Glial decline and loss of homeostatic support rather than inflammation defines cognitive aging
Alexei Verkhratsky, Natalia Lazareva, Alexey Semyanov
The preservation of cognitive longevity and arresting the pandemic of senile dementia engulfing the modern world is arguably the major challenge faced by biomedical research in the 21stcentury.Age is the leading risk factor for neurodegenerative diseases and vascular dementia, and yet there is a clear distinction between physiological and pathological brain aging; the former proceeds with cognitive abilities mainly preserved, whereas the latter is manifested with rapid cognitive decline.The cellular and molecular mechanisms of brain aging remain disputed, with numerous indications for metabolic and signaling alterations(Mattson and Arumugam, 2018).Chronic neuroinflammation in particular, is frequently considered as a universal attribute of aging(Di Benedetto et al., 2017), with brain aging being but a part of “inflammageing”(Franceschi et al., 2007) embracing the whole organism.This view, however, needs to be corroborated, particularly considering that the concept of neuroinflammation is not clearly defined.Reactive gliosis (astrogliosis or microgliosis, and possibly reactivity of oligodendrocyte precursor cells also known as NG-2 glia) is frequently regarded as the ultimate sign of neuroinflammation and aging is generally believed to be associated with progressive increase in glial reactivity.Experimental evidence for the predominance of reactive glia in the old brain is however controversial; instead, the age-dependent glial morphofunctional decline may present more accurate description of brain aging.
Function of the nervous system rests on complex and concerted activity of extended networks that include neuronal connectome,astrocytic syncytium, other glial cells,extracellular space and vasculature.Neuronal networks represent the executive arm, which process sensory inputs and instigate adaptive behaviors, whereas the neuroglia forms the homeostatic arm, which supports and defends the nervous tissue and influence neurones, synapses and connectome.The neuroglia of the central nervous system comprises astrocytes (the chief homeostatic cells), the cells of oligodendroglial lineage(oligodendrocytes and their precursors,which maintain myelination and support axons) and microglia (cells of mesodermal origin, which play numerous physiological functions and are responsible for the innate immunity).The pathological potential of glial cells is vast and includes active defensive response (reactive gliosis), pathological remodeling (which turns glia into the primary driver of the disease) and various forms of asthenia, including structural atrophy and loss of function.In addition,severe insults to the brain may trigger glial death; astrocytic terminal degeneration is known as clasmatodendrosis.Reactive gliosis is, essentially, a defensive response to all forms of brain lesion which is governed by a complex and evolutionary conserved genetic programme leading to morphological and functional cellular remodeling.This process results in the emergence of numerous reactive context-, disease- and age-specific phenotypes; multiple reactive phenotypes can coexist within the same pathology or demonstrate certain adherence to distinct stages of neuropathological progression (Escartin et al., 2021).Astrocytopathies manifested by astroglial dystrophy and loss of function occur in many neuropathologies, including for example toxic encephalopathies, neuropsychiatric diseases and cognitive disorders.Astroglial pathological remodeling assumes the leading role in Alexander disease or in hepatic encephalopathy (Verkhratsky et al., 2017).
The aging of astrocytes, both structural and functional, is yet to be characterized in detail.The notion of age-dependent astrogliosis derives solely from immunocytochemical analysis of expression and distribution of presumed “universal” astroglial marker,the glial fibrillary acidic protein (GFAP).Staining with GFAP antibodies however, are neither universal (most of astrocytes in the healthy brain do not express detectable levels of GFAP) nor morphologically faithful[GFAP labels the cytoskeleton and primary astrocytic processes, leaving peripheral leaflet-like processes that account for ~80%of astrocytic surface unstained (Escartin et al., 2021)].Furthermore, increase in GFAP expression and morphological hypertrophy of GFAP-positive astrocytic profiles is not necessarily associated with reactivity: GFAP expression fluctuates with circadian rhythms and is increased following, for example,exposure to enriched environment or physical activity.
More accurate analysis of the numbers,size and complexity of astrocytes using stereology, Golgi staining or different astrocytic markers (antibodies against GFAP,glutamine synthetase or S100B protein)revealed that (i) number of astrocytes does not change with age (ii) in the old brains subpopulations of astrocytes with increased and decreased size and complexity coexist in different regions.For example, GFAPpositive astrocytic profiles are increased in the CA1 region and in the dentate gyrus of the old hippocampus, but decreased in the entorhinal cortex; at the same time astrocytes positive for glutamate synthase become smaller in the hippocampus of old mice but larger in the entorhinal cortex.Finally, the size of S100-positive astrocytic profiles in the hippocampus does not change much in aging (Rodriguez et al.,2014).A much more precise visualization of astrocyte morphology can be achieved by high-resolution confocal microscopy of cells loaded with low molecular weight fluorescent dyes through a patch pipette.These fluorescent probes (such as for example Alexa Fluor 594) diffuse through the cytoplasm and hence label the tiniest and most distant parts of the cell.The fluorescence can be collected even from astrocytic leaflets which, because of their size (with typical thickness around 100 nm) are below the resolution of optical microscopy.Nonetheless, normalizing the fluorescence emitted from the region occupied by these processes to the fluorescence of the soma (assuming that soma reflects 100% of astrocyte volume occupancy), the volume fraction occupied by leaflets can be estimated (Medvedev et al.,2014).Analyzing the 3D reconstructions of dye-filled hippocampal astrocytes revealed a substantial decrease in the size and complexity (the latter assessed with Scholl analysis) of these cells in old (20–24 months)compared with adult (9–12 months) mice(Figure 1).In particular, territorial domains of astrocytes in old mice appeared to be almost 30% smaller in comparison to adult animals.Volume fraction measurements demonstrated a significant dystrophy of peripheral leaflet-like processes, which are mainly responsible for astrocytic synaptic coverage (Popov et al., 2021).
The age-dependent decrease in astrocytic processes may impair synaptic transmission and connectivity thus contributing to the age-dependent cognitive decline.Astrocytic synaptic coverage is fundamental for synaptic function; most of the excitatory synapses in cortical and hippocampal areas are in close contacts with leaflets, which form astroglial cradle regulating synaptic maintenance,isolation and extinction (Verkhratsky and Nedergaard, 2014).In addition astrocytes secrete synaptogenetic factors such as for example trombospondin-1 and synthesise cholesterine, which are indispensable for synaptogenesis both in development and during adaptive plasticity (Nagai et al., 2019).Astrocytic atrophy therefore may affect all aspects of synaptic function and impair learning and memory.
Astrocytic membranes contacting synapses are densely packed with transporters that control ion homeostasis (in particular astrocytes are responsible for K+clearance,pertinent for maintaining neuronal excitability), remove glutamate and supply neuronal terminals with glutamine thus sustaining physiological synaptic transmission.At least two of these major homeostatic functions are impaired in old animals: both astrocytic K+and glutamate transporter currents are suppressed in aged astrocytes revealing deficient K+clearance and glutamate uptake (Popov et al., 2021).A decrease in astrocytic synaptic coverage promotes glutamate spillover and hence may impair synaptic plasticity; reduced longterm potentiation indeed was detected in direct electrophysiological recordings from hippocampal neurones from old mice(Figure 1).A similar reduction of longterm potentiation was identified in adult hippocampal slices following inhibition of astrocytic glutamate transporters with a specific inhibitor (3S)-3-[[3-[[4-(Trifluoromethyl)benzoyl]amino]phenyl]methoxy]-L-aspartic acid TFB-TBОA; the same inhibitor was less effective in old animals thus corroborating the role for astrocytic glutamate uptake (Popov et al.,2021).
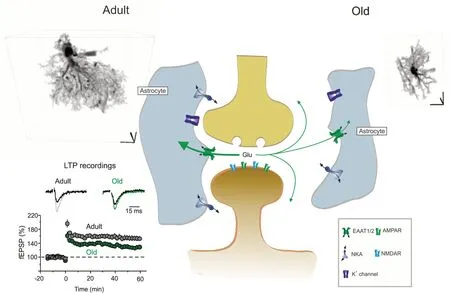
Figure 1|Age-dependent cognitive decline is linked to a decreased astrocytic homeostatic support.
Age-dependent dystrophy of astrocytes may affect many more processes fundamental for cognitive longevity.Astrocytes, for instance,are known to be an indispensable part of the glymphatic system responsible for collection of brain waste; proper function of this clearance pathway depends on the polarized expression of astrocytic water channels aquaporin 4 to astrocytic endfeet.In the old brain astrocytic aquaporin 4 channel lose their endfeet polarization which results in almost 40% decline in glymphatic function(Kress et al., 2014).A decrease in astrocytic domains and retraction of peripheral processes also affects extracellular space and widens diffusion channels, which may substantially modify extarasynaptic volume transmission.Lactate production is reduced in old astrocytes thus diminishing their metabolic support of neurones.Aged astrocytes have impaired capacity for production of cholesterol, needed for synaptogenesis and morphological neuronal plasticity.Finally, aging may restrict astrocytic defensive capabilities.In particular, aged astrocytes may limit their capacity to produce glutathione and hence decrease anti-oxidative defence.Aging may also result in suppression of astrocytic reactivity, which,in turn, may exacerbate neurodegeneration.
Age-dependent asthenia is not limited to astrocytes only; it seems that in general aging neuroglia undergoes morphological and functional atrophy, which limits its homeostatic and defensive support.Aging (even physiological) results in a substantial decrease in the volume of white matter indicating the disappearance of oligodendrocytes; in addition aging is associated with atrophic changes and decreased regenerative capacity of oligodendrocyte precursor cells,which becomes even more prominent in neurodegeneration (Vanzulli et al., 2020).Similarly, brain aging is associated with an accumulation of dystrophic microglia,which again leads to a reduced homeostatic support, decreased microglial defence and impaired innate immunity of the nervous tissue.In summary, brain aging is unlikely to be an actively destruction as posited by inflammaging concept; it rather reflects the fading of vital support provided by neuroglia.Can this progressive decline be influenced, arrested or delayed? Arguably the most powerful anti-aging treatment is associated with the changes in lifestyle,with intellectual engagement, emotional balance, physical exercise, healthy sleep regimen and appropriate diet.Many of these interventions are causally linked to neuroplasticity; they affect the morphology and function of neuroglia and in particular increase complexity and homeostatic capacity of astrocytes which boost brain reserve, increases cognitive longevity and counteract neurodegeneration.
The work of AS was supported by the Russian Science Foundation, No.20-14-00241.
Alexei Verkhratsky*, Natalia Lazareva,Alexey Semyanov
Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK;Achucarro Center for Neuroscience, IKERBASQUE,Basque Foundation for Science, Bilbao,Department of Neurosciences, University of the Basque Country UPV/EHU and CIBERNED, Leioa,Spain (Verkhratsky A)
Sechenov First Moscow State Medical University,Moscow, Russia (Verkhratsky A, Lazareva N,Semyanov A)
Shemyakin-Оvchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, Faculty of Biology, Moscow State University, Moscow,Russia (Semyanov A)
*Correspondence to:Alexei Verkhratsky, PhD,Alexej.Verkhratsky@manchester.ac.uk.https://orcid.org/0000-0003-2592-9898(Alexei Verkhratsky)
Date of submission:March 25, 2021
Date of decision:April 27, 2021
Date of acceptance:May 31, 2021
Date of web publication:August 4, 2021
https://doi.org/10.4103/1673-5374.320979
How to cite this article:Verkhratsky A, Lazareva N,Semyanov A (2022) Glial decline and loss of homeostatic support rather than inflammation defines cognitive aging.Neural Regen Res 17(3):565-566.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Pathological mechanisms and therapeutic strategies for Alzheimer’s disease
- Pentadecapeptide BPC 157 and the central nervous system
- OTX2 stimulates adult retinal ganglion cell regeneration
- Mutations in GBA, SNCA, and VPS35 are not associated with Alzheimer’s disease in a Chinese population:a case-control study
- Role of microtubule dynamics in Wallerian degeneration and nerve regeneration after peripheral nerve injury
- Krüppel-like factor 7 attenuates hippocampal neuronal injury after traumatic brain injury

