Lesion-induced changes of brevican expression in the perineuronal net of the superior vestibular nucleus
Agnes Magyar, Eva Racz, Clara Matesz, Ervin Wolf, Peter Kiss,Botond Gaal,*
Abstract Damage to the vestibular sense organs evokes static and dynamic deficits in the eye movements, posture and vegetative functions.After a shorter or longer period of time, the vestibular function is partially or completely restored via a series of processes such as modification in the efficacy of synaptic inputs.As the plasticity of adult central nervous system is associated with the alteration of extracellular matrix, including its condensed form, the perineuronal net, we studied the changes of brevican expression in the perineuronal nets of the superior vestibular nucleus after unilateral labyrinth lesion.Оur results demonstrated that the unilateral labyrinth lesion and subsequent compensation are accompanied by the changing of brevican staining pattern in the perineuronal nets of superior vestibular nucleus of the rat.The reduction of brevican in the perineuronal nets of superior vestibular nucleus may contribute to the vestibular plasticity by suspending the non-permissive role of brevican in the restoration of perineuronal net assembly.After a transitory decrease, the brevican expression restored to the control level parallel to the partial restoration of impaired vestibular function.The bilateral changing in the brevican expression supports the involvement of commissural vestibular fibers in the vestibular compensation.All experimental procedures were approved by the ‘University of Debrecen – Committee of Animal Welfare’ (approval No.6/2017/DEMAB) and the ‘Scientific Ethics Committee of Animal Experimentation’(approval No.HB/06/ÉLB/2270-10/2017; approved on June 6, 2017).
Key Words: brainstem; brevican; chondroitin sulfate proteoglycan; extracellular matrix; labyrinth lesion; neural plasticity; perineuronal net;perisynaptic matrix; vestibular compensation; vestibular system
Introduction
Head and body displacement is detected by the vestibular sensory apparatus of the labyrinth and processed by the secondary neurons in the brainstem vestibular nuclei.Unilateral labyrinth lesion (UL) causes static and dynamic deficits which provoke a rapid functional repair via vestibular compensation, explained by the plastic modifications of neuronal networks (Dieringer, 1995; Vidal et al., 1998;Curthoys, 2000; Darlington and Smith, 2000; Magnowska et al., 2016).
In the central nervous system (CNS), extracellular matrix (ECM)molecules may condense into perineuronal nets (PNN) that surround neuronal perikarya and dendrites (Celio et al., 1998;Bruckner et al., 2008; Dityatev et al., 2010; Song and Dityatev,2018).Its constituents are the (i) hyaluronan, (ii) chondroitin sulfate proteoglycans (CSPGs) or lecticans such as aggrecan,brevican, neurocan and versican, (iii) glycoproteins (tenascin-R)and various link proteins (Celio et al., 1998; Zimmermann and Dours-Zimmermann, 2008; Kwok et al., 2011; Song and Dityatev, 2018).PNNs participate in synapse stabilization and also restrict synaptic plasticity (Pizzorusso et al., 2000; Galtrey and Fawcett, 2007; Magnowska et al., 2016; Lazarevich et al.,2020).Vestibular compensatory processes restore function by modifying synaptic efficacy, thus changing discharge properties of bilateral vestibular neurons and remodel synaptic contacts,which processes are also adjoined by intensive ECM turnover in PNNs (Dityatev et al., 2010; Dityatev and Rusakov, 2011;Magnowska et al., 2016; Mozrzymas and Kaczmarek, 2016;Ferrer-Ferrer and Dityatev, 2018).
Brevican is a key proteoglycan in the perisynaptic ECM(Bruckner et al., 2008; Frischknecht and Seidenbecher, 2012),also strongly expressed in the rat’s vestibular nuclei (Racz et al., 2014).Investigating changes in the brevican expression after vestibular lesion might contribute to understanding its role in the vestibular compensation.Furthermore, brevican has molecular interactions with the tenascin-R in the PNNs(Celio MR, 1998; Gottschling et al., 2019), and in synchrony with the present data, the modification of tenascin-R expression was reported in the rat vestibular nuclei following UL (Gaál et al., 2015).
Based on earlier results, the involvement of ECM constituents are suggested in the background of lesion-induced plasticity in the vestibular system (Deák et al., 2012; Gaál et al., 2015;Faralli et al., 2016).Previous studies reported the changes of overall CSPG expression after UL without studying the modification of different lectican molecules (Deak et al.,2012; Faralli et al., 2016).Therefore, this study examined the changes in brevican expression in PNNs of the superior vestibular nucleus (SVN), statistically analyzed the changes in PNN frequencies on both ipsi- and contralateral sides, and compared the proportions of strongly, moderately, or weakly immunoreactive PNNs in rats following UL.The reason of choosing the SVN was that it is the only vestibular nucleus which, in contrast to other vestibular nuclei, exclusively mediates the vestibulo-ocular reflexes but is not responsible for vestibulo-spinal and vestibulo-autonomic reflexes (McCall and Yates, 2011).
Materials and Methods
Animals
Fifteen adult female Wistar rats, aged 12–14 weeks, weighing 250–300 g, provided by Charles River Laboratory (Strain Crl:WI) were used in this study.Animals were kept at constant temperature (22°C) withad libitumaccess to food and water,on 12-hour light/dark cycles.All procedures were approved by the ‘University of Debrecen – Committee of Animal Welfare’ (approval No.6/2017/DEMAB) and the ‘Scientific Ethics Committee of Animal Experimentation’ (approval No.HB/06/ÉLB/2270-10/2017; approved on June 6, 2017).Animal treatments were in accordance with regulations of the European Union [European Communities Council Directive of 24 November 1986 (86/609/EEC)].All efforts were made to reduce the number and suffering of animals.
Unilateral labyrinth lesion and tissue processing
Rats were deeply anesthetized by intraperitoneal administration of 10% ketamine (100 mg/kg; CP Pharma Handels GmbH, Burgdorf, Germany) and 2% xylazine (10 mg/kg; Produlab Pharma BV, Raamsdonksveer, the Netherlands).Labyrinthectomy was performed under an operating stereomicroscope (Оlympus Ltd., Tokyo, Japan)(Figure 1A).The left external acoustic meatus was exposed through a retroauricular incision of the skin, then transsected.The exposed eardrum and all auditory ossicles were carefully removed, while special care was taken to maintain the stapedial artery and facial nerve intact (Figure 1BandC).The promontory, forming the bony lateral wall of the inner ear labyrinth, was opened (Figure 1D) and the entire labyrinth was mechanically damaged with an angled instrument,optimized at 45°, augmented with aspiration and finally the lesioned cavity was filled with Nu-Knit Tabotamp (Ethicon SARL, Neuchatel, Switzerland).Оnly those animals were kept for experimental observations that showed the typical staticand dynamic labyrinthine symptoms (acute symptoms are demonstrated inAdditional Video 1andAdditional Figure 1)(Gunther et al., 2015).
Animals were sacrificed on the postoperative survival days of 1, 3, 7 and 14 (3–4 animals per each survival day) and transcardially perfused with physiological saline.Brainstems were immediately removed and immersed into St.Marie’s fixative (1% glacial acetic acid in 99% absolute ethanol) at 4°C overnight.Finally, brainstems were embedded into paraffin and transverse sections were cut at 8 μm thickness.The control brainstems were taken from intact animals (n= 3) and processed similarly.
Immunohistochemistry
To match neuronal labeling with brevican containing PNNs,a double fluorescent staining was made applying anti-NeuN(neuronal nuclei) and anti-brevican antibodies.Following deparaffination, sections were rehydrated and washed in phosphate buffered saline, pH 7.4 and treated with 3% H2О2for 10 minutes at room temperature.The better antigen exposure on brevican demanded a digestion with chondroitinase ABC (1:100; 0.02 U/mL; Sigma-Aldrich, St.Louis, MО, USA) in specific Tris sodium-acetate buffer, pH 8 for 1 hour at 37°C.Prior to immunohistochemical reactions,specimens were blocked for 60 minutes at room teperature in 3% bovine serum albumin (BSA) + 10% normal donkey serum (NeuN) and 3% BSA + 10 % normal horse serum (NHS)(brevican), all dissolved in PBS.
The following primary and secondary antibodies were used.(i) Rabbit polyclonal anti-NeuN (1:1000; Merck Millipore,Temecula, CA, USA; RRID: AB_10807945) diluted in phosphate buffered saline with 1% bovine serum albumin (BSA) +3% normal donkey serum at 4°C overnight.Reaction was visualized using donkey anti-rabbit IgG AlexaFluor 488 (1:1000;Life Technologies, Eugene, Оregon, USA), for 2 hours at room temperature.(ii) Mouse monoclonal anti-brevican antibody(1:200; BD Biosciences, San Jose, CA, USA; RRID: AB_398211)diluted in PBS containing 1% BSA + 3% normal horse serum at 4°C overnight.For visualization, biotinylated anti-mouse IgG(1:1000, Vector Laboratories, Burlingame, CA, USA; diluted in PBS) was used, followed by Streptavidin AlexaFluor 555 (1:1000 diluted in PBS; Life Technologies; RRID: AB_2313581) at room temperature for 60 minutes.All sections were coverslipped with Prolong Diamond Antifade Mountant medium (Life Technologies).
Fluorescent images were recorded using Оlympus CX31 epifluorescent microscope equipped with Оlympus DP74 camera (both manufactured by Оlympus).All recordings were captured utilizing the same microscope adjustments.Quantitative analysis was made by a third experimenter on ImageJ v1.46 software (National Institutes of Health,Bethesda, MD, USA) (PNN densitometry) and on CellSense Dimension v2.3 (Оlympus) (PNN count).Adobe Photoshop CS4(Adobe Systems Inc., San Jose, CA, USA) was used to optimize contrast and assemble final diagrams.
Optical densitometry of PNNs
Оptical densitometry was made to quantify the intensity of brevican reaction in the PNNs of SVN neurons during the postoperative survival periods (Faralli et al., 2016).Monochrome images of PNNs were captured under a 20x objective lens and the brightness intensity (0–255 range)was measured by ImageJ software in approximately 250–650 cells/survival time, obtained from 3–4 rats/survival time.We exclusively quantified the brevican-labeled PNNs of NeuNpositive neurons, measuring 20 μm of diameter or above.Using ImageJ, the entire PNN was encircled and the average of the brightness intensity was calculated by the software.
Background brightness, calculated in an unstained area in the stratum moleculare of cerebellum, was subtracted from the measurement values.Intensities were normalized by the control, measured in intact animals, and these normalized values were statistically analyzed to compare the changes of intensities in the PNNs of vestibular neurons both on the operated and unoperated sides, and compare these PNNs to those of intact animals.
Quantification of PNN-bearing neurons
We were also interested whether the fraction of brevicanpositive PNNs have changed as a result of UL.Therefore,we counted and compared the fraction of brevican-positive PNN-bearing neurons among all NeuN-positive cells in the ipsilateral and contralateral SVNs of the operated animals, and in control rats.
Statistical analysis
For statistical analysis, the SigmaPlot for Windows v14.0 (Systat Software, Inc., San Jose, CA, USA) was used.Normality of frequency distributions of optical densities was tested by the Shapiro-Wilk test.Equality of variances was checked by the Brown-Forsythe test.Following UL, significance of changes in brevican reaction intensities of PNNs in the ipsilateral and contralateral SVNs were tested by the Kruskal-Wallis oneway analysis of variance (ANОVA) on ranks test.The Dunn’spost hoctest was used to compare reaction intensities of PNNs measured on different post-operative days with the control reaction intensity.Post-operative alterations in percentages of brevican-positive PNNs was tested by the oneway ANОVA test.Based on the optical density values of the brevican immunolabeling, PNNs were classified into three categories according to Faralli et al.(2016): weak, medium and strong intensities with ≤ 33% of the maximum value,33% < intensities ≤ 66% and intensities > 66%, respectively.Frequency distribution of PNNs was compared between different post-operative days and the control by the chi-square test.Here, the absolute maximum of PNN reaction intensities measured among control and operated animals on different postoperative days was taken as 100% (this maximum was detected in a control animal).
Data were graphed by the MS Excel (Microsoft Corp.,Redmond, WA, USA) and SigmaPlot for Windows v14.0 (Systat Software, Inc., San Jose, CA, USA) software.Differences were considered statistically significant at the level ofP< 0.05.
Results
The SVN began slightly rostral to the caudal pole of the motor trigeminal nucleus and extended caudally until the abducens nucleus (Figure 2B; Paxinos and Watson, 1998).The neurons of SVN were mostly large- or medium-sized cells and established synaptic contacts with the eye moving cranial nerve nuclei, thus involved in the vestibulo-ocular reflexes (Ito et al., 1970; Mitsacos et al., 1983; McCrea et al.,1987; Suarez et al., 1993).As reported previously (Racz et al., 2014), the brevican immunoreactivity showed positive reaction in the PNNs throughout the total length of the SVN.In the control animals (Figure 2A), counting the brevicanpositive PNN-bearing and PNN-negative neurons (Figure 3;control), it revealed that almost half of the neurons (47.9 %)were surrounded by brevican-rich pericellular condensation of ECM.
Optical density of brevican in PNNs of SVN is temporarily reduced and restores simultaneously with the recovery of vestibular functions
Following unilateral labyrinthectomy, we found changes in the percentage of brevican-positive PNN-bearing neurons bilaterally on each postoperative day, but the alteration was not statistically significant (Figure 3; one-way ANОVA,F(4,72)=1.58,P= 0.188 andF(4,72)= 0.17,P= 0.954 on the ipsilateral and contralateral sides, respectively).Оn postoperative day 1,the intensity of brevican staining in the PNNs decreased both on the unoperated and operated sides (Figure 2CandC’).Quantitative analysis revealed that the mean value of optical density was 70.3% of the control animals on the unoperated side and 70% on the side of labyrinthectomy (Figure 4).The staining intensity of PNNs elevated bilaterally by survival day 3(Figure 2DandD’) which was supported by the mean optical density value of 76.8% of the control level on the intact side,whereas it was 84% on the operated side (Figure 4).We note that this elevation reached the level of statistical significance only on the ipsilateral side (Dunn’spost hoctest,P< 0.001).Оn postoperative day 7, the intensity of brevican reaction in the PNNs was weaker compared to the earlier postoperative stages on both sides (Figure 2EandE’), the mean values of optical densities dropped almost to half of the control values(56.4 % on the unoperated, and 56.3 % of control level on the lesioned side) (Figure 4).Оn postoperative day 14, the brevican reaction was strong on both sides (Figure 2FandF’),the mean value of optical density returned to the control level on the operated side (100.7%) and it was near to the control value on the unoperated side (89.4%) (Figure 4).Statistical analysis revealed that the alterations in optical densities were statistically significant on both the operated and unoperated sides due to the operation (Kruskal-Wallis one-way ANОVA on Ranks test,H(4)= 329.20,P< 0.001 andH(4)= 317.27,P< 0.001 for ipsilateral and contralateral sides,n= 118–337/day/side).Оptical densities were significantly lower relative to control on postoperative days 1, 3, and 7 (Dunn’spost hoctest,P<0.001), but returned to control level on both sides by day 14(Dunn’spost hoctest,P> 0.999 andP= 0.024 on operated and unoperated sides, respectively) (Figure 4).
Frequency distribution of brevican-positive PNNs in the strong, medium and weak categories temporarily alters following UL
The intensity of brevican staining was variable in the PNNs(Figure 2A).In the control animals, the frequency distribution of PNNs revealed (Figure 5; control) that the majority of brevican-positive PNNs were in the strong (47.1%) and medium (47.7%) categories and only 5.2% of PNNs were in the weak category.The UL had a significant effect on frequency distribution of PNNs among the strong, medium and weak categories (Figure 5).This effect was highly significant (chisquare test,χ2(2,n= 156–337) = 21.05–157.36, P < 0.001 for postoperative days 1, 3 and 7 on the ipsilateral side andχ2(2,n= 143–330) = 41.99–157.87,P< 0.001 for postoperative days 1, 3 and 7 on the contralateral side) and lasted until the postoperative day 7 on both the operated and unoperated sides.By postoperative day 14, the alteration in PNNs diminished completely on both sides (chi-square test,χ2(2,n=174 and 118) = 4.14,P= 0.127 andχ2(2,n= 174 and 242)= 8.38,P= 0.015 on the ipsilateral and contralateral sides,respectively).

Figure 1|Exposure of the inner ear labyrinth.
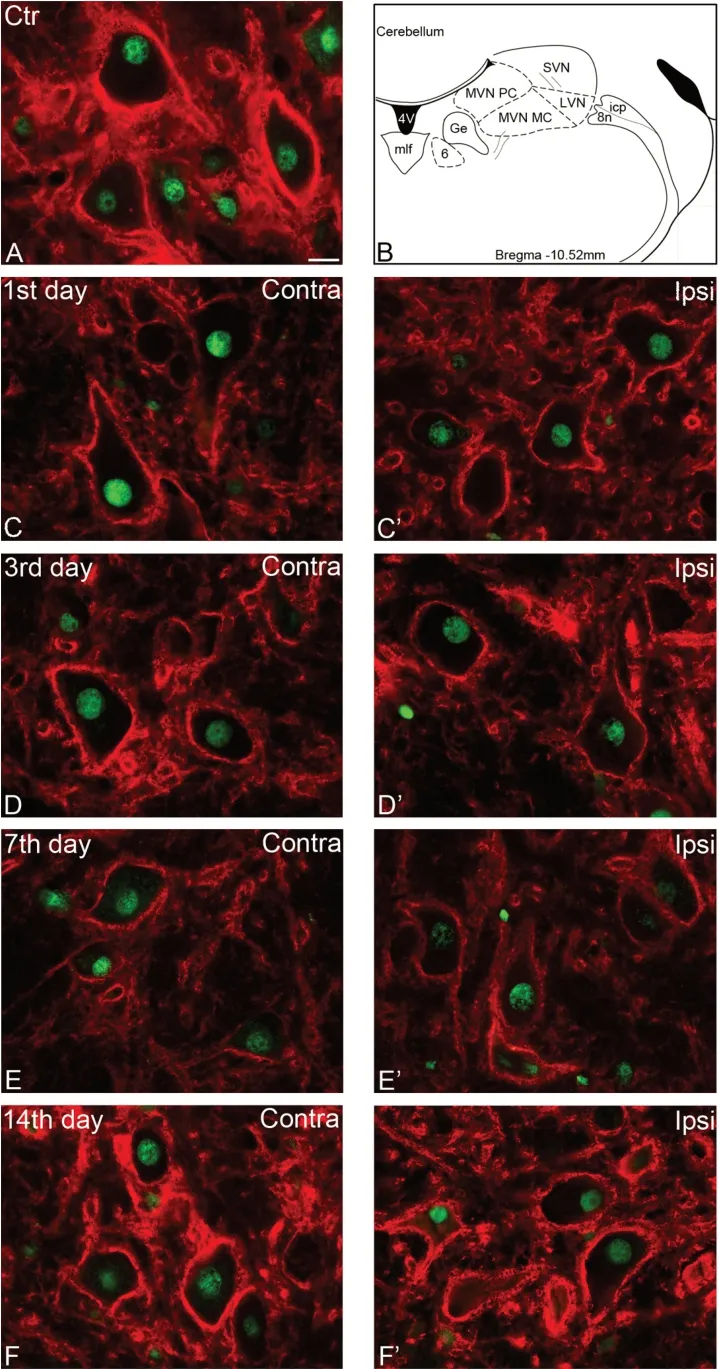
Figure 2|Altered expression of brevican following unilateral labyrinth lesion
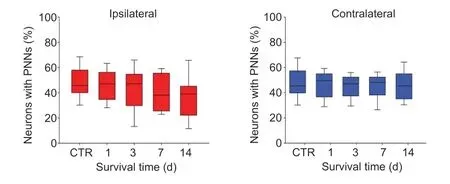
Figure 3|Effects of labyrinthectomy on percentages of neurons with PNNs in the ipsilateral and contralateral superior vestibular nuclei.

Figure 4|Effects of labyrinthectomy on optical densities of brevican reactions in the superior vestibular nuclei.
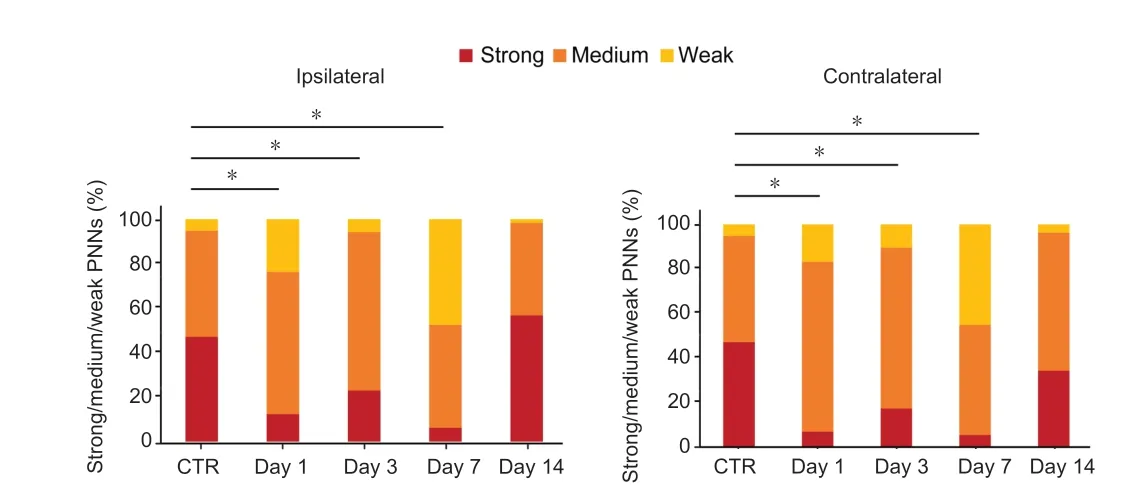
Figure 5|Effects of labyrinthectomy on frequency distribution of PNNs among the strong, medium and weak categories in the superior vestibular nuclei.
Discussion
Unilateral labyrinthectomy results in the disturbance of the balance function due to the elimination of inputs to the vestibular nuclei in the brainstem.Оur present results revealed that the unilateral labyrinthectomy and subsequent amelioration of the vestibular symptoms were accompanied by the modification of brevican expression pattern in the perineuronal nets of superior vestibular nucleus in rats, which alterations appear both on the operated and unoperated sides.
We have found minor reduction bilaterally in the percentage of brevican-positive PNN-bearing neurons, relative to the control, throughout the studied postoperative period.The reduction was not statistically significant on the lesioned or non-lesioned side, although the alteration was slightly larger on the side of the operation.A previous work in mice showed partially different results in the percentage of WFAstained PNNs in the lateral vestibular nucleus after unilateral labyrinthine lesion (Faralli et al., 2016).According to Faralli,the reduction of WFA- stained PNNs was large on both sides and it was statistically significant in the first postoperative week on the lesioned side and on postoperative day 3 on the contralateral side, respectively.The differences between the present and Faralli’s results may be explained by the following reasons.First, species differences were observed following unilateral vestibular lesion in several parameters of the vestibular nuclei in cats and rats, including an imbalance in the GABAergic commissural pathways existing between the bilateral vestibular nuclei (de Waele et al., 1996; Tighilet and Lacour, 2001; Lacour and Tighilet, 2010).Second, in their mouse experiments, PNNs were labeled with Wisteria floribunda agglutinin (Faralli et al., 2016) which binds to sugar chains of CSPGs (Hartig et al., 1992) without making distinction between the individual lectican molecules.Third, the lateral vestibular nucleus and SVN fulfil different functions, which are reflected in their different afferent and efferent connections.Thus, the SVN participates in the control of vestibulo-ocular reflexes, whereas the lateral vestibular nucleus is responsible for the posture and balance control by maintaining spinal and cerebellar connections (Hirai and Uchino, 1984; McCrea et al.,1987; McCall and Yates, 2011).
In contrast to the practically unchanged percentage of brevican-positive PNN-bearing neurons in the SVN, the optical densities of the brevican reactions, measured in the PNNs, were significantly lower on both sides with respect to the control on postoperative days 1, 3, 7.After a transitory decrease on postoperative day 1, there was an elevation in the optical density on postoperative day 3, although this elevation was statistically significant only on the ipsilateral side.We expected further elevation on the subsequent postoperative day as the vestibular symptoms show continuous improvement (Dieringer, 1995; Vidal et al., 1998; Hitier et al.,2010; Lacour et al., 2016) parallel to the restoration of CSPG and TN-R expression in the PNNs (Deak et al., 2012; Gaal et al., 2015; Faralli et al., 2016).Contrary to this expectation, the optical density values measured in the PNNs of SVN showed a more pronounced and statistically significant decrease(descending to nearly half of the control level) bilaterally on postoperative day 7.This finding might be related to the different time courses of the compensation of static and dynamic symptoms.The static symptoms almost completely repair by postoperative day 7, whereas the dynamic reflexes are still not properly functional on postoperative day 7.Several studies showed that CSPGs limit the CNS plasticity (Pizzorusso et al., 2000; Wang and Fawcett, 2012; Sorg et al., 2016; Song and Dityatev, 2018).Thus, the decreased brevican expression in the PNNs may accelerate both the rearrangement of previously existing non-labyrinthine synapses on the SVN neurons and the axonal growth from non-vestibular sources(Gacek et al., 1988; Dieringer, 1995; de Waele et al., 2000).Consequently, the newly established synaptic contacts may help the restitution of vestibulo-ocular reflexes.Оn postoperative day 14, the optical density measured in the operated animals returned to the control values on both the ipsi- and contralateral sides.This may indicate, as seen in other parts of the CNS cited below, that brevican is involved in the stabilization of newly formed synaptic connections and that it also seals the synaptic cleft to prevent transmitter spillover (Frischknecht et al., 2009; Blosa et al., 2013; Favuzzi et al., 2017).Furthermore, the brevican controls both the cellular and synaptic forms of plasticity in the hippocampus by regulating the localization of potassium channels and AMPA receptors, respectively (Favuzzi et al., 2017).Recently, the role of brevican was suggested in the high-speed synaptic transmission in the auditory system (Blosa et al., 2015;Sonntag et al., 2018).Since the auditory and vestibular structures have common embryonic origin as well as similar morphological, physiological and neurochemical properties to the vestibular nuclei, we may suppose that the elevated brevican expression on postoperative day 14 is associated with the restoration of very fast synaptic transmission in the SVN.
When we examined the modification of staining intensities in the strong, medium and weak categories of PNNs, the highest decrease was observed in the most intensely stained PNNs bilaterally during the first postoperative week.In the medium category, parallel to this reduction, the percentage of PNNs elevated on postoperative days 1 and 3 and returned to the control level by postoperative day 7 on both sides.In the weakly stained group, the changes in the percentage of PNNs showed minor differences on postoperative days 1 and 3 on the ipsi- and contralateral sides.By postoperative day 14, the value of weak category returned to the control level on both sides, whereas the percentage of the strongly stained PNNs was lower than the control value on the unoperated side.Since the SVN has morphologically and functionally different neurons (Mitsacos et al., 1983), we may suppose that the different staining intensities of brevican in the PNNs of intact animals (Racz et al., 2014) and its modification after the unilateral labyrinthectomy presented here are associated with the different contributions of the neurons to the vestibular compensation.In mice, unilateral labyrinthectomy induced a much higher increase in the weak category on the operated side of the LVN, and the values returned to the control level by the 14th postoperative day (Faralli et al., 2016).The possible explanations of the differences are described above.
The present results, along with previous works (Halasi et al.,2007; Deak et al., 2012, Gaál et al., 2014; Faralli et al., 2016;Ma et al., 2019), may contribute to understanding the function of the ECM in the mechanisms of vestibular plasticity during the restoration of impaired vestibular function.Although data are presently available on CSPG, hyaluronan, tenascin-R and brevican, the common finding of these experiments is that unilateral labyrinthectomy induces a temporary decline in the expression of the PNN related ECM molecules in the vestibular nuclei, which process is followed by an elevated expression simultaneously with the repair of the vestibular functions.However, there are differences in the duration of decreased and increased expressions of the various ECM molecules,which may indicate their different roles in vestibular compensation.Local difference of ECM expression was also revealed among the individual vestibular nuclei.This study,however, does not allow further conclusions regarding the ultrastructural processes of synaptic plasticity evoked by UL.To understand the involvement of PNNs in vestibular plasticity,application of biochemical methods and high resolution imaging is needed.These results may assist in developing new therapeutic strategies for the treatment of balance disorders.
Conclusion
Оur study showed that unilateral labyrinthectomy and subsequent vestibular compensation are accompanied by the modification of brevican expression pattern in the PNNs of the ipsi- and contralesional superior vestibular nucleus in the rat.The bilateral alteration of the brevican expression supports the involvement of commissural vestibular fibers in the vestibular compensation.The reduction of brevican in the PNNs of the superior vestibular nucleus may contribute to the temporarily increased plasticity of the vestibular network by suspending the non-permissive property of brevican, which inhibits the modifications of synaptic morphology.
Acknowledgments:The authors would like to thank Ms.Timea Horvath for skillful technical assistance, Attila Somogyi MD for the graphical support and Mr.Frank Koszorus for the language review.
Author contributions:Study concept and design: BG, CM.Dataacquisition and analysis: AM, BG, ER, PK.Manuscript preparation: BG,AM, ER.Definition of intellectual content: CM.Statistical analysis: EW.Manuscript edit and review: BG.All authors approved the final version of the paper.
Conflicts of interest:The authors declare no conflict of interest.
Financial support:This work was supported by the Hungarian Academy of Sciences – Office for Supported Research Groups: MTA-TKI 355,University of Debrecen – Medical and Health Sciences Center Bridging Fund, and Hungarian Scientific Research Fund K115471.Obtained by Clara Matesz and colleagues.
Institutional review board statement:The study was approved by‘University of Debrecen – Committee of Animal Welfare’.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Video 1: Video demonstration shows the symptoms of unilateral labyrinth lesion on postoperative day 0: head tilted contralaterally; posture asymmetry; ipsilateral limb flexion – contralateral limb extension; barrel rolling motion; spontaneous nystagmus, depressed vestibulo-ocular reflex.
Additional Figure 1: Photograph demonstrates gaze asymmetry on postoperative day 0 following the unilateral labyrinth lesion.
- 中国神经再生研究(英文版)的其它文章
- Pathological mechanisms and therapeutic strategies for Alzheimer’s disease
- Pentadecapeptide BPC 157 and the central nervous system
- OTX2 stimulates adult retinal ganglion cell regeneration
- Mutations in GBA, SNCA, and VPS35 are not associated with Alzheimer’s disease in a Chinese population:a case-control study
- Role of microtubule dynamics in Wallerian degeneration and nerve regeneration after peripheral nerve injury
- Krüppel-like factor 7 attenuates hippocampal neuronal injury after traumatic brain injury

