.ZnAl2O4was observed at relatively high temperatures.However,it is difficult for the normal methanol synthesis conditions to achieve the temperature of ZnAl2O4formation[49].To obtain the Cu/ZnO/Al2O3catalyst with high thermostability,zinc malachite with high Zn content should be employed as the main catalyst precursor,which could be realized by fractional precipitation.Additionally,over reduction of ZnO should be avoided during the methanol synthesis reaction.It may be realized by the addition of Mg,which was reported to be adverse to the reducibility of ZnO[50].This part of work will be reported in a separated paper.
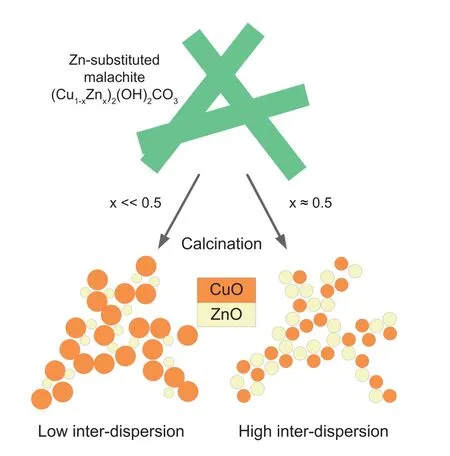
Fig.8.The role of precursor composition for the Cu dispersion in the final catalyst [6,45].

Fig.9.In situ XRD patterns of ZM-1 showing the transformation of Cu(c)and Zn species(b)during the deactivation test(2°C min-1;10 min per one XRD test;33.3%H2-N2 atmosphere,30 mL min-1).
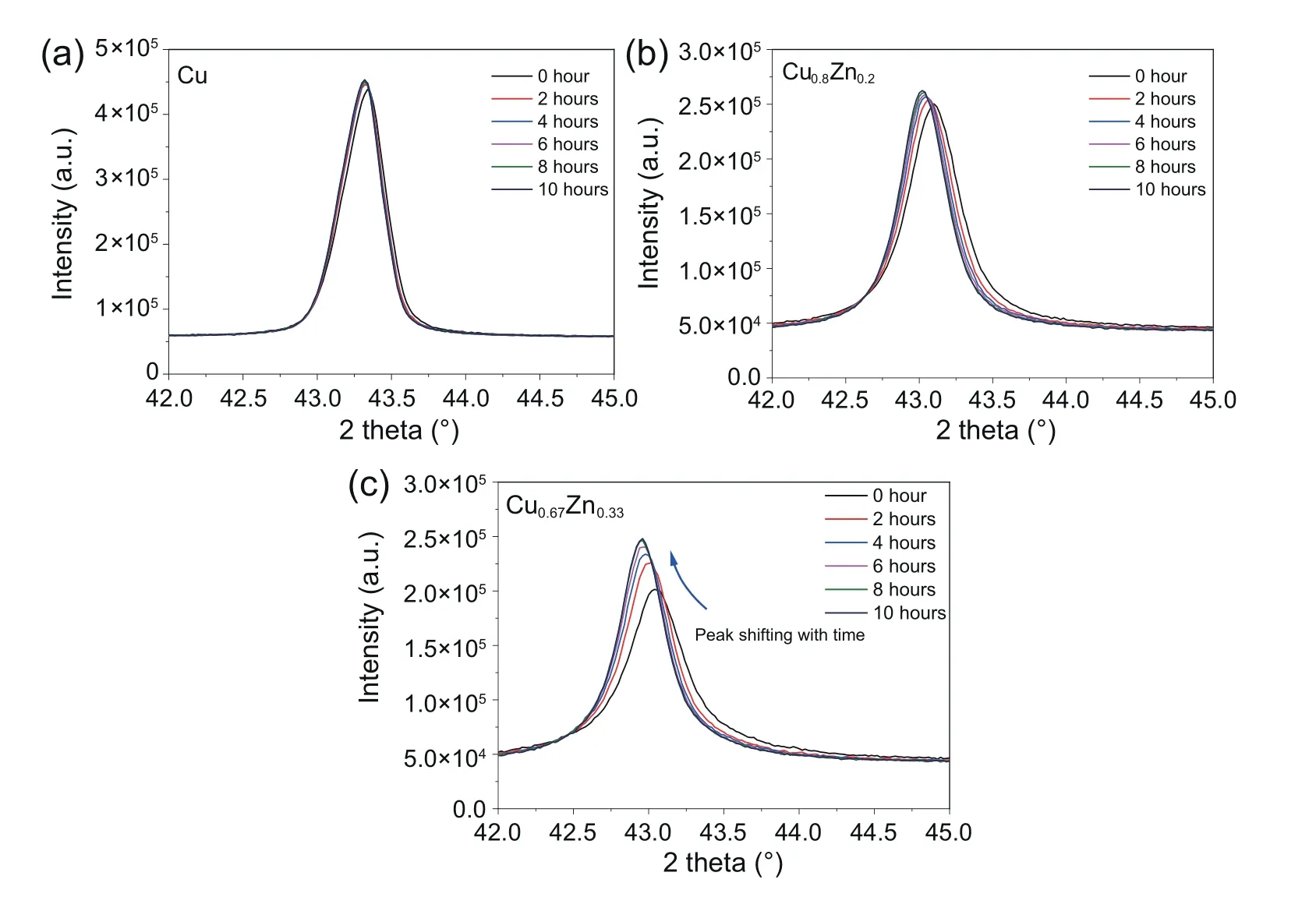
Fig.10.The in-situ XRD results of Cu/ZnO samples at 320°C with different Zn contents.
4.Conclusions
Cu/ZnO/Al2O3catalysts with designed precursor components were successfully prepared.The catalyst derived from zinc malachite with high zinc contents exhibited the best catalytic performance.Htlc precursor led to the low zinc content of the co-produced zinc malachite,the low specific surface area and surface Cu concentration of the final catalysts.Fraction precipitation,which could eliminate the Htlc precursor and promote the formation of malachite with higher zinc contents,was an effective preparation method of the catalysts.The reduction of ZnO and the formation of CuZn alloy were observed when the catalyst was treated at relatively high temperatures.Preventing ZnO from over-reduction is crucial to the catalyst's thermostability.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by the National Key R&D Program of China (2018YFB0604701),the CHN ENERGY Group Corp.Ltd.(CF9300200004).The authors thank Professor Haiquan Su and Jianli Li from Inner Mongolia University for their kind assistances.
 Green Energy & Environment2022年4期
Green Energy & Environment2022年4期
- Green Energy & Environment的其它文章
- Multivariate MOF for optimizing atmospheric water harvesting
- Lignin-based carbon fibers: Formation,modification and potential applications
- Charactering and optimizing cathode electrolytes interface for advanced rechargeable batteries: Promises and challenges
- Metal-organic frameworks-derived metal phosphides forelectrochemistry application
- Surface-mediated iron on porous cobalt oxide with high energy state for efficient water oxidation electrocatalysis
- Oxygen-deficient SnO2 nanoparticles with ultrathin carbon shell for efficient electrocatalytic N2 reduction
