KAI1/CD82 gene and autotaxin-lysophosphatidic acid axis in gastrointestinal cancers
lNTRODUCTlON
The
/
gene is an important tumor suppressor gene.As a metastasis-related suppressor gene of prostate cancer discovered by Dong
[1] in 1995,
/
is located on human chromosome 11p11.2 and consists of l0 exons and 9 introns with a length of about 80 kb.The protein encoded by this gene is composed of 267 amino acids residues and has a relative molecular weight of 29600 Da.KAI1/CD82 is a member of the transmembrane 4 superfamily (TM4SF).TM4SF proteins promote the interactions between cells and the extracellular matrix,enhance the cohesion between tumor cells,reduce phagocytosis and invasion,and inhibit tumor cell metastasis.Cell dysmetabolism is an important cause of tumor occurrence,development,and metastasis.As one of the hallmarks of cancer,cell dysmetabolism has increasingly attracted the attention of researchers in recent years.Phospholipid is an indispensable substance in cell metabolism and participates in the metabolism of various tumor cells.Phospholipid metabolites have become important cell signaling molecules.Lysophosphatidic acid(LPA) is secreted by platelets,fibroblasts,cancer cells,and fat cells and is a multifunctional “phospholipid messenger”.In tumor tissues,LPA induces intracellular signal transduction by binding G protein-coupled LPA receptors (LPARs) on the cell surface and regulates tumor cell proliferation,adhesion,migration,and invasion.Autotaxin (ATX) is a key enzyme catalyzing LPA synthesis.Clarifying the role and molecular mechanism of ATX-LPA and LPARs in cancer invasion and metastasis is necessary.According to our previous experimental results and recent pre-experimental results,as well as current reports on ATX-LPA,KAI1/CD82 might inhibit the cancer cell migration and metastasis by regulating the ATX-LPA axis.The abnormal metabolism of the ATX-LPA axis may be associated with the high metastasis characteristics of cancer.The ATX-LPA axis and their receptors may serve as molecular markers for cancer metastasis and prognosis.Clarifying the mechanism of the ATX-LPA axis in the inhibition of cancer metastasis by KAI1/CD82 will provide an important theoretical basis for targeted cancer therapy and further research.
MOLECULAR COMPOSlTlON OF THE KAI1/CD82 GENE AND THE ATX-LPA AXlS
Molecular composition of KAI1/CD82
(named after Anticancer Kang Ai) is a tumor-suppressor gene first discovered by Dong
[1] in 1995 on chromosome 11 of rabbit AT6.1 metastatic prostate cancer cells.Later,researchers confirmed that
has the same structure as the
gene; therefore,it was named
/
.The 5’-end promoter region of the
gene is 735 bp long and rich in CpG island with nine transcription factor-specific protein SPI binding sites,five AP2 binding sites,and tcF-1,Myb,and MEP.1 binding sites,which suggests that the gene is regulated by multiple mechanisms[2,3].KAI1/CD82 is located on the cell membrane and is a member of TM4SF,which comprises four conservative hydrophobic transmembrane domains (TM1–TM4) and one extracellular glycosyl-based binding site.This structure indicates that KAI1/CD82,like other TM4SF members,can affect plasma membrane molecular rearrangement,cell aggregation,adhesion,and migration,and other physiological and pathological activities through various mechanisms,as well as inhibit the migration and metastasis of various malignant tumors[4].
。,。,,、----。We used to take it in turn to carry small delicacies4 which my mother had made down from the big house to the little cottage where Aunt Stephia and an old colored maid spent their days
Molecular composition of the ATX-LPA axis
ATX is a secretory glycoprotein called autocrine motility factor.ATX was first identified in A2058 melanoma cells and induces cell migration through the pertussis toxin G protein[5].ATX has phosphodiesterase activity[6],and LPA is catalyzed by lysophosphatidylcholine (LPC)[7].LPA is a multifunctional “phospholipid messenger” secreted by platelets,fibroblasts,adipocytes,and cancer cells.Although LPA is the simplest phospholipid,it is not a simple biomolecule.LPA has six G-proteincoupled receptors that mediate several physiological and pathological processes,including embryogenesis,wound healing,chronic inflammation,cancer progression,and treatment tolerance[8].In tumor tissues,LPA binds to LPARs on the cell surface to induce intracellular signal transduction,which in turn regulates tumor cell proliferation,adhesion,migration,and invasion[7].At present,ATX-LPA target inhibitors are not yet used as a therapeutic measure clinically,and the therapeutic effects of LPA monoclonal antibodies,LPAR antagonists,and ATX inhibitors are still being explored.
ATX is also called extracellular pyrophosphatase/phosphodiesterase (ENPP)
because of its 47%-55%homology with pc-1/NPP1 and B-10/NPP3 amino acid sequences in the ENPP family.ATX is a multidomain protein[9],and lysophospholipase D (lysoPLD) catalyzes LPA formation[10].ATX has a slightly U-shaped hydrophobic pocket in the catalytic region,which tends to contain unsaturated substrates,such as unsaturated fatty acids[11],and all five selective splicing isomers have catalytic activity[12,13].Therefore,its affinity with LPC is strong.Although LPA can be produced by other processes,such as phospholipase A2,Ca
-independent phospholipase A2,and phosphatidate[14-16],ATX is still the main pathway of extracellular LPA generation.
Serum contains 2-20 μm LPA,and its metabolites extensively affect biological activities inside and outside cells[17].LPA is one of the smallest glycerophosphatides and comprises three domains:Phosphate head,linker,and lipophilic terminal.The function of the phosphoric head is to activate the receptor; the lipophilic terminal sequence determines its biological activity; and the head and tail are linked by acyl,alkyl,or alkenyl groups[18].Its free hydroxyl and phosphate groups make LPA more soluble in water than long-chain phospholipids,which likely contributes to its biological activities.The family of lipid phosphate phosphohydrolases (LPPs) dephosphorylates LPA[19,20].
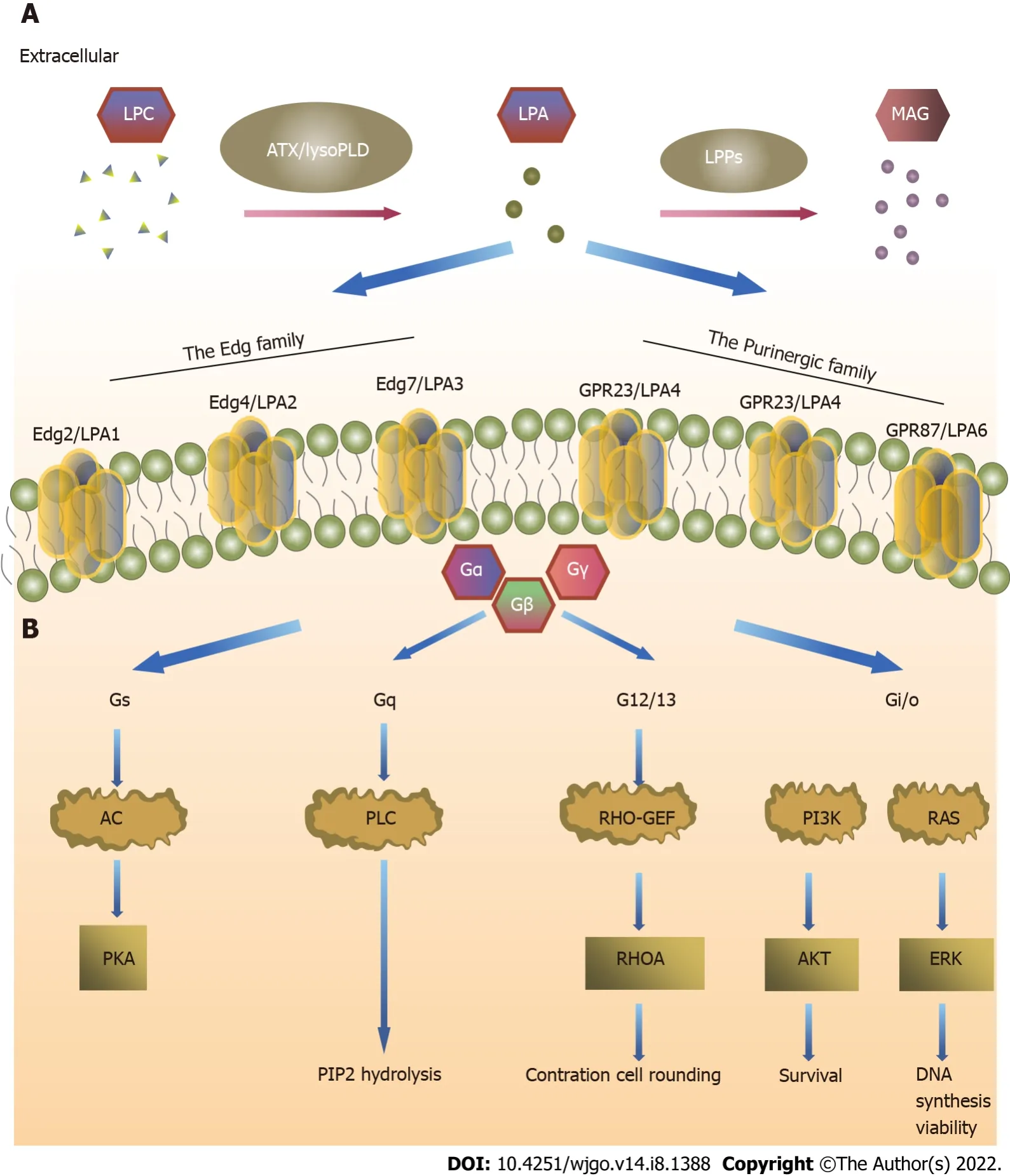
LPARs are divided into two subfamilies: LPA
receptors belonging to the endothelial cell differentiation gene (Edg) family,and LPA
receptors belonging to the purine (P2Y) receptor family[9,21].LPA
(Edg
) has 50%-60% amino acid homology with LPA
(Edg4) and LPA
(Edg
).LPA
and LPA
need to pass through the G
,G
,and G
signaling pathways,whereas LPA
passes only through the G
and G
signaling pathways[22].The function of G
is to stimulate mitotic division through the Ras-Raf-MAPK signaling pathway and promote tumor cell survival through the PI3K-Akt signaling pathway[23,24].LPA
(P2Y9/GPR23),LPA
(GPR92),and LPA
(P2Y5) have 35%-55% amino acid homology.LPA
acts through the G
,G
,G
,and G
signaling pathways and is the only LPAR that activates adenosine cyclase and leads to cyclic adenosine monophosphate elevation.LPA
plays a role through the G
and G
signaling pathways,whereas LPA
plays a role through the G
activation of the Rho signaling pathways[22].The effect of LPARs on tumors depends on the G protein signaling pathway that it activates[25].
PHYSlOLOGlCAL FUNCTlONS OF THE KAI1/CD82 GENE AND THE ATX-LPA-LPP AXlS lN CANCERS
Inhibition of the KAI1/CD82 gene in cancers
Low KAI1 expression accelerates tumor invasion and metastasis[26].In 2017,a meta-analysis involving 31 studies showed that high KAI1 expression is significantly associated with overall survival (OS)[hazard ratio (HR) = 0.56,95% confidence interval (CI): 0.47-0.67] and disease-free/relapsefree/progression-free survival (PFS) (HR = 0.42,95%CI: 0.30-0.59) in patients with cancer.In addition,they performed a subgroup analysis showing that KAI1/CD82 is associated with a good prognosis in patients with cancer.KAI1/CD82 may be a promising biomarker for predicting the prognosis of patients with malignant tumors,and its biological function has important research value for this topic[27].The Human Protein Atlas is an outstanding initiative associated to the Human Proteome Project,which has made available valuable information about the functional and pathological aspects of about 17000 proteins.In particular,they are able to propose scores that suggest the prognostic value of proteins in diseases based on the expression levels of these proteins in healthy and diseased tissues.Considering that only 31 studies were included in the meta-analysis,more studies may be needed in the future to verify whether KAI1 can be used as a prognostic factor.KAI1/CD82 may inhibit cell metastasis and migration through two pathways.The first is that KAI1/CD82 inhibits cell migration as an initiating signal.However,the possibility of this pathway is low because of the simple structure of KAI1/CD82 and the lack of corresponding enzymes in the cytoplasm.However,evidence also indicates that KAI1/CD82 may be an initiating signal[28,29].KAI1/CD82 is crosslinked with monoclonal antibody to induce morphological changes and signal transduction[30].Integrins are also essential for cell adhesion and migration,and KAI1/CD82 is associated with several integrins,including α3β1,α4β1,α5β1,α6β1,and αLβ2[31-35],which may also be one of the pathways through which KAI1/CD82 inhibits tumor.Epidermal growth factor receptor (EGFR) is a member of the ErbB family.In tumor tissues,the receptors and ligand of the ErbB pathway are overproduced and overactivated.Odintsova
[36]found that KAI1/CD82 is correlated with EGFR,ErbB2,and ErbB3 and inhibits the endocytosis of the EGF signaling pathway and EGFR.KAI1/CD82 redistributes molecules on the cell membrane surface;KAI1/CD82 overexpression results in the redistribution and aggregation of urokinase-type plasminogen activator receptor (uPAR) into a stable α5β1 complex.Moreover,KAI1/CD82 overexpression also results in the redistribution of EGFR and gangliosides in the plasma membrane.However,whether the redistribution of these substances is related to KAI1/CD82 tumor inhibition remains unknown[37].
Physiological function of the ATX-LPA-LPP axis in cancers
LPA signals can be roughly divided into three parts,namely,ATX,LPARs,and LPP of extracellular LPA[38,39].ATX has lysoPLD activity and promotes LPA generation in blood[40,41].Many tumor cells secrete ATX[42],LPAR expression is higher on tumor cell surfaces than on normal cells,and LPP expression is lower in tumor cells than in normal cells.Understanding the metabolic pathway of the ATX-LPA-LPP axis in the tumor microenvironment (TME) is important to study its target therapy(Figure 1).
The TME is produced by tumor cells,such as neuroblastoma[43],glioblastoma[44],liver cancer[45],Bcell lymphoma[46],melanoma[47],kidney cancer[48],thyroid cancer[49],breast cancer,and non-small cell lung cancer[50],as well as stromal cells such as fibroblasts and adipocytes[51-53].How to regulate ATX expression remains unclear.ENPP overexpression may be one of the reasons for ATX upregulation in cancer tissues[54].The Cancer Genome Atlas shows that ENPP overexpression is present in serous ovarian cystadenocarcinoma (about 33%) and invasive breast carcinoma (about 20%).The
ENPP
gene is overexpressed in hepatocellular carcinoma (HCC; about 20%),lung adenocarcinoma (about 11%),bladder transitional cell carcinoma (about 10%),and head and neck squamous cell carcinoma (about 10%)[13].Moreover,ATX is involved in the physiological wound-healing response,and ATX levels are increased in some inflammatory diseases[55].Park
[56] found that the levels of interleukin (IL)-4,IL-5,and ATX increase in patients with asthma who received bronchoalveolar lavage fluid when stimulated by allergens.ATX induces pro-inflammatory cytokines,such as tumor necrosis factor (TNF)-α and IL-1β[57,58],NOD receptor family (NLRP3),ATM kinase,ATR protein kinase,and nuclear transcription factor-kappa B (NF-κB)[59].At present,although ATX research has made some progress,the overall understanding remains limited.
‘Madam,’ answered one of the attendants present, who had been with the prince at the farm, Donkey Skin is, next to the wolf, the most disgusting creature on the face of the earth. She is a girl who wears a black, greasy35 skin, and lives at your farmer’s as hen-wife.’
Thirsty as he was, he did not wait to drink, but he told his mother that he smelt the blood of a Christian man, and that she had better bring him out at once and make him ready to be eaten
LPA is present in intracellular and extracellular fluids (blood,ascites,follicular fluid,saliva,
)[56].In 1989,van Corven
[60] found that LPA may be involved in cell diffusion and migration.Two years later,Merchant
[61] found increased LPA levels in malignant colon tumor tissues.LPA may be a simple lipid,but it is involved in all aspects of tumor development; it stimulates proliferative signals[62],prevents growth inhibition and resists apoptosis[63,64],regulates telomerase[64],promotes vascular endothelial growth factor (VEGF)-A and VEGF-C,and induces angiogenesis[65-67].LPA induces the gene instability caused by reactive oxygen species and stimulates the production of inflammatory factors,such as COX-2,IL,and TNF-α[68,69].LPA activates at least three signaling pathways: (1)Promotes phosphoinositol hydrolysis and therefore activates protein kinase C (PKC) and Ca
mobilization; (2) Promotes the release of guanosine triphosphate (GTP); and (3) Inhibits adenylate cyclase activity.In recent years,the activation of the downstream signaling Ras pathway may promote LPA fibrogenesis[70].Moreover,MAK-related kinase,as an effector of RhoC,regulates LPA-induced cell invasion through myosin,extracellular signal-regulated kinase (ERK),and P38[71],whereas LPA induces the G12/13-Rhoa-Rock signaling pathway to mediate focal adhesion kinase autophosphorylation and promote tumor cell migration[72].Furthermore,Lee
[73] found that LPA interacts with T lymphocytes,B lymphocytes,acidic granulocytes,neutrophils,macrophages,mast cells,dendritic cells,and natural killer cells in the immune system and blood.Currently,no clinical treatment for LPA target is available,and the study of TME’s molecular mechanism is helpful to guide clinical treatment.
GC is one of the most common malignant tumors.Although GC-related morbidity has shown a downward trend in recent years,the mortality rate remains high[120,121].KAI1 has been studied to identify novel therapeutic targets[122-126].Ilhan
[122] and Knoener
[123] found that KAI1/CD82 is negative in all tissues with distant metastasis or tissues in stage IV GC with statistical significance (
< 0.05).KAI1 inhibits tumor growth and metastasis and is a prognostic factor for patients with GC.Hinoda
[124] found that the positive rate of KAI1/CD82 in patients with stages Ia-IIIa GC is 16.6% (8/48),and all patients with stages IIIb-IVb GC are negative for KAI1/CD82 (0%,0/25;
=0.05).KAI1/CD82 is highly expressed in normal gastric epithelial cells.In GC,KAI1/CD82 expression decreases with increased tumor differentiation,tumor invasion depth,and lymph node metastasis[127,128].Guan
[129] found that reduced KAI1/CD82 expression promotes lymph node metastasis and liver metastasis in patients with GC.The detection of
mRNA expression level can be used as a prognostic index for patients with GC.
Comparative analysis of LPAR-mediated signals in tumors
All the authors report no relevant conflicts of interest for this article.
LPA:
LPA
is elevated in tumor tissue[84].Studies showed that LPA
is associated with many human tumors,and the binding of LPA
with its ligand,LPA,can activate the LPA signaling pathway and promote cell proliferation and malignant transformation.For example,the high expression level of the LPA
receptor in breast cancer suggests a poor prognosis[85].The high expression of
LPA
mRNA in HCC is related to the low differentiation of cancer cells[86],and the high expression of LPA
receptor in colon cancer cells promotes the acquisition of drug resistance and the failure of anticancer drugs[87].LPA
-mediated signaling plays an important role in the enhancement of the chemoresistance of A375 cells treated with anticancer drugs[78].Ren
[88] transfected SGC-7901 gastric cancer (GC) cells with LPA
expression vector and found that the expression of E-cadherin gradually decreases and the expression of vimentin gradually increases with the increase in LPA
level.These findings suggest that LPA
is involved in the epithelial-mesenchymal transition (EMT) process of GC cells.GC cells with increased LPA
level are likely to metastasize.Dong
[89] believed that an effective drug that can inhibit
LPA
gene expression,inhibit GC cell proliferation,and promote apoptosis might be a potential new target for GC treatment.Xu
[90] found that thyroid receptor interacting protein 6 activates LPA
and its downstream signal and therefore promotes cell adhesion and migration.The carcinogenic mechanism of LPA
is still unknown,and most studies have focused on the LPA stimulation of the expression of cytokines,such as IL-6,VEGF,hypoxia-inducible factor 1α,C-MyC,cyclin D1,Kruppellike factor 5,and COX-2.Moreover,Na
/H
regulatory factor 2 (NHERF-2) may enhance
LPA
gene expression and other LPA-induced cellular processes[91].
Many LPA receptor antagonists have been found,but few work
.LPA receptor antagonists are divided into lipid and small-molecule inhibitors,which are derived from fibrosis model studies[194].BrP-LPA,a pan-LPAR antagonist,was used to treat breast MDA-MB-231 cancer cells[195].Through LPAR2,BrP-LPA may also sensitize vascular endothelial cells in mouse GL-261 glioma cells to improve malignant glioma response to radiation therapy[183].LPA accelerates pulmonary fibrosis through LPA
,and the LPA
antagonist AM966 can inhibit bleomycin-induced idiopathic pulmonary fibrosis.Zhao
[196] found that Ki16425 (LPA
and LPA
antagonist) and ono7300243 (LPA
antagonist)completely block LPA-induced actions.Recently,lysophospholipid
genes have been used to develop receptor subtype-selective agonists and antagonists.The discovery of FTY720,a novel immune modulator,along with other chemical tools,has provided a means of elucidating the functions of each lysophospholipid GPCR on an organ and the whole body level[197].In some cancers,targeting LPAR
is considered a good option against cancer development[87,198].LPAR
antagonist TCLPA5 attenuates the proliferation and migration of thyroid carcinoma cells[199].In addition,the loss of LPA
in mouse B16-F10 melanoma results in fewer lung metastases[200],which suggests that the drug inhibition of LPA
can also control melanoma-mediated metastasis.MP-LPA analogs exhibit an unanticipated pattern of partial agonist/antagonist activity for the LPA G protein-coupled receptor family and the intracellular LPA receptor peroxisome proliferator-activated receptors-γ[201].Currently,all are based on LPA
,LPA
,or LPA
dual antagonists[194].However,the development of PAN-LPA receptor antagonists may be a more effective approach owing to the complexity of LPAR signals[202].
LPA:
LPA
may be involved in the invasion and metastasis of breast cancer cells,and the migration and invasion ability may involve the regulation of MMP2 and MMP9 protein expression.Takara
[98]found that LPA
is involved in the formation of vascular networks.LPA
activation induces the subcellular binding of circumferential actin and enhances the linear adhesion of vascular-endothelial cadherin in endothelial cells.Studies found that LPA
knockout cells show high motor activity.The gelatinase spectrum shows that LPA
inhibits the activation of MMP2.LPA
also inhibits the cellular motility of endothelial cells,which is correlated to the expression level of the
gene[99].However,Tsujino
[100] found no mutation in the
LPA
gene in colon cancer cells DLD1,SW480,HCT116,CACO-2,SW48,and LoVo.LPA
-mediated tube formation,which reflects the stabilization of barrier integrity,was confirmed by
angiogenesis assay.By contrast,LPA
-mediated protective actions are associated with the activation of Src and Rap1 and attenuated by the abrogation of their activities[101].A considerable correlation between LPA6 and PIM-3 expression levels is also observed in patients with HCC.Furthermore,the biological roles of LPA
remain unknown[102,103].
THE KAI1/CD82 GENE AND ATX–LPA AXlS lN GASTROlNTESTlNAL CANCERS
KAI1/CD82 in pancreatic cancer
Pancreatic cancer (PC) is the seventh most common cancer worldwide and causes more than 300000 deaths a year[104].The 5-year survival rate of PC is only 3%-5%.In the early stages of PC,it directly invades peripancreatic tissues or metastasizes to organs near and far
lymphatic and/or blood vessels.More than 80% of patients with PC are initially diagnosed at advanced stages,lose the chance of surgical treatment,and have poor radiotherapy and chemotherapy effects.In 1996,Guo
[105] found that the expression of
mRNA in early pancreatic tumors (I and II) is significantly higher than that in advanced tumors (III and IV) with lymph node metastasis or distant metastasis (
< 0.01),and the
mRNA level in poorly differentiated tumors is significantly higher than that in moderately differentiated or well-differentiated tumors (
< 0.05).Friess
[106] and Xu
[107] also found similar results.Subsequent studies have shown that low KAI1/CD82 level is associated with the inhibition of PC cell invasion and metastasis,and the
gene may control PC cell metastasis by inhibiting cancer cell invasion and motor function[108-111].
When he saw her well-disposed towards him, he exclaimed: Madam, I have a most important secret to confide49 to you, and I beg you not to be alarmed by what I am about to say
KAIl/CD82 protein,a member of TM4SF,has been accepted for its inhibitory effect on tumor metastasis; the mechanism of this effect has not yet been clearly explained,but it may be related to its localization on the cell membrane,extensive glycosylation,and cell-cell and cell-extracellular matrix interactions.Mashimo
[112] found that the loss of p53 leads to the downregulation of the
gene and promotes cancer metastasis.KAI1 may inhibit the metastasis of the PC cells PANC-1 and Miapaca-2,caused by hepatocyte growth factor (HGF) by downregulating sphingosine kinase (SphK)expression.After they were infected with the
gene,the PANC-1 and Miapaca-2 cells induced by HGF had decreased invasive ability in the Boyden chamber assay.KAI1 overexpression in cells leads to the deactivation of SphK and a decreased level of intracellular sphingosine-1-phosphate[108].Liu
[108] found that KAI1/CD82 induces the downregulation of VEGF-C expression through the Src/STAT3 signaling pathway,which may also inhibit the lymph node metastasis of PC.Wu
[111]found that KAI1 induces the expression of the autophagy proteins LC3 and Beclin1,and further confirmed that KAI1 could induce autophagy in the human PC cell line MiAPACA-2 and therefore promote cell apoptosis and inhibit proliferation.EMT plays an important role in the pathogenesis of PC.KAI1 reverses the expression of EMT-related factors,such as Snail,Vimentin,MMP2,and MMP9 (
<0.05),and inhibits PC cell metastasis and invasion.In conclusion,KAI1 may be a new potential therapeutic target for PC in the future.
KAI1/CD82 in HCC
The main risk factors for HCC are hepatitis virus infection; alcohol consumption; and metabolic disorders,such as obesity,diabetes,and non-alcoholic fatty liver disease[149].The abnormal expression of the ATX-LPA axis may cause liver metabolism disorder and induce steatohepatitis and liver cancer[150,151].The ATX-LPA axis is currently considered one of the most promising signaling pathways in liver cancer[152].Watanabe
[153] found elevated ATX and LPA levels in hepatic fibrosis tissues.Memet
[149] found that high expression of ATX in HCC is an independent prognostic factor (HR =13.70,95%CI: 3.26-57.62,
= 0.0004),and high expression of ATX (+3) also increases the risk of death by eight-fold.Wu
[154] found that ATX is significantly elevated in Hep3B and Huh7 cells.Park
[155] found that LPA
is significantly elevated in liver cancer.LPA
may be highly expressed in HCC tissues through the lPA3-GI-ERK signaling pathway[156].Enooku
[86] found that increased
LPA
mRNA level may be associated with the low differentiation degree of HCC.Okabe
[157] found that LPA
induces the invasion of rabbit RH7777 hepatoma cells.LPA
is not expressed in normal tissues but is expressed in liver cancer tissues.Zheng
[158] found that nuclear receptor coactivator 3 induces the acetylation of histone 3-LYS-27 at the LPA
site after HGF treatment and inhibits LPA
transcription.High LPA
expression promotes HCC proliferation.Lippolis
[159] found that high LPA
expression promotes the development of HCC with poor prognosis.Gnocchi
[160] and Mazzocca
[161]found that LPA
may be an important therapeutic target for HCC,although LPA
overexpression promotes HCC cell growth.
KAI1/CD82 in GC
LPA is hydrolyzed and inactivated by LPPs.Studies have found that LPP1 and LPP3 are reduced in various tumor tissues[74].LPPs activate ERK signaling by thrombin; induce LPP1 and LPP2 overexpression; and attenuate cell migration,cell differentiation,and angiogenesis[75].Pilquil
[76] found that increased LPP
expression weakens PLD activation,which is an intermediate substance necessary for LPA to stimulate cell migration.LPP
also weakens fibroblast migration.Tanyi
[77] found that LPP
reduces cell apoptosis,decreases the migration ability of transfected LPP
cells,and slows down tumor growth
and
.
KAI1/CD82 in colorectal cancer
Colorectal cancer (CRC) is a common malignant tumor,and metastasis is the main cause of its poor prognosis.KAI1 may affect cellular connectivity and may be related to its metastasis.KAI1 may be a new therapeutic target for CRC[130,131].KAI1 mRNA and protein are increased in early CRC tumors,decreased in late CRC tumors,and no longer expressed in distant metastasis[132].Integrin-α3 and TAp73 regulate CRC invasion and metastasis by regulating KAI1 transcription[133,134].
ATX-LPA in PC
The expression of ATX in PC remains unclear,and its molecular biological mechanism has not yet been reported.Ryder
[135] and Nakai
[136] found that ATX expression is increased in PC tissues,but it is more increased in chronic pancreatitis or pancreatic cysts than in PC.Quan
[137] found that TNF-α,NF-κB,Wnt/β-catenin pathway,V-Jun,EGF,and B-FGF are all activated or abnormally expressed in PC tissues,which may provide a direction for future research on mechanisms.LPA activates downstream signaling pathways,such as PI3K/AKT,RAS/ERK,Rho,and Hippo,and promotes PC cell proliferation,migration,and invasion[138,139].Additionally,LPA is remarkably increased in the serum and ascites[140,141],which suggests that ATX activity is elevated in patients with PC.
ATX catalyzes LPA synthesis from LPC and exerts biological effects through the receptors LPA
.Fukushima
[142] found that the invasion ability of PANC-R9 cells is 15 times that of PANC-1 cells,LPA
expression in PANC-R9 cells is remarkably higher than that in PANC-1 cells,and LPA
is decreased.Kato
[143] also found that LPA
and LPA
play opposite roles in PC cell migration.Tsujiuchi
[144],Komachi
[145],and Yamada
[146] found that LPA
induces PC cell migration.Liao
[141] and Yoshikawa
[147] found that LPA
may induce PC cell migration by enhancing the proto-oncogene K-RAS pathway.However,Komachi
[145] found that LPA
may inhibit PC cell migration through the conjugated G12/13/Rho signaling pathway.Ishii
[148]conducted a cell activity assay after LPARs were knocked out from PANC-1 cells (PANC-SH4,PANCSH5,and PANC-SH6 cells).They found that PANC-SH4 and PANC-SH5 enhance cell migration ability,whereas PANC-SH6 inhibits cell migration.Currently,few studies have been conducted on the molecular biology of LPAR and PC,and further research is needed.
ATX-LPA axis in HCC
HCC is a common malignant tumor with the second highest mortality rate in China.Rapid intrahepatic and extrahepatic metastases lead to poor prognosis[113].Zhang
[114] found that the combined detection of KAI1 and VEGF can greatly improve the diagnostic efficiency for HCC.Mu
[115] found that KAI1/CD82 suppresses the HGF-induced migration of hepatoma cells
SphK1 downregulation.HGF induces hepatoma cell migration through cellular SphK1 activation.The adenovirus-mediated gene transfer of KAI1 downregulates SphK1 expression and suppresses the HGF-induced migration of SMMC-7721 human HCC cells.Guo
[116] found that the
fusion gene and JunB inhibit tumor cell invasiveness and promote tumor cell apoptosis by regulating KAI1/CD82 expression.Si
[117]and Yang
[118] found that changing KAI1 expression could alter the migration and invasion ability of MHCC97-H in HCC cells.Xu
[119] found that KAI1 is negatively correlated with tumor grade,venous invasion,lymph node metastasis,intrahepatic metastasis,and TNM stage and positively correlated with patients’ OS.KAI1/CD82 may also play an important role in HCC metastasis and prognosis.
She replied, Baby, not now, but if you are a good girl maybe Santa will bring it to you. But Mama, I want that telephone right now. Her eyes narrowed and her hand tightened7 on mine. Becky, you can t have that telephone today, but if you misbehave you can have a spanking8() .
ATX-LPA axis in GC
The role of ATX-LPA axis in GC invasion and metastasis remains to be explored.Zeng
[162] found that LPA is increased in GC tissue samples with peritoneal metastasis (
= 0.046) and is significantly increased in ascites (
< 0.001).Serum LPA decreases after chemotherapy (
= 0.028).PFS and OS are significantly decreased in an ascites LPA > 24000 ng/mL group (
< 0.001).Ramachandran
[163]and Shida
[164] found that LPA upregulates SphK1 through the ERK1 signaling pathway.Kim
[165] found that LPA can induce uPAR to stimulate the downstream signaling pathways,rho-family GTPase,JNK,AP-1,and NF-κB.Budnik[18] found that LPA upregulates human epidermal growth factor receptor 2 expression in GC cells and promotes GC cell invasion.LPA promotes cell proliferation,but the molecular biological mechanism between LPA and GC still needs further exploration,and LPA may become a new target for GC treatment.
ATX-LPA in CRC
CRC is the fourth leading cause of cancer deaths in the world[166].Kazama
[167] found that ATX overexpression is associated with tumor angiogenesis in the early stage of colon cancer.LPA may stimulate the proliferation and migration of CRC cells through the EGFR pathway.It may also promote hcT-116 colon cancer cell migration by regulating the cell cycle through the rho-Rock and STAT3 pathways.Whether LPA
stimulates colon cancer cell proliferation remains controversial.A study found that HCT116 and LS174T cells with LPA
knockout do not affect the spread of cancer cells[168],and DLD cancer cells are affected when they spread[169,170].LPA
promotes the spread and migration of colon cancer by regulating the NHERF-2 pathway[171,172]; therefore,LPA
may be one of the therapeutic targets for CRC in the future[173].Shida
[174] found that
LPA
mRNA is microexpressed in normal and tumor tissues.Fukui
[175] found that the expression levels of VEGF-A and VEGF-C are increased in HCT-SH3-3 cells with LPA
knockout,and LPA
inhibits the metastasis of HCT116 colon cancer cells.Takahashi
[176] found that LPA
and LPA
inhibit the activities of DLD1 and HCT116 colon cancer cells.Studies on ATX-LPA axis target inhibitors and colon malignancies are still few and require further exploration.
KAl1/CD82 AND ATX-LPA AXlS TARGET THERAPY
KAI1/CD82 target therapy
Most studies have shown that KAI1/CD82 inhibits tumor metastasis and migration,but knowledge about KAI1/CD82 antibody reagents is still lacking.Custer
[177] found that the KAI1 polyclonal antibody produced by rabbits is expressed similarly in normal tissues of mice and humans and could specifically detect mouse KAI1/CD82 protein.KAI1/CD82 is a novel tumor therapeutic target,and more KAI1/CD82 antibodies are expected to be developed in the future[178,179].
ATX inhibitors
ATX inhibitors decrease serum LPA levels by more than 95%[180].Oral ATX inhibitors have better bioavailability owing to their low hydrophobicity and slow degradation
[181].PF-8380 is the first ATX inhibitor to permanently reduce LPA levels
.Bhave
[182] and Schleicher
[183] found that PF-8380 reduces lPA-induced inflammation and delays tumor growth for more than 20 d in a mouse model of glioblastoma multiforme.Tang
[184] found that the inhibition of GLPG1690 on ATX enhances the efficacy of chemoradiotherapy in mouse breast cancer models.ONO-8430506 is also a highly effective ATX inhibitor,and the oral administration of 30 mg/kg ONO-8430506 effectively reduces serum ATX and LPA levels in rats[185].ONO-8430506 in combination with adriamycin delays the growth time of orthotopic 4T1 breast tumors in 60% Balb/C mice by about 10 d and reduces the growth time of 70% tumors by about 17 d[186,187].Cholera toxin treatment increases the expression of the anti-inflammatory cytokines IL-4 and IL-10 and inhibits
mRNA[188],and the knockdown of
mRNA inhibits the growth of Hep3B and Huh7 hepatoma cells[189].Gupte
[190] found that ATX inhibitors,such as 4-pentadecylbenzylphosphonic acid,reduce plasma LPA levels by 50%.Plasma LPA in ATX-KO mice lacking dominant heterozygosity is reduced by 50%.ATX inhibitors have not shown remarkable side effects to date.
Winter came, and the plant was covered with snow, but the snowglittered over it as if it had sunshine beneath as well as above.When spring came, the plant appeared in full bloom: a morebeautiful object than any other plant in the forest. And now theprofessor of botany presented himself, one who could explain hisknowledge in black and white. He examined and tested the plant, but it did not belong to his system of botany, nor could he possibly find out to what class it did belong. It must be some degenerate3 species, said he; I do not know it, and it is not mentioned in any system. Not known in any system! repeated the thistles and the nettles4.
LPA monoclonal antibody and LPA receptor antagonist
Antibody interventional therapy is superior to traditional therapy,and its antibody bioavailability and receptor binding are longer than other therapies[191].Goldshmit
[192] found that monoclonal antibody B3 can reduce inflammation and glial cell death and improve neuronal function.Monoclonal antibody B3,also known as lpathomab,reduces IL-6 expression and the lesion area and has improved function in a mouse model of traumatic brain injury[193].
LPA:
Research found that LPA
promotes cancer cell proliferation and metastasis.Zhao
[92] found that the high expression of the LPA
protein is considerably correlated with the occurrence and recurrence of epithelial ovarian cancer.Hayashi
[93] and Kitayoshi
[94] found that LPA
inhibits tumor cell migration.Sun
[95] found that LPA
overexpression is associated with lymph node metastasis and the loss of the expression of estrogen receptor,progesterone receptor,and human EGFR2.Studies found that LPA
may be related to the activation of the YAP protein in breast cancer and that LPA
overexpression may promote the activation of YAP protein and the proliferation and metastasis of breast cancer cells.Fang
[96] found that LPA
affects B cell lymphoma (Bcl)-2 and Bax expression; therefore,it affects the Bcl-2/Bax ratio,inhibits the apoptosis of ovarian cancer cells,and promotes the development of ovarian cancer.The vasodilator-stimulated phospho-protein phosphorylation induced by LPA receptor is a key mediator of migration initiation.LPA
plays a role in cellular motility and may contribute to cell invasion and metastasis[97].
CONCLUSlON
This paper systematically reviews the physiological functions of the
gene and the ATX-LPA axis in tumors,as well as their roles in digestive system tumors and targeted therapies.The results demonstrate that KAI1/CD82 is indeed an important inhibitor of tumor metastasis.Further elucidation of the molecular mechanism and regulatory network of KAI1/CD82 and the inhibition of tumor metastasis is needed to discover the molecular markers of pancreatic tumor metastasis,adopt effective strategies to treat PC and prevent PC metastasis,and provide a new approach for the diagnosis and treatment of patients with refractory PC.Although the ATX-LPA axis is considered an important target of cancer,its clinical application is still faced with obstacles.LPA is degraded quickly in the body,and many other factors,such as diet,smoking,and alcohol consumption,can affect the detection results.Other lipids may also generate LPA during extraction,storage,and detection.Therefore,many technical problems need to be overcome in LPA detection.In recent years,clinical trials on the ATX-LPA axis have begun.LPA monoclonal antibodies,LPA receptor antagonists,and ATX inhibitors may become feasible treatment measures.Moreover,ATX-LPA axis-targeted therapy may affect the efficacy of existing chemical drugs.Therefore,an in-depth exploration of specific biomarkers related to LPA activity should be conducted to track disease progression during LPA treatment and ensure the rational application of drugs.
Wang S wrote the original draft; Chen J and Guo XZ contributed to the review and manuscript editing.
the National Natural Science Foundation of China,No.81672465; and the Science and Technology Program of Liaoning Province,No.2019JH8/10300080.
Because they knew they were being put to the test, answered the Lion; and so they made an effort; but just have a dozen spinning- wheels placed in the ante-room
LPA:
LPA
is the most widely expressed Edg LPAR in tissues[69].LPA signaling through LPA
regulates a variety of malignant properties in cancer cells[78].Murph
[79] found that LPA
downregulates the tumor suppressor gene
and weakens its inhibitory effect.Marshall
[80] found that the tumor-suppressor gene
could inhibit LPA
expression.Additionally,Stadler
[81] found that LPA
is a signaling receptor downstream of fibroblast growth factor receptor 4 (FGFR4) that promotes cell transformation of cells into fibroblasts,which are one of the main components of TME matrix.LPA
preferentially binds to Gα Q proteins in tumors to activate PKC.PKC is involved in many cellular processes,including proliferation and metastasis.Valdés-Rives
[82] found that when the LPA
/PKC α signaling pathway is blocked,the number of cells is reduced; this finding suggests a correlation between LPA
and PKCα in glioblastoma multiforme growth.Stadler
[81] found that patients with high expression of the LPA
receptor for R388 FGFR4 phenotype are more likely to develop cancer.Lin
[83] found that LPA
signaling mediates tumor lymphangiogenesis by promoting calreticulin expression in prostate cancer.Elevated LPA
receptors also contribute to cancer development.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
China
Shuo Wang 0000-0003-0848-433X; Jiang Chen 0000-0002-5836-6342; Xiao-Zhong Guo 0000-0001-5434-9273.
11 The Devil took off his green coat, gave it to the soldier, and said, If thou hast this coat on thy back and puttest thy hand into the pocket, thou wilt always find it full of money
“I wished that you and Grandma could have church. And I just knew that it would come true. Look! There’s the pastor, and everyone from church is coming up the walk.”
30.At every word you speak there shall come out of your mouth a snake or a toad: Once again, the punishment fits the crime. Since the girl s words are rude and disgusting, she will have disgusting objects issue from her mouth whenever she speaks.
Director,chief physician,Department of Gastroenterology,General Hospital of Northern Theater Command; Director of biliopancreatic and Endoscopic Diagnosis and Treatment Center of PLA and Key Laboratory of Liaoning Province; Director of Liaoning Institute of Digestive Diseases; Vice chairman of Pancreatic Disease Branch,Chinese Medical Doctor Association; Standing member of Gastroenterology Society,Chinese Medical Association; Standing member of Gastroenterologist Branch,Chinese Medical Doctor Association; Leader of pancreatology Group,Gastroenterology Branch,Chinese Medical Association.
Wang JJ
And therefore, said the eel-breeder in conclusion, it is alwaysthe proper thing to drink brandy after eating eels. This story was the tinsel thread, the most humorous recollectionof Jurgen s life. He also wanted to go a little way farther out and upthe bay- that is to say, out into the world in a ship- but hismother said, like the eel-breeder, There are so many bad people-eel spearers! He wished to go a little way past the sand-hills, outinto the dunes, and at last he did: four happy days, the brightestof his childhood, fell to his lot, and the whole beauty andsplendour of Jutland, all the happiness and sunshine of his home, were concentrated in these. He went to a festival, but it was a burialfeast.
Wang TQ
Wang JJ
1 Dong JT,Lamb PW,Rinker-Schaeffer CW,Vukanovic J,Ichikawa T,Isaacs JT,Barrett JC.KAI1,a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2.
1995; 268: 884-886 [PMID: 7754374 DOI:10.1126/science.7754374]
2 Malik FA,Sanders AJ,Jiang WG.KAI-1/CD82,the molecule and clinical implication in cancer and cancer metastasis.
2009; 24: 519-530 [PMID: 19224455 DOI: 10.14670/HH-24.519]
3 Dong JT,Isaacs WB,Barrett JC,Isaacs JT.Genomic organization of the human KAI1 metastasis-suppressor gene.
1997; 41: 25-32 [PMID: 9126478 DOI: 10.1006/geno.1997.4618]
4 Zhang XA,He B,Zhou B,Liu L.Requirement of the p130CAS-Crk coupling for metastasis suppressor KAI1/CD82-mediated inhibition of cell migration.
2003; 278: 27319-27328 [PMID: 12738793 DOI:10.1074/jbc.M303039200]
5 Tang X,Benesch MGK,Brindley DN.Role of the autotaxin-lysophosphatidate axis in the development of resistance to cancer therapy.
2020; 1865: 158716 [PMID: 32305571 DOI:10.1016/j.bbalip.2020.158716]
6 Lee HY,Bae GU,Jung ID,Lee JS,Kim YK,Noh SH,Stracke ML,Park CG,Lee HW,Han JW.Autotaxin promotes motility
G protein-coupled phosphoinositide 3-kinase gamma in human melanoma cells.
2002; 515: 137-140 [PMID: 11943209 DOI: 10.1016/s0014-5793(02)02457-2]
7 Leblanc R,Peyruchaud O.New insights into the autotaxin/LPA axis in cancer development and metastasis.
2015; 333: 183-189 [PMID: 25460336 DOI: 10.1016/j.yexcr.2014.11.010]
8 Benesch MG,Tang X,Venkatraman G,Bekele RT,Brindley DN.Recent advances in targeting the autotaxinlysophosphatidate-lipid phosphate phosphatase axis in vivo.
2016; 30: 272-284 [PMID: 27533936 DOI:10.7555/JBR.30.20150058]
9 Houben AJ,Moolenaar WH.Autotaxin and LPA receptor signaling in cancer.
2011; 30: 557-565[PMID: 22002750 DOI: 10.1007/s10555-011-9319-7]
10 Stefan C,Jansen S,Bollen M.NPP-type ectophosphodiesterases: unity in diversity.
2005; 30: 542-550 [PMID: 16125936 DOI: 10.1016/j.tibs.2005.08.005]
11 Nishimasu H,Okudaira S,Hama K,Mihara E,Dohmae N,Inoue A,Ishitani R,Takagi J,Aoki J,Nureki O.Crystal structure of autotaxin and insight into GPCR activation by lipid mediators.
2011; 18: 205-212 [PMID:21240269 DOI: 10.1038/nsmb.1998]
12 Boutin JA,Ferry G.Autotaxin.
2009; 66: 3009-3021 [PMID: 19506801 DOI:10.1007/s00018-009-0056-9]
13 Hashimoto T,Okudaira S,Igarashi K,Hama K,Yatomi Y,Aoki J.Identification and biochemical characterization of a novel autotaxin isoform,ATXδ,with a four-amino acid deletion.
2012; 151: 89-97 [PMID: 21994952 DOI:10.1093/jb/mvr126]
14 Li H,Zhao Z,Wei G,Yan L,Wang D,Zhang H,Sandusky GE,Turk J,Xu Y.Group VIA phospholipase A2 in both host and tumor cells is involved in ovarian cancer development.
2010; 24: 4103-4116 [PMID: 20530749 DOI:10.1096/fj.10-161356]
15 Zhao X,Wang D,Zhao Z,Xiao Y,Sengupta S,Zhang R,Lauber K,Wesselborg S,Feng L,Rose TM,Shen Y,Zhang J,Prestwich G,Xu Y.Caspase-3-dependent activation of calcium-independent phospholipase A2 enhances cell migration in non-apoptotic ovarian cancer cells.
2006; 281: 29357-29368 [PMID: 16882668 DOI:10.1074/jbc.M513105200]
16 Fourcade O,Simon MF,Viodé C,Rugani N,Leballe F,Ragab A,Fournié B,Sarda L,Chap H.Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells.
1995; 80: 919-927 [PMID: 7697722 DOI: 10.1016/0092-8674(95)90295-3]
17 Moolenaar WH.Lysophosphatidic acid,a multifunctional phospholipid messenger.
1995; 270: 12949-12952[PMID: 7768880 DOI: 10.1074/jbc.270.22.12949]
18 Budnik LT.Lysophosphatidic acid,LPA: a bad boy becomes good.
2003; 1: 37 [PMID:12740030 DOI: 10.1186/1477-7827-1-37]
19 Wang FQ,Ariztia EV,Boyd LR,Horton FR,Smicun Y,Hetherington JA,Smith PJ,Fishman DA.Lysophosphatidic acid(LPA) effects on endometrial carcinoma in vitro proliferation,invasion,and matrix metalloproteinase activity.
2010; 117: 88-95 [PMID: 20056268 DOI: 10.1016/j.ygyno.2009.12.012]
20 Aoki J.Mechanisms of lysophosphatidic acid production.
2004; 15: 477-489 [PMID: 15271293 DOI:10.1016/j.semcdb.2004.05.001]
21 Aoki J,Inoue A,Okudaira S.Two pathways for lysophosphatidic acid production.
2008; 1781:513-518 [PMID: 18621144 DOI: 10.1016/j.bbalip.2008.06.005]
22 Yung YC,Stoddard NC,Chun J.LPA receptor signaling: pharmacology,physiology,and pathophysiology.
2014; 55: 1192-1214 [PMID: 24643338 DOI: 10.1194/jlr.R046458]
23 Zhang G,Cheng Y,Zhang Q,Li X,Zhou J,Wang J,Wei L.ATXLPA axis facilitates estrogeninduced endometrial cancer cell proliferation
MAPK/ERK signaling pathway.
2018; 17: 4245-4252 [PMID: 29328374 DOI:10.3892/mmr.2018.8392]
24 Riaz A,Huang Y,Johansson S.G-Protein-Coupled Lysophosphatidic Acid Receptors and Their Regulation of AKT Signaling.
2016; 17: 215 [PMID: 26861299 DOI: 10.3390/ijms17020215]
25 Valdés-Rives SA,González-Arenas A.Autotaxin-Lysophosphatidic Acid: From Inflammation to Cancer Development.
2017; 2017: 9173090 [PMID: 29430083 DOI: 10.1155/2017/9173090]
26 Jackson P,Marreiros A,Russell PJ.KAI1 tetraspanin and metastasis suppressor.
2005; 37: 530-534 [PMID: 15618009 DOI: 10.1016/j.biocel.2004.08.009]
27 Zhu J,Miao C,Liu S,Tian Y,Zhang C,Liang C,Xu A,Cao Q,Wang Z.Prognostic role of CD82/KAI1 in multiple human malignant neoplasms: a meta-analysis of 31 studies.
2017; 10: 5805-5816 [PMID: 29263677 DOI: 10.2147/OTT.S150349]
28 Waterhouse R,Ha C,Dveksler GS.Murine CD9 is the receptor for pregnancy-specific glycoprotein 17.
2002;195: 277-282 [PMID: 11805154 DOI: 10.1084/jem.20011741]
29 Crotta S,Stilla A,Wack A,D'Andrea A,Nuti S,D'Oro U,Mosca M,Filliponi F,Brunetto RM,Bonino F,Abrignani S,Valiante NM.Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein.
2002; 195: 35-41 [PMID: 11781363 DOI: 10.1084/jem.20011124]
30 Nojima Y,Hirose T,Tachibana K,Tanaka T,Shi L,Doshen J,Freeman GJ,Schlossman SF,Morimoto C.The 4F9 antigen is a member of the tetra spans transmembrane protein family and functions as an accessory molecule in T cell activation and adhesion.
1993; 152: 249-260 [PMID: 8242765 DOI: 10.1006/cimm.1993.1285]
31 Ono M,Handa K,Withers DA,Hakomori S.Motility inhibition and apoptosis are induced by metastasis-suppressing gene product CD82 and its analogue CD9,with concurrent glycosylation.
1999; 59: 2335-2339 [PMID: 10344740]
32 Lee JH,Seo YW,Park SR,Kim YJ,Kim KK.Expression of a splice variant of KAI1,a tumor metastasis suppressor gene,influences tumor invasion and progression.
2003; 63: 7247-7255 [PMID: 14612520]
33 Iwata S,Kobayashi H,Miyake-Nishijima R,Sasaki T,Souta-Kuribara A,Nori M,Hosono O,Kawasaki H,Tanaka H,Morimoto C.Distinctive signaling pathways through CD82 and beta1 integrins in human T cells.
2002; 32:1328-1337 [PMID: 11981820 DOI: 10.1002/1521-4141(200205)32:5<1328::AID-IMMU1328>3.0.CO;2-6]
34 Mannion BA,Berditchevski F,Kraeft SK,Chen LB,Hemler ME.Transmembrane-4 superfamily proteins CD81 (TAPA-1),CD82,CD63,and CD53 specifically associated with integrin alpha 4 beta 1 (CD49d/CD29).
1996; 157:2039-2047 [PMID: 8757325]
35 Sugiura T,Berditchevski F.Function of alpha3beta1-tetraspanin protein complexes in tumor cell invasion.Evidence for the role of the complexes in production of matrix metalloproteinase 2 (MMP-2).
1999; 146: 1375-1389 [PMID:10491398 DOI: 10.1083/jcb.146.6.1375]
36 Odintsova E,Sugiura T,Berditchevski F.Attenuation of EGF receptor signaling by a metastasis suppressor,the tetraspanin CD82/KAI-1.
2000; 10: 1009-1012 [PMID: 10985391 DOI: 10.1016/s0960-9822(00)00652-7]
37 Liu WM,Zhang XA.KAI1/CD82,a tumor metastasis suppressor.
2006; 240: 183-194 [PMID: 16260083 DOI: 10.1016/j.canlet.2005.08.018]
38 Brindley DN,Lin FT,Tigyi GJ.Role of the autotaxin-lysophosphatidate axis in cancer resistance to chemotherapy and radiotherapy.
2013; 1831: 74-85 [PMID: 22954454 DOI: 10.1016/j.bbalip.2012.08.015]
39 Bekele R,David S.Role of autotaxin and lysophosphatidate in cancer progression and resistance to chemotherapy and radiotherapy.
2012; 7: 313-328 [DOI: 10.2217/clp.12.30]
40 Tokumura A,Majima E,Kariya Y,Tominaga K,Kogure K,Yasuda K,Fukuzawa K.Identification of human plasma lysophospholipase D,a lysophosphatidic acid-producing enzyme,as autotaxin,a multifunctional phosphodiesterase.
2002; 277: 39436-39442 [PMID: 12176993 DOI: 10.1074/jbc.M205623200]
41 Umezu-Goto M,Kishi Y,Taira A,Hama K,Dohmae N,Takio K,Yamori T,Mills GB,Inoue K,Aoki J,Arai H.Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production.
2002; 158: 227-233 [PMID: 12119361 DOI: 10.1083/jcb.200204026]
42 Gotoh M,Fujiwara Y,Yue J,Liu J,Lee S,Fells J,Uchiyama A,Murakami-Murofushi K,Kennel S,Wall J,Patil R,Gupte R,Balazs L,Miller DD,Tigyi GJ.Controlling cancer through the autotaxin-lysophosphatidic acid receptor axis.
2012; 40: 31-36 [PMID: 22260662 DOI: 10.1042/BST20110608]
43 Kawagoe H,Stracke ML,Nakamura H,Sano K.Expression and transcriptional regulation of the PD-Ialpha/autotaxin gene in neuroblastoma.
1997; 57: 2516-2521 [PMID: 9192834]
44 Hoelzinger DB,Mariani L,Weis J,Woyke T,Berens TJ,McDonough WS,Sloan A,Coons SW,Berens ME.Gene expression profile of glioblastoma multiforme invasive phenotype points to new therapeutic targets.
2005; 7: 7-16 [PMID: 15720813 DOI: 10.1593/neo.04535]
45 Kostadinova L,Shive CL,Anthony DD.Elevated Autotaxin and LPA Levels During Chronic Viral Hepatitis and Hepatocellular Carcinoma Associate with Systemic Immune Activation.
2019; 11 [PMID: 31769428 DOI: 10.3390/cancers11121867]
46 Masuda A,Nakamura K,Izutsu K,Igarashi K,Ohkawa R,Jona M,Higashi K,Yokota H,Okudaira S,Kishimoto T,Watanabe T,Koike Y,Ikeda H,Kozai Y,Kurokawa M,Aoki J,Yatomi Y.Serum autotaxin measurement in haematological malignancies: a promising marker for follicular lymphoma.
2008; 143: 60-70 [PMID:18710386 DOI: 10.1111/j.1365-2141.2008.07325.x]
47 Stracke ML,Krutzsch HC,Unsworth EJ,Arestad A,Cioce V,Schiffmann E,Liotta LA.Identification,purification,and partial sequence analysis of autotaxin,a novel motility-stimulating protein.
1992; 267: 2524-2529 [PMID:1733949]
48 Stassar MJ,Devitt G,Brosius M,Rinnab L,Prang J,Schradin T,Simon J,Petersen S,Kopp-Schneider A,Zöller M.Identification of human renal cell carcinoma associated genes by suppression subtractive hybridization.
2001;85: 1372-1382 [PMID: 11720477 DOI: 10.1054/bjoc.2001.2074]
49 Kehlen A,Englert N,Seifert A,Klonisch T,Dralle H,Langner J,Hoang-Vu C.Expression,regulation and function of autotaxin in thyroid carcinomas.
2004; 109: 833-838 [PMID: 15027116 DOI: 10.1002/ijc.20022]
50 Yang Y,Mou Lj,Liu N,Tsao MS.Autotaxin expression in non-small-cell lung cancer.
1999;21: 216-222 [PMID: 10423404 DOI: 10.1165/ajrcmb.21.2.3667]
51 Su SC,Hu X,Kenney PA,Merrill MM,Babaian KN,Zhang XY,Maity T,Yang SF,Lin X,Wood CG.Autotaxinlysophosphatidic acid signaling axis mediates tumorigenesis and development of acquired resistance to sunitinib in renal cell carcinoma.
2013; 19: 6461-6472 [PMID: 24122794 DOI: 10.1158/1078-0432.CCR-13-1284]
52 Federico L,Ren H,Mueller PA,Wu T,Liu S,Popovic J,Blalock EM,Sunkara M,Ovaa H,Albers HM,Mills GB,Morris AJ,Smyth SS.Autotaxin and its product lysophosphatidic acid suppress brown adipose differentiation and promote dietinduced obesity in mice.
2012; 26: 786-797 [PMID: 22474126 DOI: 10.1210/me.2011-1229]
53 Benesch MG,Tang X,Dewald J,Dong WF,Mackey JR,Hemmings DG,McMullen TP,Brindley DN.Tumor-induced inflammation in mammary adipose tissue stimulates a vicious cycle of autotaxin expression and breast cancer progression.
2015; 29: 3990-4000 [PMID: 26071407 DOI: 10.1096/fj.15-274480]
54 Benesch MGK,MacIntyre ITK,McMullen TPW,Brindley DN.Coming of Age for Autotaxin and Lysophosphatidate Signaling: Clinical Applications for Preventing,Detecting and Targeting Tumor-Promoting Inflammation.
2018; 10 [PMID: 29543710 DOI: 10.3390/cancers10030073]
55 Benesch MG,Ko YM,McMullen TP,Brindley DN.Autotaxin in the crosshairs: taking aim at cancer and other inflammatory conditions.
2014; 588: 2712-2727 [PMID: 24560789 DOI: 10.1016/j.febslet.2014.02.009]
56 Park GY,Lee YG,Berdyshev E,Nyenhuis S,Du J,Fu P,Gorshkova IA,Li Y,Chung S,Karpurapu M,Deng J,Ranjan R,Xiao L,Jaffe HA,Corbridge SJ,Kelly EA,Jarjour NN,Chun J,Prestwich GD,Kaffe E,Ninou I,Aidinis V,Morris AJ,Smyth SS,Ackerman SJ,Natarajan V,Christman JW.Autotaxin production of lysophosphatidic acid mediates allergic asthmatic inflammation.
2013; 188: 928-940 [PMID: 24050723 DOI:10.1164/rccm.201306-1014OC]
57 Benesch MG,Zhao YY,Curtis JM,McMullen TP,Brindley DN.Regulation of autotaxin expression and secretion by lysophosphatidate and sphingosine 1-phosphate.
2015; 56: 1134-1144 [PMID: 25896349 DOI:10.1194/jlr.M057661]
58 Balogh A,Shimizu Y,Lee SC,Norman DD,Gangwar R,Bavaria M,Moon C,Shukla P,Rao R,Ray R,Naren AP,Banerjee S,Miller DD,Balazs L,Pelus L,Tigyi G.The autotaxin-LPA2 GPCR axis is modulated by γ-irradiation and facilitates DNA damage repair.
2015; 27: 1751-1762 [PMID: 26027517 DOI: 10.1016/j.cellsig.2015.05.015]
59 Meng G,Tang X,Yang Z,Benesch MGK,Marshall A,Murray D,Hemmings DG,Wuest F,McMullen TPW,Brindley DN.Implications for breast cancer treatment from increased autotaxin production in adipose tissue after radiotherapy.
2017; 31: 4064-4077 [PMID: 28539367 DOI: 10.1096/fj.201700159R]
60 van Corven EJ,Groenink A,Jalink K,Eichholtz T,Moolenaar WH.Lysophosphatidate-induced cell proliferation:identification and dissection of signaling pathways mediated by G proteins.
1989; 59: 45-54 [PMID: 2551506 DOI:10.1016/0092-8674(89)90868-4]
61 Merchant TE,Kasimos JN,de Graaf PW,Minsky BD,Gierke LW,Glonek T.Phospholipid profiles of human colon cancer using 31P magnetic resonance spectroscopy.
1991; 6: 121-126 [PMID: 1875121 DOI:10.1007/BF00300208]
62 Xu Y,Gaudette DC,Boynton JD,Frankel A,Fang XJ,Sharma A,Hurteau J,Casey G,Goodbody A,Mellors A.Characterization of an ovarian cancer activating factor in ascites from ovarian cancer patients.
1995; 1:1223-1232 [PMID: 9815916]
63 Deng W,Wang DA,Gosmanova E,Johnson LR,Tigyi G.LPA protects intestinal epithelial cells from apoptosis by inhibiting the mitochondrial pathway.
2003; 284: G821-G829 [PMID: 12684213 DOI: 10.1152/ajpgi.00406.2002]
64 Sui Y,Yang Y,Wang J,Li Y,Ma H,Cai H,Liu X,Zhang Y,Wang S,Li Z,Zhang X,Liu R,Yan Y,Xue C,Shi X,Tan L,Ren J.Lysophosphatidic Acid Inhibits Apoptosis Induced by Cisplatin in Cervical Cancer Cells.
2015;2015: 598386 [PMID: 26366416 DOI: 10.1155/2015/598386]
65 Sutphen R,Xu Y,Wilbanks GD,Fiorica J,Grendys EC Jr,LaPolla JP,Arango H,Hoffman MS,Martino M,Wakeley K,Griffin D,Blanco RW,Cantor AB,Xiao YJ,Krischer JP.Lysophospholipids are potential biomarkers of ovarian cancer.
2004; 13: 1185-1191 [PMID: 15247129]
66 Kim KS,Sengupta S,Berk M,Kwak YG,Escobar PF,Belinson J,Mok SC,Xu Y.Hypoxia enhances lysophosphatidic acid responsiveness in ovarian cancer cells and lysophosphatidic acid induces ovarian tumor metastasis in vivo.
2006; 66: 7983-7990 [PMID: 16912173 DOI: 10.1158/0008-5472.CAN-05-4381]
67 Ren J,Xiao YJ,Singh LS,Zhao X,Zhao Z,Feng L,Rose TM,Prestwich GD,Xu Y.Lysophosphatidic acid is constitutively produced by human peritoneal mesothelial cells and enhances adhesion,migration,and invasion of ovarian cancer cells.
2006; 66: 3006-3014 [PMID: 16540649 DOI: 10.1158/0008-5472.CAN-05-1292]
68 Barekzi E,Roman J,Hise K,Georas S,Steinke JW.Lysophosphatidic acid stimulates inflammatory cascade in airway epithelial cells.
2006; 74: 357-363 [PMID: 16725318 DOI:10.1016/j.plefa.2006.03.004]
69 Spangelo BL,Jarvis WD.Lysophosphatidylcholine stimulates interleukin-6 release from rat anterior pituitary cells in vitro.
1996; 137: 4419-4426 [PMID: 8828503 DOI: 10.1210/endo.137.10.8828503]
70 Seufferlein T,Rozengurt E.Lysophosphatidic acid stimulates tyrosine phosphorylation of focal adhesion kinase,paxillin,and p130.Signaling pathways and cross-talk with platelet-derived growth factor.
1994; 269: 9345-9351[PMID: 7510708]
71 Korkina O,Dong Z,Marullo A,Warshaw G,Symons M,Ruggieri R.The MLK-related kinase (MRK) is a novel RhoC effector that mediates lysophosphatidic acid (LPA)-stimulated tumor cell invasion.
2013; 288: 5364-5373[PMID: 23319595 DOI: 10.1074/jbc.M112.414060]
72 Bian D,Mahanivong C,Yu J,Frisch SM,Pan ZK,Ye RD,Huang S.The G12/13-RhoA signaling pathway contributes to efficient lysophosphatidic acid-stimulated cell migration.
2006; 25: 2234-2244 [PMID: 16301993 DOI:10.1038/sj.onc.1209261]
73 Lee SC,Dacheux MA,Norman DD,Balázs L,Torres RM,Augelli-Szafran CE,Tigyi GJ.Regulation of Tumor Immunity by Lysophosphatidic Acid.
2020; 12 [PMID: 32397679 DOI: 10.3390/cancers12051202]
74 Chatterjee I,Humtsoe JO,Kohler EE,Sorio C,Wary KK.Lipid phosphate phosphatase-3 regulates tumor growth
βcatenin and CYCLIN-D1 signaling.
2011; 10: 51 [PMID: 21569306 DOI: 10.1186/1476-4598-10-51]
75 Samadi N,Bekele R,Capatos D,Venkatraman G,Sariahmetoglu M,Brindley DN.Regulation of lysophosphatidate signaling by autotaxin and lipid phosphate phosphatases with respect to tumor progression,angiogenesis,metastasis and chemo-resistance.
2011; 93: 61-70 [PMID: 20709140 DOI: 10.1016/j.biochi.2010.08.002]
76 Pilquil C,Dewald J,Cherney A,Gorshkova I,Tigyi G,English D,Natarajan V,Brindley DN.Lipid phosphate phosphatase-1 regulates lysophosphatidate-induced fibroblast migration by controlling phospholipase D2-dependent phosphatidate generation.
2006; 281: 38418-38429 [PMID: 17057224 DOI: 10.1074/jbc.M601670200]
77 Tanyi JL,Hasegawa Y,Lapushin R,Morris AJ,Wolf JK,Berchuck A,Lu K,Smith DI,Kalli K,Hartmann LC,McCune K,Fishman D,Broaddus R,Cheng KW,Atkinson EN,Yamal JM,Bast RC,Felix EA,Newman RA,Mills GB.Role of decreased levels of lipid phosphate phosphatase-1 in accumulation of lysophosphatidic acid in ovarian cancer.
2003; 9: 3534-3545 [PMID: 14506139]
78 Minami K,Ueda N,Ishimoto K,Tsujiuchi T.Lysophosphatidic acid receptor-2 (LPA
)-mediated signaling enhances chemoresistance in melanoma cells treated with anticancer drugs.
2020; 469: 89-95 [PMID: 32301060 DOI: 10.1007/s11010-020-03730-w]
79 Murph MM,Hurst-Kennedy J,Newton V,Brindley DN,Radhakrishna H.Lysophosphatidic acid decreases the nuclear localization and cellular abundance of the p53 tumor suppressor in A549 lung carcinoma cells.
2007; 5:1201-1211 [PMID: 18025263 DOI: 10.1158/1541-7786.MCR-06-0338]
80 Marshall JC,Collins J,Marino N,Steeg P.The Nm23-H1 metastasis suppressor as a translational target.
2010; 46: 1278-1282 [PMID: 20304626 DOI: 10.1016/j.ejca.2010.02.042]
81 Stadler CR,Knyazev P,Bange J,Ullrich A.FGFR4 GLY388 isotype suppresses motility of MDA-MB-231 breast cancer cells by EDG-2 gene repression.
2006; 18: 783-794 [PMID: 16109476 DOI: 10.1016/j.cellsig.2005.07.002]
82 Valdés-Rives SA,de la Fuente-Granada M,Velasco-Velázquez MA,González-Flores O,González-Arenas A.LPA
receptor activation induces PKCα nuclear translocation in glioblastoma cells.
2019; 110: 91-102[PMID: 30849522 DOI: 10.1016/j.biocel.2019.03.003]
83 Lin YC,Chen CC,Chen WM,Lu KY,Shen TL,Jou YC,Shen CH,Ohbayashi N,Kanaho Y,Huang YL,Lee H.LPA1/3 signaling mediates tumor lymphangiogenesis through promoting CRT expression in prostate cancer.
2018; 1863: 1305-1315 [PMID: 30053596 DOI: 10.1016/j.bbalip.2018.07.005]
84 Kitayama J,Shida D,Sako A,Ishikawa M,Hama K,Aoki J,Arai H,Nagawa H.Over-expression of lysophosphatidic acid receptor-2 in human invasive ductal carcinoma.
2004; 6: R640-R646 [PMID: 15535846 DOI:10.1186/bcr935]
85 Li M,Xiao D,Zhang J,Qu H,Yang Y,Yan Y,Liu X,Wang J,Liu L,Duan X.Expression of LPA2 is associated with poor prognosis in human breast cancer and regulates HIF-1α expression and breast cancer cell growth.
2016;36: 3479-3487 [PMID: 27805252 DOI: 10.3892/or.2016.5206]
86 Enooku K,Uranbileg B,Ikeda H,Kurano M,Sato M,Kudo H,Maki H,Koike K,Hasegawa K,Kokudo N,Yatomi Y.Higher LPA2 and LPA6 mRNA Levels in Hepatocellular Carcinoma Are Associated with Poorer Differentiation,Microvascular Invasion and Earlier Recurrence with Higher Serum Autotaxin Levels.
2016; 11: e0161825[PMID: 27583415 DOI: 10.1371/journal.pone.0161825]
87 Ishimoto K,Minami A,Minami K,Ueda N,Tsujiuchi T.Different effects of lysophosphatidic acid receptor-2 (LPA2)and LPA5 on the regulation of chemoresistance in colon cancer cells.
2021; 41: 93-98[PMID: 32672083 DOI: 10.1080/10799893.2020.1794002]
88 Ren Z,Zhang C,Ma L,Zhang X,Shi S,Tang D,Xu J,Hu Y,Wang B,Zhang F,Zheng H.Lysophosphatidic acid induces the migration and invasion of SGC-7901 gastric cancer cells through the LPA2 and Notch signaling pathways.
2019; 44: 67-78 [PMID: 31115486 DOI: 10.3892/ijmm.2019.4186]
89 Dong S,Li GX,Fang JH,Chen X,Sun YT.Advances in understanding of relationship between Hhip and Lpar2 gene expression and gastric cancer.
2021; 29: 1049-1054 [DOI: 10.11569/wcjd.v29.i18.1049]
90 Xu J,Lai YJ,Lin WC,Lin FT.TRIP6 enhances lysophosphatidic acid-induced cell migration by interacting with the lysophosphatidic acid 2 receptor.
2004; 279: 10459-10468 [PMID: 14688263 DOI:10.1074/jbc.M311891200]
91 Lin FT,Lai YJ.Regulation of the LPA2 receptor signaling through the carboxyl-terminal tail-mediated protein-protein interactions.
2008; 1781: 558-562 [PMID: 18501721 DOI: 10.1016/j.bbalip.2008.04.013]
92 Zhao P,Yun Q,Li R,Yan Y,Wang Y,Sun H,Damirin A.LPA3 is a precise therapeutic target and potential biomarker for ovarian cancer.
2022; 39: 17 [PMID: 34982278 DOI: 10.1007/s12032-021-01616-5]
93 Hayashi M,Okabe K,Yamawaki Y,Teranishi M,Honoki K,Mori T,Fukushima N,Tsujiuchi T.Loss of lysophosphatidic acid receptor-3 enhances cell migration in rat lung tumor cells.
2011;405: 450-454 [PMID: 21255556 DOI: 10.1016/j.bbrc.2011.01.051]
94 Kitayoshi M,Fukui R,Tanabe E,Kato K,Yoshikawa K,Fukushima N,Tsujiuchi T.Different effects on cell proliferation and migration abilities of endothelial cells by LPA
and LPA
in mammary tumor FM3A cells.
2012; 32: 209-213 [PMID: 22686188 DOI: 10.3109/10799893.2012.692121]
95 Sun K,Cai H,Duan X,Yang Y,Li M,Qu J,Zhang X,Wang J.Aberrant expression and potential therapeutic target of lysophosphatidic acid receptor 3 in triple-negative breast cancers.
2015; 15: 371-380 [PMID: 25209561 DOI: 10.1007/s10238-014-0306-5]
96 Fang X,Yu S,Bast RC,Liu S,Xu HJ,Hu SX,LaPushin R,Claret FX,Aggarwal BB,Lu Y,Mills GB.Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells.
2004; 279: 9653-9661 [PMID:14670967 DOI: 10.1074/jbc.M306662200]
97 Hasegawa Y,Murph M,Yu S,Tigyi G,Mills GB.Lysophosphatidic acid (LPA)-induced vasodilator-stimulated phosphoprotein mediates lamellipodia formation to initiate motility in PC-3 prostate cancer cells.
2008; 2: 54-69 [PMID: 19081821 DOI: 10.1016/j.molonc.2008.03.009]
98 Takara K,Eino D,Ando K,Yasuda D,Naito H,Tsukada Y,Iba T,Wakabayashi T,Muramatsu F,Kidoya H,Fukuhara S,Mochizuki N,Ishii S,Kishima H,Takakura N.Lysophosphatidic Acid Receptor 4 Activation Augments Drug Delivery in Tumors by Tightening Endothelial Cell-Cell Contact.
2017; 20: 2072-2086 [PMID: 28854359 DOI:10.1016/j.celrep.2017.07.080]
99 Araki M,Kitayoshi M,Dong Y,Hirane M,Ozaki S,Mori S,Fukushima N,Honoki K,Tsujiuchi T.Inhibitory effects of lysophosphatidic acid receptor-5 on cellular functions of sarcoma cells.
2014; 32: 117-122 [PMID:24798396 DOI: 10.3109/08977194.2014.911294]
100 Tsujino M,Fujii M,Okabe K,Mori T,Fukushima N,Tsujiuchi T.Differential expressions and DNA methylation patterns of lysophosphatidic acid receptor genes in human colon cancer cells.
2010; 457: 669-676 [PMID:20890765 DOI: 10.1007/s00428-010-0960-2]
101 Kimura T,Mogi C,Sato K,Tomura H,Ohta H,Im DS,Kuwabara A,Kurose H,Murakami M,Okajima F.p2y5/LPA(6)attenuates LPA(1)-mediated VE-cadherin translocation and cell-cell dissociation through G(12/13) protein-Src-Rap1.
2011; 92: 149-158 [PMID: 21632882 DOI: 10.1093/cvr/cvs087]
102 Mukherjee A,Wu J,Barbour S,Fang X.Lysophosphatidic acid activates lipogenic pathways and de novo lipid synthesis in ovarian cancer cells.
2012; 287: 24990-25000 [PMID: 22665482 DOI: 10.1074/jbc.M112.340083]
103 Fukushima N,Ishii S,Tsujiuchi T,Kagawa N,Katoh K.Comparative analyses of lysophosphatidic acid receptormediated signaling.
2015; 72: 2377-2394 [PMID: 25732591 DOI: 10.1007/s00018-015-1872-8]
104 Wu DH,Liu L,Chen LH,Ding YQ.Expression of KAI1/CD82 in human colorectal tumor.
2003; 23: 714-715,719 [PMID: 12865229]
105 Guo X,Friess H,Graber HU,Kashiwagi M,Zimmermann A,Korc M,Büchler MW.KAI1 expression is up-regulated in early pancreatic cancer and decreased in the presence of metastases.
1996; 56: 4876-4880 [PMID: 8895737]
106 Friess H,Guo XZ,Berberat P,Graber HU,Zimmermann A,Korc M,Büchler MW.Reduced KAI1 expression in pancreatic cancer is associated with lymph node and distant metastases.
1998; 79: 349-355 [PMID: 9699525 DOI: 10.1002/(sici)1097-0215(19980821)79:4<349::aid-ijc7>3.0.co;2-v]
107 Xu JH,Guo XZ,Ren LN,Shao LC,Liu MP.KAI1 is a potential target for anti-metastasis in pancreatic cancer cells.
2008; 14: 1126-1132 [PMID: 18286698 DOI: 10.3748/wjg.14.1126]
108 Liu X,Guo XZ,Zhang WW,Lu ZZ,Zhang QW,Duan HF,Wang LS.KAI1 inhibits HGF-induced invasion of pancreatic cancer by sphingosine kinase activity.
2011; 10: 201-208 [PMID: 21459729 DOI:10.1016/s1499-3872(11)60032-5]
109 Li H,Li J,Liu X,Chen J,Wu C,Guo X.Effect of PTEN and KAI1 gene overexpression on the proliferation,metastasis and radiosensitivity of ASPC1 pancreatic cancer cells under hypoxic conditions.
2014; 10: 1973-1977[PMID: 25051346 DOI: 10.3892/mmr.2014.2404]
110 Liu X,Guo XZ,Li HY,Chen J,Ren LN,Wu CY.KAI1 inhibits lymphangiogenesis and lymphatic metastasis of pancreatic cancer in vivo.
2014; 13: 87-92 [PMID: 24463085 DOI:10.1016/s1499-3872(14)60012-6]
111 Wu CY,Guo XZ,Li HY.Hypoxia and Serum deprivation protected MiaPaCa-2 cells from KAI1-induced proliferation inhibition through autophagy pathway activation in solid tumors.
2015; 17: 201-208 [PMID: 25199507 DOI: 10.1007/s12094-014-1211-9]
112 Mashimo T,Watabe M,Hirota S,Hosobe S,Miura K,Tegtmeyer PJ,Rinker-Shaeffer CW,Watabe K.The expression of the KAI1 gene,a tumor metastasis suppressor,is directly activated by p53.
1998; 95: 11307-11311 [PMID: 9736732 DOI: 10.1073/pnas.95.19.11307]
113 Tang ZY.Hepatocellular carcinoma surgery-review of the past and prospects for the 21
century.
2005; 91:95-96 [PMID: 16028278 DOI: 10.1002/jso.20291]
114 Zhang W,Zhao CG,Sun HY,Zheng WE,Chen H.Expression characteristics of KAI1 and vascular endothelial growth factor and their diagnostic value for hepatocellular carcinoma.
2014; 8: 536-542 [PMID: 25071074 DOI:10.5009/gn13331]
115 Mu Z,Wang H,Zhang J,Li Q,Wang L,Guo X.KAI1/CD82 suppresses hepatocyte growth factor-induced migration of hepatoma cells
upregulation of Sprouty2.
2008; 51: 648-654 [PMID: 18622748 DOI:10.1007/s11427-008-0086-1]
116 Guo C,Liu Q,Zhang L,Yang X,Song T,Yao Y.Double lethal effects of fusion gene of wild-type p53 and JunB on hepatocellular carcinoma cells.
2012; 32: 663-668 [PMID: 23259178 DOI:10.1007/s11596-012-1014-6]
117 Si SH,Yang JM,Peng ZH,Luo YH,Zhou P.Effects of KAI1 gene on growth and invasion of human hepatocellular carcinoma MHCC97-H cells.
2004; 10: 2019-2023 [PMID: 15237426 DOI:10.3748/wjg.v10.i14.2019]
118 Yang JM,Peng ZH,Si SH,Liu WW,Luo YH,Ye ZY.KAI1 gene suppresses invasion and metastasis of hepatocellular carcinoma MHCC97-H cells in vitro and in animal models.
2008; 28: 132-139 [PMID: 18028322 DOI:10.1111/j.1478-3231.2007.01620.x]
119 Xu J,Zhang Y,Wang Y,Tao X,Cheng L,Wu S,Tao Y.Correlation of KAI1,CD133 and vasculogenic mimicry with the prediction of metastasis and prognosis in hepatocellular carcinoma.
2018; 11: 3638-3646 [PMID:31949744]
120 Lai JF,Xu WN,Noh SH,Lu WQ.Effect of World Health Organization (WHO) Histological Classification on Predicting Lymph Node Metastasis and Recurrence in Early Gastric Cancer.
2016; 22: 3147-3153 [PMID: 27595490 DOI: 10.12659/msm.897311]
121 Ang TL,Fock KM.Clinical epidemiology of gastric cancer.
2014; 55: 621-628 [PMID: 25630323 DOI:10.11622/smedj.2014174]
122 Ilhan O,Celik SY,Han U,Onal B.Use of KAI-1 as a prognostic factor in gastric carcinoma.
2009; 21: 1369-1372 [PMID: 19506480 DOI: 10.1097/MEG.0b013e328323aac9]
123 Knoener M,Krech T,Puls F,Lehmann U,Kreipe H,Christgen M.Limited value of KAI1/CD82 protein expression as a prognostic marker in human gastric cancer.
2012; 32: 337-342 [PMID: 22684230 DOI:10.3233/DMA-2012-0896]
124 Hinoda Y,Adachi Y,Takaoka A,Mitsuuchi H,Satoh Y,Itoh F,Kondoh Y,Imai K.Decreased expression of the metastasis suppressor gene KAI1 in gastric cancer.
1998; 129: 229-234 [PMID: 9719466 DOI:10.1016/s0304-3835(98)00112-8]
125 Chen M,Towers LN,O'Connor KL.LPA2 (EDG4) mediates Rho-dependent chemotaxis with lower efficacy than LPA1(EDG2) in breast carcinoma cells.
2007; 292: C1927-C1933 [PMID: 17496233 DOI:10.1152/ajpcell.00400.2006]
126 Xu L,Hou Y,Tu G,Chen Y,Du YE,Zhang H,Wen S,Tang X,Yin J,Lang L,Sun K,Yang G,Liu M.Nuclear Drosha enhances cell invasion
an EGFR-ERK1/2-MMP7 signaling pathway induced by dysregulated miRNA-622/197 and their targets LAMC2 and CD82 in gastric cancer.
2017; 8: e2642 [PMID: 28252644 DOI:10.1038/cddis.2017.5]
127 Tsutsumi S,Shimura T,Morinaga N,Mochiki E,Asao T,Kuwano H.Loss of KAI1 expression in gastric cancer.
2005; 52: 281-284 [PMID: 15783050]
128 Zheng HC,Wang MC,Li JY,Yang XF,Sun JM,Xin Y.Expression of maspin and kai1 and their clinicopathological significance in carcinogenesis and progression of gastric cancer.
2004; 19: 193-198 [PMID: 15506646]
129 Guan-Zhen Y,Ying C,Can-Rong N,Guo-Dong W,Jian-Xin Q,Jie-Jun W.Reduced protein expression of metastasisrelated genes (nm23,KISS1,KAI1 and p53) in lymph node and liver metastases of gastric cancer.
2007;88: 175-183 [PMID: 17504447 DOI: 10.1111/j.1365-2613.2006.00510.x]
130 Zhu B,Zhou L,Yu L,Wu S,Song W,Gong X,Wang D.Evaluation of the correlation of vasculogenic mimicry,ALDH1,KAI1 and microvessel density in the prediction of metastasis and prognosis in colorectal carcinoma.
2017; 17:47 [PMID: 28431527 DOI: 10.1186/s12893-017-0246-6]
131 Lu G,Zhou L,Zhang X,Zhu B,Wu S,Song W,Gong X,Wang D,Tao Y.The expression of metastasis-associated in colon cancer-1 and KAI1 in gastric adenocarcinoma and their clinical significance.
2016; 14: 276[PMID: 27793161 DOI: 10.1186/s12957-016-1033-z]
132 Yang JL,Jackson P,Yu Y,Russell PJ,Markovic B,Crowe PJ.Expression of the KAI1 metastasis suppressor gene in non-metastatic versus metastatic human colorectal cancer.
2002; 22: 3337-3342 [PMID: 12530084]
133 Bae WK,Hong CS,Park MR,Sun EG,Lee JH,Kang K,Ryu KH,Shim HJ,Hwang JE,Cho SH,Chung IJ.TAp73 inhibits cell invasion and migration by directly activating KAI1 expression in colorectal carcinoma.
2018;415: 106-116 [PMID: 29222041 DOI: 10.1016/j.canlet.2017.12.002]
134 Hashida H,Takabayashi A,Tokuhara T,Taki T,Kondo K,Kohno N,Yamaoka Y,Miyake M.Integrin alpha3 expression as a prognostic factor in colon cancer: association with MRP-1/CD9 and KAI1/CD82.
2002; 97: 518-525[PMID: 11802216 DOI: 10.1002/ijc.1625]
135 Ryder NM,Guha S,Hines OJ,Reber HA,Rozengurt E.G protein-coupled receptor signaling in human ductal pancreatic cancer cells: neurotensin responsiveness and mitogenic stimulation.
2001; 186: 53-64 [PMID: 11147814 DOI: 10.1002/1097-4652(200101)186:1<53::AID-JCP1004>3.0.CO;2-Q]
136 Nakai Y,Ikeda H,Nakamura K,Kume Y,Fujishiro M,Sasahira N,Hirano K,Isayama H,Tada M,Kawabe T,Komatsu Y,Omata M,Aoki J,Koike K,Yatomi Y.Specific increase in serum autotaxin activity in patients with pancreatic cancer.
2011; 44: 576-581 [PMID: 21439952 DOI: 10.1016/j.clinbiochem.2011.03.128]
137 Quan M,Cui JJ,Feng X,Huang Q.The critical role and potential target of the autotaxin/lysophosphatidate axis in pancreatic cancer.
2017; 39: 1010428317694544 [PMID: 28347252 DOI: 10.1177/1010428317694544]
138 Yang S,Zhang L,Purohit V,Shukla SK,Chen X,Yu F,Fu K,Chen Y,Solheim J,Singh PK,Song W,Dong J.Active YAP promotes pancreatic cancer cell motility,invasion and tumorigenesis in a mitotic phosphorylation-dependent manner through LPAR3.
2015; 6: 36019-36031 [PMID: 26440309 DOI: 10.18632/oncotarget.5935]
139 Tveteraas IH,Aasrum M,Brusevold IJ,Ødegård J,Christoffersen T,Sandnes D.Lysophosphatidic acid induces both EGFR-dependent and EGFR-independent effects on DNA synthesis and migration in pancreatic and colorectal carcinoma cells.
2016; 37: 2519-2526 [PMID: 26386720 DOI: 10.1007/s13277-015-4010-1]
140 Gardner JA,Ha JH,Jayaraman M,Dhanasekaran DN.The gep proto-oncogene Gα13 mediates lysophosphatidic acidmediated migration of pancreatic cancer cells.
2013; 42: 819-828 [PMID: 23508014 DOI:10.1097/MPA.0b013e318279c577]
141 Liao Y,Mu G,Zhang L,Zhou W,Zhang J,Yu H.Lysophosphatidic acid stimulates activation of focal adhesion kinase and paxillin and promotes cell motility,
LPA1-3,in human pancreatic cancer.
2013; 58: 3524-3533[PMID: 24061591 DOI: 10.1007/s10620-013-2878-4]
142 Fukushima K,Otagaki S,Takahashi K,Minami K,Ishimoto K,Fukushima N,Honoki K,Tsujiuchi T.Promotion of cellinvasive activity through the induction of LPA receptor-1 in pancreatic cancer cells.
2018;38: 367-371 [PMID: 30396320 DOI: 10.1080/10799893.2018.1531889]
143 Kato K,Yoshikawa K,Tanabe E,Kitayoshi M,Fukui R,Fukushima N,Tsujiuchi T.Opposite roles of LPA1 and LPA3 on cell motile and invasive activities of pancreatic cancer cells.
2012; 33: 1739-1744 [PMID: 22678979 DOI:10.1007/s13277-012-0433-0]
144 Tsujiuchi T,Furukawa M,Obo Y,Yamasaki A,Hotta M,Kusunoki C,Suyama N,Mori T,Honoki K,Fukushima N.Infrequent mutation of lysophosphatidic Acid receptor-1 gene in hamster pancreatic duct adenocarcinomas and established cell lines.
2009; 22: 89-92 [PMID: 22271981 DOI: 10.1293/tox.22.89]
145 Komachi M,Tomura H,Malchinkhuu E,Tobo M,Mogi C,Yamada T,Kimura T,Kuwabara A,Ohta H,Im DS,Kurose H,Takeyoshi I,Sato K,Okajima F.LPA1 receptors mediate stimulation,whereas LPA2 receptors mediate inhibition,of migration of pancreatic cancer cells in response to lysophosphatidic acid and malignant ascites.
2009; 30:457-465 [PMID: 19129242 DOI: 10.1093/carcin/bgp011]
146 Yamada T,Sato K,Komachi M,Malchinkhuu E,Tobo M,Kimura T,Kuwabara A,Yanagita Y,Ikeya T,Tanahashi Y,Ogawa T,Ohwada S,Morishita Y,Ohta H,Im DS,Tamoto K,Tomura H,Okajima F.Lysophosphatidic acid (LPA) in malignant ascites stimulates motility of human pancreatic cancer cells through LPA1.
2004; 279: 6595-6605[PMID: 14660630 DOI: 10.1074/jbc.M308133200]
147 Yoshikawa K,Tanabe E,Shibata A,Inoue S,Kitayoshi M,Okimoto S,Fukushima N,Tsujiuchi T.Involvement of oncogenic K-ras on cell migration stimulated by lysophosphatidic acid receptor-2 in pancreatic cancer cells.
2013; 319: 105-112 [PMID: 23041208 DOI: 10.1016/j.yexcr.2012.09.014]
148 Ishii S,Hirane M,Fukushima K,Tomimatsu A,Fukushima N,Tsujiuchi T.Diverse effects of LPA4,LPA5 and LPA6 on the activation of tumor progression in pancreatic cancer cells.
2015; 461: 59-64 [PMID:25849892 DOI: 10.1016/j.bbrc.2015.03.169]
149 Memet I,Tsalkidou E,Tsaroucha AK,Lambropoulou M,Chatzaki E,Trypsianis G,Schizas D,Pitiakoudis M,Simopoulos C.Autotaxin Expression in Hepatocellular Carcinoma.
2018; 31: 359-365 [PMID: 28598712 DOI: 10.1080/08941939.2017.1331280]
150 Ertle J,Dechêne A,Sowa JP,Penndorf V,Herzer K,Kaiser G,Schlaak JF,Gerken G,Syn WK,Canbay A.Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis.
2011; 128:2436-2443 [PMID: 21128245 DOI: 10.1002/ijc.25797]
151 Alexander J,Torbenson M,Wu TT,Yeh MM.Non-alcoholic fatty liver disease contributes to hepatocarcinogenesis in non-cirrhotic liver: a clinical and pathological study.
2013; 28: 848-854 [PMID: 23302015 DOI:10.1111/jgh.12116]
152 Lopane C,Agosti P,Gigante I,Sabbà C,Mazzocca A.Implications of the lysophosphatidic acid signaling axis in liver cancer.
2017; 1868: 277-282 [PMID: 28591560 DOI: 10.1016/j.bbcan.2017.06.002]
153 Watanabe N,Ikeda H,Nakamura K,Ohkawa R,Kume Y,Tomiya T,Tejima K,Nishikawa T,Arai M,Yanase M,Aoki J,Arai H,Omata M,Fujiwara K,Yatomi Y.Plasma lysophosphatidic acid level and serum autotaxin activity are increased in liver injury in rats in relation to its severity.
2007; 81: 1009-1015 [PMID: 17850827 DOI:10.1016/j.lfs.2007.08.013]
154 Wu JM,Xu Y,Skill NJ,Sheng H,Zhao Z,Yu M,Saxena R,Maluccio MA.Autotaxin expression and its connection with the TNF-alpha-NF-kappaB axis in human hepatocellular carcinoma.
2010; 9: 71 [PMID: 20356387 DOI:10.1186/1476-4598-9-71]
155 Park SY,Jeong KJ,Panupinthu N,Yu S,Lee J,Han JW,Kim JM,Lee JS,Kang J,Park CG,Mills GB,Lee HY.Lysophosphatidic acid augments human hepatocellular carcinoma cell invasion through LPA1 receptor and MMP-9 expression.
2011; 30: 1351-1359 [PMID: 21102517 DOI: 10.1038/onc.2010.517]
156 Zuckerman V,Sokolov E,Swet JH,Ahrens WA,Showlater V,Iannitti DA,Mckillop IH.Expression and function of lysophosphatidic acid receptors (LPARs) 1 and 3 in human hepatic cancer progenitor cells.
2016; 7: 2951-2967 [PMID: 26701886 DOI: 10.18632/oncotarget.6696]
157 Okabe K,Hayashi M,Yamawaki Y,Teranishi M,Honoki K,Mori T,Fukushima N,Tsujiuchi T.Possible involvement of lysophosphatidic acid receptor-5 gene in the acquisition of growth advantage of rat tumor cells.
2011; 50:635-642 [PMID: 21374735 DOI: 10.1002/mc.20750]
158 Zheng X,Jia Y,Qiu L,Zeng X,Xu L,Wei M,Huang C,Liu C,Chen L,Han J.A potential target for liver cancer management,lysophosphatidic acid receptor 6 (LPAR6),is transcriptionally up-regulated by the NCOA3 coactivator.
2020; 295: 1474-1488 [PMID: 31914406 DOI: 10.1074/jbc.RA119.009899]
159 Lippolis R,Gnocchi D,Santacroce L,Siciliano RA,Mazzeo MF,Scacco S,Sabbà C,Mazzocca A.A distinctive protein signature induced by lysophosphatidic acid receptor 6 (LPAR6) expression in hepatocellular carcinoma cells.
2020; 526: 1150-1156 [PMID: 32321639 DOI: 10.1016/j.bbrc.2020.04.036]
160 Gnocchi D,Kapoor S,Nitti P,Cavalluzzi MM,Lentini G,Denora N,Sabbà C,Mazzocca A.Novel lysophosphatidic acid receptor 6 antagonists inhibit hepatocellular carcinoma growth through affecting mitochondrial function.
2020; 98: 179-191 [PMID: 31863151 DOI: 10.1007/s00109-019-01862-1]
161 Mazzocca A,Dituri F,De Santis F,Filannino A,Lopane C,Betz RC,Li YY,Mukaida N,Winter P,Tortorella C,Giannelli G,Sabbà C.Lysophosphatidic acid receptor LPAR6 supports the tumorigenicity of hepatocellular carcinoma.
2015; 75: 532-543 [PMID: 25589345 DOI: 10.1158/0008-5472.CAN-14-1607]
162 Zeng R,Li B,Huang J,Zhong M,Li L,Duan C,Zeng S,Liu W,Lu J,Tang Y,Zhou L,Liu Y,Li J,He Z,Wang Q,Dai Y.Lysophosphatidic Acid is a Biomarker for Peritoneal Carcinomatosis of Gastric Cancer and Correlates with Poor Prognosis.
2017; 21: 641-648 [PMID: 28910191 DOI: 10.1089/gtmb.2017.0060]
163 Ramachandran S,Shida D,Nagahashi M,Fang X,Milstien S,Takabe K,Spiegel S.Lysophosphatidic acid stimulates gastric cancer cell proliferation
ERK1-dependent upregulation of sphingosine kinase 1 transcription.
2010;584: 4077-4082 [PMID: 20804754 DOI: 10.1016/j.febslet.2010.08.035]
164 Shida D,Fang X,Kordula T,Takabe K,Lépine S,Alvarez SE,Milstien S,Spiegel S.Cross-talk between LPA1 and epidermal growth factor receptors mediates up-regulation of sphingosine kinase 1 to promote gastric cancer cell motility and invasion.
2008; 68: 6569-6577 [PMID: 18701480 DOI: 10.1158/0008-5472.CAN-08-0411]
165 Kim MH,Park JS,Chang HJ,Baek MK,Kim HR,Shin BA,Ahn BW,Jung YD.Lysophosphatidic acid promotes cell invasion by up-regulating the urokinase-type plasminogen activator receptor in human gastric cancer cells.
2008; 104: 1102-1112 [PMID: 18247343 DOI: 10.1002/jcb.21696]
166 Arnold M,Sierra MS,Laversanne M,Soerjomataram I,Jemal A,Bray F.Global patterns and trends in colorectal cancer incidence and mortality.
2017; 66: 683-691 [PMID: 26818619 DOI: 10.1136/gutjnl-2015-310912]
167 Kazama S,Kitayama J,Aoki J,Mori K,Nagawa H.Immunohistochemical detection of autotaxin(ATX)/lysophospholipase D (lysoPLD) in submucosal invasive colorectal cancer.
2011; 42: 204-211 [PMID: 20623382 DOI: 10.1007/s12029-010-9186-4]
168 Yang M,Zhong WW,Srivastava N,Slavin A,Yang J,Hoey T,An S.G protein-coupled lysophosphatidic acid receptors stimulate proliferation of colon cancer cells through the {beta}-catenin pathway.
2005; 102:6027-6032 [PMID: 15837931 DOI: 10.1073/pnas.0501535102]
169 Shida D,Kitayama J,Yamaguchi H,Okaji Y,Tsuno NH,Watanabe T,Takuwa Y,Nagawa H.Lysophosphatidic acid(LPA) enhances the metastatic potential of human colon carcinoma DLD1 cells through LPA1.
2003; 63:1706-1711 [PMID: 12670925]
170 Takahashi K,Fukushima K,Otagaki S,Ishimoto K,Minami K,Fukushima N,Honoki K,Tsujiuchi T.Effects of LPA
and LPA
on the regulation of colony formation activity in colon cancer cells treated with anticancer drugs.
2018; 38: 71-75 [PMID: 29369010 DOI: 10.1080/10799893.2018.1426608]
171 Lee SJ,Ritter SL,Zhang H,Shim H,Hall RA,Yun CC.MAGI-3 competes with NHERF-2 to negatively regulate LPA2 receptor signaling in colon cancer cells.
2011; 140: 924-934 [PMID: 21134377 DOI:10.1053/j.gastro.2010.11.054]
172 Yun CC,Sun H,Wang D,Rusovici R,Castleberry A,Hall RA,Shim H.LPA2 receptor mediates mitogenic signals in human colon cancer cells.
2005; 289: C2-11 [PMID: 15728708 DOI:10.1152/ajpcell.00610.2004]
173 Lin S,Wang D,Iyer S,Ghaleb AM,Shim H,Yang VW,Chun J,Yun CC.The absence of LPA2 attenuates tumor formation in an experimental model of colitis-associated cancer.
2009; 136: 1711-1720 [PMID:19328876 DOI: 10.1053/j.gastro.2009.01.002]
174 Shida D,Watanabe T,Aoki J,Hama K,Kitayama J,Sonoda H,Kishi Y,Yamaguchi H,Sasaki S,Sako A,Konishi T,Arai H,Nagawa H.Aberrant expression of lysophosphatidic acid (LPA) receptors in human colorectal cancer.
2004;84: 1352-1362 [PMID: 15220934 DOI: 10.1038/labinvest.3700146]
175 Fukui R,Tanabe E,Kitayoshi M,Yoshikawa K,Fukushima N,Tsujiuchi T.Negative regulation of cell motile and invasive activities by lysophosphatidic acid receptor-3 in colon cancer HCT116 cells.
2012; 33: 1899-1905[PMID: 22763559 DOI: 10.1007/s13277-012-0450-z]
176 Takahashi K,Fukushima K,Onishi Y,Inui K,Node Y,Fukushima N,Honoki K,Tsujiuchi T.Lysophosphatidic acid(LPA) signaling
LPA4 and LPA6 negatively regulates cell motile activities of colon cancer cells.
2017; 483: 652-657 [PMID: 27993681 DOI: 10.1016/j.bbrc.2016.12.088]
177 Custer MC,Risinger JI,Hoover S,Simpson RM,Patterson T,Barrett JC.Characterization of an antibody that can detect the Kai1/CD82 murine metastasis suppressor.
2006; 66: 567-577 [PMID: 16372335 DOI: 10.1002/pros.20386]
178 Iiizumi M,Bandyopadhyay S,Watabe K.Interaction of Duffy antigen receptor for chemokines and KAI1: a critical step in metastasis suppression.
2007; 67: 1411-1414 [PMID: 17308076 DOI: 10.1158/0008-5472.CAN-06-3801]
179 Tonoli H,Barrett JC.CD82 metastasis suppressor gene: a potential target for new therapeutics?
2005;11: 563-570 [PMID: 16271511 DOI: 10.1016/j.molmed.2005.10.002]
180 Gierse J,Thorarensen A,Beltey K,Bradshaw-Pierce E,Cortes-Burgos L,Hall T,Johnston A,Murphy M,Nemirovskiy O,Ogawa S,Pegg L,Pelc M,Prinsen M,Schnute M,Wendling J,Wene S,Weinberg R,Wittwer A,Zweifel B,Masferrer J.A novel autotaxin inhibitor reduces lysophosphatidic acid levels in plasma and the site of inflammation.
2010; 334: 310-317 [PMID: 20392816 DOI: 10.1124/jpet.110.165845]
181 North EJ,Howard AL,Wanjala IW,Pham TC,Baker DL,Parrill AL.Pharmacophore development and application toward the identification of novel,small-molecule autotaxin inhibitors.
2010; 53: 3095-3105 [PMID:20349977 DOI: 10.1021/jm901718z]
182 Bhave SR,Dadey DY,Karvas RM,Ferraro DJ,Kotipatruni RP,Jaboin JJ,Hallahan AN,Dewees TA,Linkous AG,Hallahan DE,Thotala D.Autotaxin Inhibition with PF-8380 Enhances the Radiosensitivity of Human and Murine Glioblastoma Cell Lines.
2013; 3: 236 [PMID: 24062988 DOI: 10.3389/fonc.2013.00236]
183 Schleicher SM,Thotala DK,Linkous AG,Hu R,Leahy KM,Yazlovitskaya EM,Hallahan DE.Autotaxin and LPA receptors represent potential molecular targets for the radiosensitization of murine glioma through effects on tumor vasculature.
2011; 6: e22182 [PMID: 21799791 DOI: 10.1371/journal.pone.0022182]
184 Tang X,Wuest M,Benesch MGK,Dufour J,Zhao Y,Curtis JM,Monjardet A,Heckmann B,Murray D,Wuest F,Brindley DN.Inhibition of Autotaxin with GLPG1690 Increases the Efficacy of Radiotherapy and Chemotherapy in a Mouse Model of Breast Cancer.
2020; 19: 63-74 [PMID: 31548293 DOI:10.1158/1535-7163.MCT-19-0386]
185 Saga H,Ohhata A,Hayashi A,Katoh M,Maeda T,Mizuno H,Takada Y,Komichi Y,Ota H,Matsumura N,Shibaya M,Sugiyama T,Nakade S,Kishikawa K.A novel highly potent autotaxin/ENPP2 inhibitor produces prolonged decreases in plasma lysophosphatidic acid formation in vivo and regulates urethral tension.
2014; 9: e93230 [PMID:24747415 DOI: 10.1371/journal.pone.0093230]
186 Venkatraman G,Benesch MG,Tang X,Dewald J,McMullen TP,Brindley DN.Lysophosphatidate signaling stabilizes Nrf2 and increases the expression of genes involved in drug resistance and oxidative stress responses: implications for cancer treatment.
2015; 29: 772-785 [PMID: 25398768 DOI: 10.1096/fj.14-262659]
187 Benesch MG,Tang X,Maeda T,Ohhata A,Zhao YY,Kok BP,Dewald J,Hitt M,Curtis JM,McMullen TP,Brindley DN.Inhibition of autotaxin delays breast tumor growth and lung metastasis in mice.
2014; 28: 2655-2666[PMID: 24599971 DOI: 10.1096/fj.13-248641]
188 Kehlen A,Lauterbach R,Santos AN,Thiele K,Kabisch U,Weber E,Riemann D,Langner J.IL-1 beta- and IL-4-induced down-regulation of autotaxin mRNA and PC-1 in fibroblast-like synoviocytes of patients with rheumatoid arthritis (RA).
2001; 123: 147-154 [PMID: 11168012 DOI: 10.1046/j.1365-2249.2001.01432.x]
189 Xia Q,Deng AM,Wu SS,Zheng M.Cholera toxin inhibits human hepatocarcinoma cell proliferation in vitro
suppressing ATX/LPA axis.
2011; 32: 1055-1062 [PMID: 21765444 DOI: 10.1038/aps.2011.31]
190 Gupte R,Patil R,Liu J,Wang Y,Lee SC,Fujiwara Y,Fells J,Bolen AL,Emmons-Thompson K,Yates CR,Siddam A,Panupinthu N,Pham TC,Baker DL,Parrill AL,Mills GB,Tigyi G,Miller DD.Benzyl and naphthalene methylphosphonic acid inhibitors of autotaxin with anti-invasive and anti-metastatic activity.
2011; 6: 922-935 [PMID:21465666 DOI: 10.1002/cmdc.201000425]
191 Beck A,Wurch T,Bailly C,Corvaia N.Strategies and challenges for the next generation of therapeutic antibodies.
2010; 10: 345-352 [PMID: 20414207 DOI: 10.1038/nri2747]
192 Goldshmit Y,Matteo R,Sztal T,Ellett F,Frisca F,Moreno K,Crombie D,Lieschke GJ,Currie PD,Sabbadini RA,Pébay A.Blockage of lysophosphatidic acid signaling improves spinal cord injury outcomes.
2012; 181: 978-992[PMID: 22819724 DOI: 10.1016/j.ajpath.2012.06.007]
193 Crack PJ,Zhang M,Morganti-Kossmann MC,Morris AJ,Wojciak JM,Fleming JK,Karve I,Wright D,Sashindranath M,Goldshmit Y,Conquest A,Daglas M,Johnston LA,Medcalf RL,Sabbadini RA,Pébay A.Anti-lysophosphatidic acid antibodies improve traumatic brain injury outcomes.
2014; 11: 37 [PMID: 24576351 DOI:10.1186/1742-2094-11-37]
194 Budd DC,Qian Y.Development of lysophosphatidic acid pathway modulators as therapies for fibrosis.
2013; 5: 1935-1952 [PMID: 24175745 DOI: 10.4155/fmc.13.154]
195 Zhang H,Xu X,Gajewiak J,Tsukahara R,Fujiwara Y,Liu J,Fells JI,Perygin D,Parrill AL,Tigyi G,Prestwich GD.Dual activity lysophosphatidic acid receptor pan-antagonist/autotaxin inhibitor reduces breast cancer cell migration in vitro and causes tumor regression in vivo.
2009; 69: 5441-5449 [PMID: 19509223 DOI:10.1158/0008-5472.CAN-09-0302]
196 Zhao PF,Wu S,Li Y,Bao G,Pei JY,Wang YW,Ma Q,Sun HJ,Damirin A.LPA receptor1 antagonists as anticancer agents suppress human lung tumours.
2020; 868: 172886 [PMID: 31866407 DOI:10.1016/j.ejphar.2019.172886]
197 Im DS.Pharmacological tools for lysophospholipid GPCRs: development of agonists and antagonists for LPA and S1P receptors.
2010; 31: 1213-1222 [PMID: 20729877 DOI: 10.1038/aps.2010.135]
198 Minami K,Ueda N,Maeda H,Ishimoto K,Otagaki S,Tsujiuchi T.Modulation of chemoresistance by lysophosphatidic acid (LPA) signaling through LPA
in melanoma cells treated with anticancer drugs.
2019;517: 359-363 [PMID: 31362892 DOI: 10.1016/j.bbrc.2019.07.092]
199 Zhao WJ,Zhu LL,Yang WQ,Xu SJ,Chen J,Ding XF,Liang Y,Chen G.LPAR5 promotes thyroid carcinoma cell proliferation and migration by activating class IA PI3K catalytic subunit p110β.
2021; 112: 1624-1632 [PMID:33540491 DOI: 10.1111/cas.14837]
200 Lee SC,Fujiwara Y,Liu J,Yue J,Shimizu Y,Norman DD,Wang Y,Tsukahara R,Szabo E,Patil R,Banerjee S,Miller DD,Balazs L,Ghosh MC,Waters CM,Oravecz T,Tigyi GJ.Autotaxin and LPA1 and LPA5 receptors exert disparate functions in tumor cells versus the host tissue microenvironment in melanoma invasion and metastasis.
2015; 13: 174-185 [PMID: 25158955 DOI: 10.1158/1541-7786.MCR-14-0263]
201 Prestwich GD,Gajewiak J,Zhang H,Xu X,Yang G,Serban M.Phosphatase-resistant analogues of lysophosphatidic acid: agonists promote healing,antagonists and autotaxin inhibitors treat cancer.
2008; 1781: 588-594 [PMID: 18454946 DOI: 10.1016/j.bbalip.2008.03.008]
202 Swaney JS,Chapman C,Correa LD,Stebbins KJ,Bundey RA,Prodanovich PC,Fagan P,Baccei CS,Santini AM,Hutchinson JH,Seiders TJ,Parr TA,Prasit P,Evans JF,Lorrain DS.A novel,orally active LPA(1) receptor antagonist inhibits lung fibrosis in the mouse bleomycin model.
2010; 160: 1699-1713 [PMID: 20649573 DOI:10.1111/j.1476-5381.2010.00828.x]
 World Journal of Gastrointestinal Oncology2022年8期
World Journal of Gastrointestinal Oncology2022年8期
- World Journal of Gastrointestinal Oncology的其它文章
- lnfluence of SCENlC recommendations on terminology used for histopathologic diagnosis of inflammatory bowel disease-associated dysplasia
- Poorly cohesive cells gastric carcinoma including signet-ring cell cancer:Updated review of definition,classification and therapeutic management
- Lymph node regression grading of locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy
- lmmunotherapy in biliary tract cancers:Current evidence and future perspectives
- Crosstalk between gut microbiota and COVlD-19 impacts pancreatic cancer progression
- Angiogenesis in gastrointestinal stromal tumors:From bench to bedside
