Phytochemical analysis and inhibitory effects of Calligonum polygonoides on pancreatic α-amylase and β-glucosidase enzymes
Mushtaq Ahmed,Naila Sher,Nadia Mushtaq,Rahmat Ali Khan
Mushtaq Ahmed,Naila Sher,Rahmat Ali Khan,Department of Biotechnology,Faculty of Biological Sciences,University of Science&Technology,Bannu 28100,KPK-Pakistan
Nadia Mushtaq,Department of Botany,Faculty of Biological Sciences,University of Science &Technology,Bannu 28100,KPKPakistan
Abstract OBJECTIVES:To estimate the existence of phytochemicals and then to determine the antidiabetic activity against α-amylase and β-glucosidase inhibition in vitro.METHODS:The study was carried out by following standard procedures.RESULTS:Phytochemicals analysis indicated the presence of different phytochemicals.The total phenolic content was 6.055 mg GAE/g and the total flavonoid content was 5.706 mg RU/g in the plant extract.The total saponins,alkaloids,and tannins contents were (0.044%),(2.88%) and (2.862 nm) respectively.α-amylase inhibition activity of Calligonum polygonoides (CP) extract was 70% with IC50 of 610 μg/mL and that of β-glucosidase inhibition activity was 65% with IC50 of 640 µg/mL.CONCLUSION:The findings reported for the first time the antidiabetes-promoting effects of an extract of CP in vitro,thus validating their promising anti-diabetes potential.
Keywords:Calligonum polygonoides;phytochemicals;alphaamylases;beta-glucosidase
1.INTRODUCTION
Medicinal plants contain numerous biologically active compounds such as carbohydrates,proteins,minerals,vitamins,alkaloids,quinones,terpenoids,flavonoids,carotenoids,sterols,tannins,saponins,and polyphenols,etc.that can be used for the treatment of different diseases.1,2Diabetes mellitus is a complex disease characterized by gross instability in carbohydrate,protein,and fat metabolism;this may be due to deficiency in insulin secretion and/or action.3The reasons may be lifestyle and genetic factors.4Mammalian α-amylase is a prominent enzyme in the pancreatic juice,breaking down large and insoluble starch molecules into absorbable molecules,ultimately maltose.5α-glucosidase,on the other hand,anchored in the mucosal brush border of the small intestine,catalyzes the end step of digestion of starch and disaccharides that are abundant in the human diet.6The inhibition of these two prominent enzymes has been found as a useful and effective strategy to lower the levels of postprandial hyperglycemia by delaying the breakdown of carbohydrate.7At present,the use of insulin secretagogues and sensitizers constitute the predominant line of therapy,however,the use of carbohydrate digesting enzyme inhibitors play a vital role in controlling hyperglycemia by reducing the intestinal absorption of glucose.8Acarbose is one of the leading inhibitor of carbohydrate metabolic enzymes in the gastrointestinal tract,but it is associated with side effects such as diarrhea and other intestinal disturbances such as bloating,flatulence,cramping,and abdominal pain.9Predominantly herbal drugs have been widely used globally for diabetic treatment over thousands of years due to their traditional acceptability and lesser side effects.
Calligonum polygonoides(CP) belong to the familyPolygonaceae.Many compounds with biological activity are produced by the plants which can be used for defense against microorganisms and insects and much disease.10,11The current study was conducted to investigate the phytochemical profiles and to evaluate the anti-diabetic potential of CP extracts.
2.MATERIALS AND METHODS
2.1.Preparation of extract
The fresh plant materials werecollected locally.The plant was botanically identified asCPProf.Dr.Sultan Mehmood,DeanFaculty of Biological Sciences UST Bannu.A voucher specimen was deposited (number 1574).The plant was washed,shade dried and pulverized mechanically to 0.1 mm size.About 300 mg of the powdered plant was taken and subjected to soxlet extraction with 1 L of 80% methanol.The extract was then concentrated under reduced pressure in rotary vacuum evaporator to obtain crude extract and was stored at 4℃ for further use.
2.2.Phytochemical screening
Qualitative analysis
Various qualitative chemical tests were conducted for the identification of the phytochemicals in the CP according to the procedures of.12-19
2.2.1.Tests for saponins (Frothing test)
About 10 mg of each sample was boiled in 10 mL of distilled water in a water bath for five minutes and filtered.About 5 mL of each filtrate was mixed with 5 mL of distilled water and shaken vigorously for froth formation.About 4 drops of olive oil were mixed in the froth,shaken vigorously and observed for the formation of an emulsion.
2.2.2.Test for tannins (Ferric chloride test)
About 25 mg of the plant was boiled in 10 mL of distilled water in a test tube and was filtered.A few drops of 0.1%ferric chloride were added in each test tube and observed for the color change,the presence of brownish-green or a blue-black coloration shows the presence of tannins.
2.2.3.Determination of flavonoids
About 50 mg of each extract was suspended in 100 mL of distilled water to get the filtrate.5 mL of dilute ammonia solution was added to 10 mL of aqueous filtrate followed by a few drops of concentrated H2SO4.A yellow coloration observed in each fraction indicated the presence of flavonoids.
2.2.4.Test for alkaloids (Wagner’s test)
About 0.4 g of each extract was mixed with 8 mL of 1%HCl,warmed and filtered.To the filtrate 2 mL of wagner’s reagent was added drop by drop.The formation of a reddish-brown precipitate indicates the presence of alkaloids.
2.2.5.Coumarins identification (Alkali reagent test)
About 0.3 g of plant extract was taken in a small test tube and covered it with filter paper moistened with 1 N NaOH.The test tube was placed,for a few minutes,in a boiling water bath.After removing the filter paper,it was examined under UV light,yellow fluorescence indicated the presence of coumarins.
2.2.6.Test for Steroids
About 100 mg powdered plant was mixed with 5 mL methanol and filtered.About 2 mL of acetic anhydride was added to the filtrate followed by the addition of a few drops of H2SO4(concentrated).The green coloration shows the presence of steroids.
2.2.7 Test for anthraquinones (Hydrochloric acid test)
About 20 mg plant extract was boiled with 1 mL 1% HCl and then filtered.The filtrate was then vigorously shaken with 5 mL of C6H6.The layer was removed and 10%NH4OH was added.Formation of pink,violet or red color indicated the presence of anthraquinone.
2.3.Quantitative analysis
2.3.1.Determination of alkaloids
About 200 mL of 10% CH3COOH in C2H5OH was added to the 5 mg/mL of the plant sample.The mixture was covered and kept for 4 hrs.It was then filtered until it reached ¼ of the original volume.Concentrated ammonium hydroxide was added till the precipitate completion.The collected precipitate was then washed with NH4OH (diluted) and filtered.The residue was alkaloid,which was then dried and weighed.Total alkaloids were calculated by using the formula;
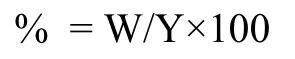
Where W is the weight of alkaloids extracted and Y is the plant material taken
2.3.2.Tannins determination
Brief 500 mg of the plant sample was taken in the bottle and 50 mL of the distilled water was added.It was shaken in a mechanical shaker for 1 h and filtered 5 mL of the filtrate was taken in a test tube and mixed with 2 mL of 0.1 M FeCl3in 0.1N HCl and 0.008M K4Fe(CN)6(Potassium ferrocyanide).After 10 min absorbance was recorded at 395 nm by a spectrophotometer.
2.3.3.Saponins estimation
About 50 mg/mL of the plant extract was mixed 50 mL of 20% methanol.It was then heated for 4 h with continuous stirring over a hot water bath at 55°C.The mixture was filtered and the residue re-extracted with another 50 mL of 20% aqueous methanol.The combined filtrate was then reduced to 40 mL at 90°C in water bath.The concentrated filtrate was transferred into a separatory funnel and 10 mL of C2H5OC2H5was added and shaken vigorously.The C2H5OC2H5layer was discorded while the aqueous was recovered.The process of purification was repeated.Added 30 mL n-C4H9OH to the aqueous layer and washed with 5 mL of 5% NaCl.The remaining solution was evaporated in a water bath and dried in the oven to a constant weight and saponins contents were calculated as a percentage of the dried fraction.Total saponins were calculated by using the formula;
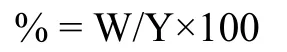
Where W is the weight of saponins extracted and Y is the plant material taken
2.3.4.Determination of the total phenolic contents in plant extract
About 200 µL (1-5 mg in methanol) concentration of plant was reacted with 130 µL of 1∶10 folin-Ciocalteu reagent.The mixture was incubated for 5 min and then 2.5 mL of saturated Na2CO3(0.115 mg/mL) was added into the reaction mixture.The absorbance reading was recorded at 765 nm after 2 hr at room temperature.Gallic acid chemical (1-5 mg/mL) was used as a reference standard.The results were expressed as milligram gallic acid equivalent (mg GAE)/g of the dried fraction.Total phenolic content is expressed in terms of Gallic acid equivalent (mg/g of extracted compounds) by using the formula;
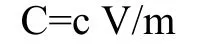
Where C=Total phenolic content mg GAE/g dry extract,c=concentration of GA obtained from calibration curve in mg/mL,V=volume of extract in mL,m=mass of extract in gram
2.3.5.Determination of total flavonoid contents in plant extract
About 250 µL (1-5 in methanol) concentration of plant and rutin (1-5 mg in methanol) as standard was mixed with 1250 µL dH2O followed by the addition of 75 µL of 5% (w/v) NaNO2(sodium nitrite) solution.After incubation for 6 min,150 µL of 10% (w/v) AlCl3.6H2O(aluminum chloride) was added and allowed to stand for further a 5 min.Brief 500 µL of 1 M NaOH (sodium hydroxide) was added and the mixture was raised up to 2500 µL with dH2O and shaken vigorously.The absorbance reading was recorded immediately at 510 nm.The results were expressed as milligram rutin equivalent(mg RU)/g of the dried fraction.The flavonoid content is expressed in terms of rutin equivalent (mg/g of extracted compounds) by using the formula;
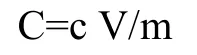
Where C=Total flavonoid content mg RUE/g dry extract,c=concentration of RU obtained from calibration curve in mg/mL,V=volume of extract in mL,m=mass of extract in gram
2.4.Assessment of in vitro anti-diabetic activity
2.4.1.Inhibition of α-amylase enzyme assay
In-vitroα-amylase inhibitory activity of CP extract was determined by following the method described by Malik and Gupta.20,21A starch solution (0.1% w/v) was prepared by mixing 0.1 gm of starch in 100 ml sodium acetate buffer (16 mM).By adding 300 µL of α-amylase from stock (250 units/mL) to 700 µL distilled water,the enzyme solution was obtained.After adding 200 µL both the sample and control ((200-1000 µg/mL) the starch solution was left to react with the α-amylase enzyme at 25°C under alkaline condition.The reaction was measured over 3 min and the quantification was measured by the reduction of 3,5 dinitro salicylic acid to 3-amino-5-nitro salicylic acid.This was then detected at 450 nm using a spectrophotometer.The percentage of inhibition of both control and sample was calculated by using the formula;
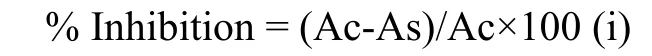
Where Ac is enzyme activity of control and As is enzyme activity of plant extract;
2.4.2.Inhibition of β-glucosidaselase enzyme assay
The assay was initiated by incubating 980 µL solution of β-D glucopyranoside (pNPGlc) (290 mM) with 20 mM citrate buffer in the pH of 5.6 at various concentration of both sample and standard (tagipmet) for 5 min at 37°C and by adding 20 µL of a β-glucosidase enzyme (IU/mL)to this solution.This was again allowed to incubate for 40 min at 35 ℃.Then the reaction ends by measuring the intensity of the colour at 540 nm using spectrophotometer after the addition of 200 µL of 6N-HCl mL of 6N HCl to it.The experiment was repeated twice.%inhibition was calculated by using the formula (i).22
2.5.Statistical analysis
All Calculations and Table representation were performed in Slide Write Plus and Microsoft Excel 2010 respectively.
3.RESULTS
3.1.Phytochemical analysis
3.1.1 Qualitative analysis
The remarkable concentration of alkaloids,flavonoids,tannins,saponins,and steroids are +ve,while anthraquinones and coumarin were absent.
3.1.2.Quantitative analysis
The standard curve of Rutin indicated the equation of y=0.161x+0.0572 and R²=0.9987 in Figure 1;Where Y-Absorbance at 510 nm and X-Total flavonoid in the extract.The total flavonoid content of the plant was 5.706 mg RU/g in the plant extract.The standard curve of Gallic acid indicated the equation of y=0.1435x+0.0511and R²=0.9787 clarified in Figure 2;Where YAbsorbance at 765 nm and X-Total phenol in the extract.The total phenolic content of the plant was 6.055 mg GAE/g in the plant extract.Methanolic extract of CP was analyzed quantitatively for Saponins (0.044%),Alkaloids (2.88%),and tannins (2.862 nm).
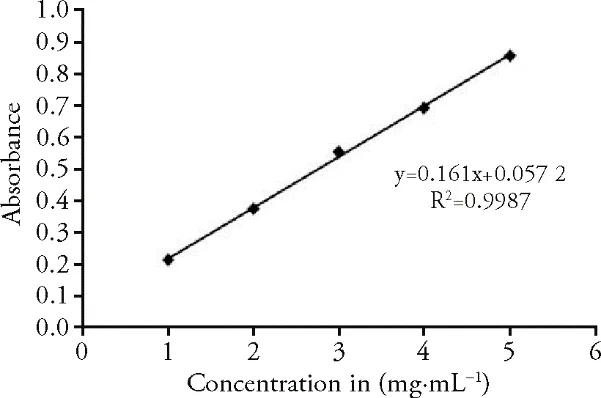
Figure 1 Standard calibration curve of Rutin
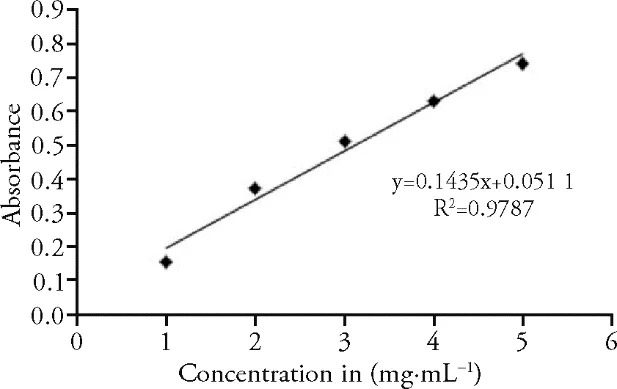
Figure 2 Standard calibration curve of Gallic acid
3.2.In-vitro antidiabetic activity
3.2.1.α-amylase assay
Among all concentrations,the highest percentage inhibition 70% and 80% were observed at 1000 µg/mL for CP and tagipmet.The IC50values were found to be 610 and 424 μg/mL for CP tagipmet respectively(Figures 3A and 3B).Similarly,the graph between the concentration of the sample and the percentage of inhibition of α-amylase was plotted (Figure 4).
Figure 3 IC50 was calculated for Calligonum polygonoides and tagipmet from the simple plot of % activity vs % inhibition.
3.2.2.β-glucosidase assay
Figures 5A and 5B indicated that 1000 μg/mL of CP and tagipmet revealed the maximum percentage of inhibition 65 and 75% with IC50of 640 and 470 μg/mL respectively.Similarly,the graph between the concentration of CP extract and the percent inhibition of β-glucosidase was plotted (Figure 6).
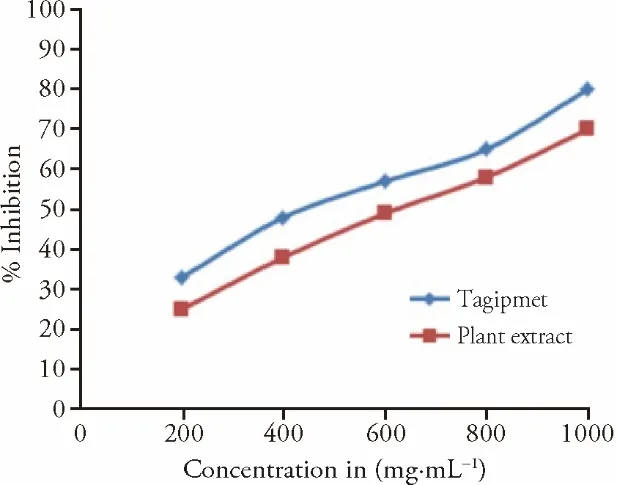
Figure 4 In-vitro antidiabetic activities by α-amylase method
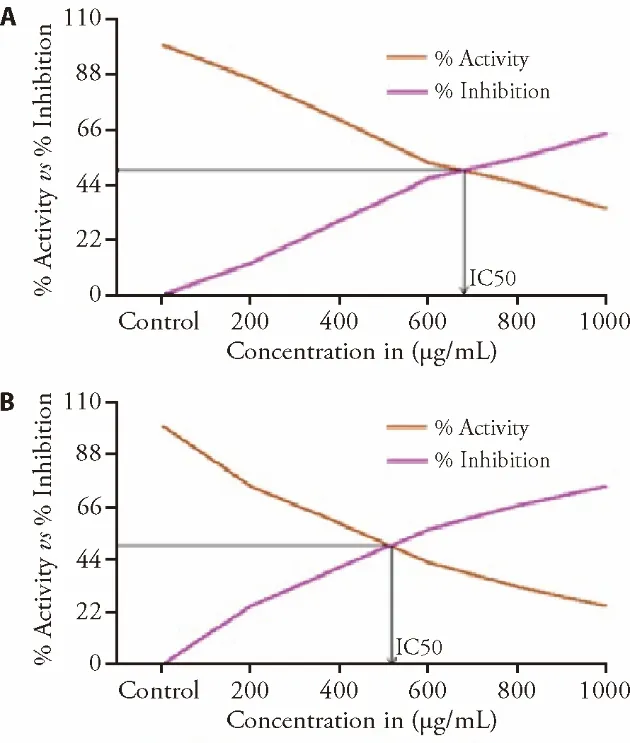
Figure 5 IC50 was calculated for Calligonum polygonoides and tagipmet from the simple plot of % activity vs % inhibition.
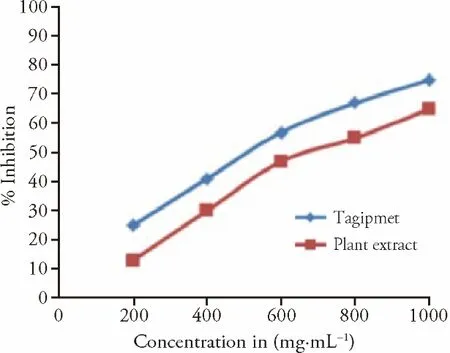
Figure 6 In-vitro antidiabetic activities by β-glucosidase method
4.DISCUSSION
The use of herbal drugs as complementary approaches in existing medications for the treatment of diabetes and its complications is growing worldwide and many plants in different countries are known to have antidiabetic effects.23The present project assessment for qualitative estimation of the CPdemonstrated the occurrence of different secondary metabolites including phenols,alkaloids,tannins,steroids,coumarins,flavonoids and saponins.These results were following the literature review on the plants which exhibited these chemical compounds to be present.24,25The quantitative analysis indicated that the total phenolic and flavonoid contents of the CP were higher compared to alkaloids,saponins,and tannins.Alkaloids are credited for analgesic and antibacterial properties.26,27Saponins have been known to induce antioxidant enzymes like catalase and superoxide dismutase (SOD) which are generally lowered in the diabetic animal model.28,29Phenolic phytochemicals function as chemo preventive agents against oxidative damage have attracted growing public attention.Consumption of fruits and vegetables rich of phenolic has been linked to the decreasing risk of developing chronic diseases by the reduction of oxidative stress and inhibition of macromolecular oxidation.30Several plant-derived flavonoids,apart from possessing their common antioxidant activity,have been reported to inhibit aldose reductase activity and impart beneficial action in diabetic complications.31,32
Herbal drugs are prescribed widely because of their effectiveness,fewer side effects,and relatively low cost.33In our investigation,CP showed appreciable inhibition against α-amylase and β-glucosidase.Medicinal plants and herbal extracts containing,phenols,steroids,tannins,flavonoids,etc.,have been testified to demonstrate antidiabetic activity.34CPpossessedtannins which inhibit the starch by binding to the digestive enzymes α-amylase due to the presence of OH groups which are suitable for hydrophobic association.35CP possessed high content of flavonoids and phenols which are responsible for the antidiabetic activity.36The antidiabetic effect of flavonoids is due to insulin secretion enhancement and by regulation of glucose metabolism.37Phenols exist in vegetables,fruits and medicinal herbs38including CP.39Phenols are potent inhibitor of α-amylase based on the structure of C ring,the degree of hydroxylation and the linkage between benzopyran and phenols group.The 2,3 double bonds,5-OH,the linkage of the B ring at the 3 positions and the hydroxyl substitution on the B ring enhanced the inhibitory activity while 3-OH group reduced it.40Flavonoid is also a strong inhibitor of β-glucosidase.41Lopezet al42reported the existence of flavonoid inPolygonum ferrugineum(Polygonaceae).The unsaturated C ring,3-OH,4-CO,the linkage of the B ring at the 3 positions and the hydroxyl substitution on the B ring of flavonoid enhanced the inhibitory activity of flavonoid.40Many bioactive compounds from different plants have been reported to have hypoglycemic effect,in that mostly phenolics and flavonoids have a positive correlation as antidiabetic agents.43,44Thus the presence of flavonoids and phenolics in the CP extract might have attributed to the highest enzyme inhibition activity as compared to other bioactive compounds.The presence of flavonoid compounds in CP extract may act against diabetes mellitus either through their capacity to avoid glucose absorption or to improve glucose tolerance by competitive inhibition of sodium-dependent glucose transporter-1.Another possible mechanism followed by flavonoid to control blood glucose levels is the inhibition of α-amylase and β-glucosidase activity in the intestine.In conclusion,medicinal plants are neither harmful to the environment nor expensive.The present work was designed to estimate the existence of phytochemicals of methanolic extract of CP.Moreover,its potential to inhibit α-amylase and β-glucosidase was also examined and was found significant results.In order to study the hidden potential of CP,further research is required.
5.REFERENCES
1.Farnsworth NR.The role of ethnopharmacology in drug development.Ciba Found Symp 1990;154:2-11.
2.Anandarajagopal K,Sudhahar D,Ajaykumar TV,et al.Evaluation of CNS depressant activity of aerial parts of Basella alba Linn.J Pharmacol Toxicol 2011;1.
3.Luo Q,Cai Y,Yan J,et al.Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum.Life Sci 2004;76:137-49.
4.Risérus U,Willett WC,Hu FB.Dietary fats and prevention of type 2 diabetes.Prog.Lipid Res 2009;48:44-51.
5.Gupta R,Gigras P,Mohapatra H,et al.Microbial α-amylases:a biotechnological perspective.Process Biochem 2003;38:1599-616.
6.Anam K,Widharna RM,Kusrini D.α-Glucosidase inhibitor of Terminalia species.Int J Pharmacol 2009;5:277-80.
7.Fred-Jaiyesimi A,Kio A,Richard W.α-Amylase inhibitory effect of 3β-olean-12-en-3-yl (9Z)-hexadec-9-enoate isolated from Spondias mombin leaf.Food Chem 2009;116:285-8.
8.Ghadyale V,Takalikar S,Haldavnekar V,et al.Effective control of postprandial glucose level through inhibition of intestinal alpha glucosidase by Cymbopogon martinii (Roxb.).J Evid Based Complementary Altern Med 2012;2012:372909.
9.Singh SK,Rai PK,Jaiswal D,et al.Evidence-based Critical Evaluation of Glycemic Potential of Cynodon dactylon.J Evid Based Complementary Altern Med 2008;5:415-20.
10.Katewa SS,Galav PK.Traditional herbal medicines from Shekhawati region of Rajasthan.Indian J Tradit Knowl 2005;4:237-45.
11.Lichterman BL.Aspirin:the story of a wonder drug.Bri Med J 2004;329:1408.
12.Sofowora A.Medicinal plants and traditional medicine in Africa.Ibadan [[u.a.]:Spectrum Books Ltd.;1993;191-289.
13.Harborne JB.Phytochemical methods:a guide to modern techniques of plant analysis.London:Chapman and Hall;1973;1-36.
14.Trease GE,Evans MD.A Textbook of Pharmacognosy.13th ed.Builler Tindall and Caussel London1989;44.
15.Siddiqui S,Verma A,Rather AA,et al.Preliminary phytochemicals analysis of some important medicinal and aromatic plants.Adv Bio Res 2009;3:188-95.
16.Van-Burden TP,Robinson WC.Formation of complexes between protein and tannin acid.J Agri Food Chem 1981;1:77-82.
17.Obadoni BO,Ochuko PO.Phytochemical studies and comparative efficacy of the crude extract of some homeostatic plants in Edo and Delta states of Nigeria.Global J Pure Appl Sci 2001;8:203-8.
18.Singleton VL,Rossi JA.Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents.Am J Enol Vitic 1965;16:144-58.
19.Sakanaka S,Tachibana Y,Okada Y.Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea(kakinohacha).Food Chem 2005;89:569-75.
20.Malik CP,Singh MB.Plant Enzymology and Histoenzymology.Kalyani publishers,New Delhi 1981:278.
21.Gupta R,Misra A.In vitroantidiabetic activity of pentacyclic tritrprnoida and fatty acid ester from bauhinia purpurea.Br J Diabetes Vasc 2007;7:16-25.
22.Krishnaveni S,Balasubramanian T,Sadasivam S.Sugar distribution in sweet stalk sorghum.Food Chem 1984;15:229-32.
23.Hasani-Ranjbar S,Larijani B,Abdollah M.A systematic review of Iranian medicinal plants useful in diabetes mellitus.Arch Med Sci 2008:285-92.
24.Tsakala O,Pen RS,Miemanang K,et al.Paullinoside A and Paullinomide a:a new cerebroside and a new ceramide from the leaves of Paullinia pinnata.Z Naturfosch 2006;61:1123-7.
25.Okwu DE,Ekeke O.Phytochemical screening and mineral composition of chewingsticks in South Eastern Nigeria.Int J Pure Appl Sci 2003;9:235-8.
26.Pietta PG.Flavonoids as antioxidants.J Nat Prod 2000;63:1035-42.
27.Shehzad A,Khan S,Sup LY.Future oncology.Precis Future Med 82012:179-90.
28.Elekofehinti OO,Kamdem JP,Kade IJ,et al.Saponins fromSolanum anguivi lam.fruit exhibitin vitroandin vivoantioxidant activities in alloxan-induced oxidative stress.Asian J Pharm Clin Res 2012;6:249-54.
29.Betteridge DJ.What is oxidative stress.Metabolism 2000;49:3-8.
30.Velioglu YS,Mazza G,Gao L,et al.Antioxidant activity and total phenolics in selected fruits,vegetables,and grain products.J Agric Food Chem 1998;46 :4113-7.
31.Thielecke F,Boschmann M.The potential role of green tea catechins in the prevention of the metabolic syndrome -a review.Phytochemistry 2009;70:11-24.
32.Lim SS,Jung SH,Ji J,et al.Synthesis of flavonoids and their effects on aldose reductase and sorbitol accumulation in streptozotocininduced diabetic rat tissues.Pharm Pharmacol 2001;53:653-68.
33.Venkatesh S,Reddy GD,Reddy BM,et al.Antihyperglycemic activity of Caralluma attenuata.Fitoterapia 2003;74:274-9.
34.Suba V,Murugesan T,Arunachalam G,et al.Anti-diabetic potential of Barleria lupulina extract in rats.Int.J.Phytomedicine 2004;11:202-5.
35.Barrett A,Ndou T,Hughey CA,et al.Inhibition of alpha-amylase and glucoamylase by tannins extracted from cocoa,pomegranates,cranberries,and grapes.J Agric Food Chem 2013;61:1477-86.
36.Jadhav R,Puchchakayala G.Hypoglycemic and antidiabetic activity of flavonoids:boswellic acid,ellagic acid,quercetin,rutin on streptozotocinnicotinamide induced type 2 diabetic rats.Int J Pharm Pharm Sci 2012;1:251-6.
37.Babu PV,Liu D,Gilbert ER.Recent advances in understanding the anti-diabetic actions of dietary flavonoids.J Nutr Biochem 2013;24:1777-89.
38.Lin Y,Shi R,Wang X,et al.Luteolin,a flavonoid with potential for cancer prevention and therapy.Curr Cancer Drug Targets 2008;8:634-46.
39.Bannour M,Fellah B,Rocchetti G,et al.Phenolic profiling and antioxidant capacity of Calligonum azel Maire,a Tunisian desert plant.Food Res Int 2017;101:148-54.
40.Tadera K,Minami Y,Takamatsu K,et al.Inhibition of alphaglucosidase and alpha-amylase by flavonoids.J Nutr Sci Vitaminol (Tokyo) 2006;52:149-53.
41.Dendup T,Prachyawarakorn V,Pansanit A,et al.Alpha-Glucosidase inhibitory activities of isoflavanones,isoflavones,and pterocarpans from Mucuna pruriens.Planta Med 2014;80:604-8.
42.Lopez SN,Sierra MG,Gattuso SJ,et al.An unusual homoisoflavanone and a structurally-related dihydrochalcone from Polygonum ferrugineum (Polygonaceae).Phytochemistry 2006;67:2152-8.
43.Tundis R,Loizzo MR,Menichini F.Natural products as alphaamylase and alpha-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes:an update.Mini Rev Med Chem 2010;10:315-31.
44.Sales PM,Souza PM,Simeoni LA,et al.α-Amylase inhibitors:a review of raw material and isolated compounds from plant source.J Pharm Pharm Sci:a publication of the Canadian Society for Pharmaceutical Sciences,Societe canadienne des sciences pharmaceutiques 2012;15:141-83.
 Journal of Traditional Chinese Medicine2022年3期
Journal of Traditional Chinese Medicine2022年3期
- Journal of Traditional Chinese Medicine的其它文章
- Efficacy of meridian massage for motor function after a stroke:a systematic review and Meta-analysis
- Antiviral Activity of Medicinal Plants against Human Coronavirus:a systematic scoping review of in vitro and in vivo experimentations
- Fuzheng Kang' ai decoction (扶正抗癌方) inhibits cell proliferation,migration and invasion by modulating mir-21-5p/human phosphatase and tensin homology deleted on chromosome ten in lung cancer cells
- Correlation between slow transit constipation and spleen Qi deficiency,and gut microbiota:a pilot study
- Efficacy of Kushen decoction (苦参汤) on high-fat-diet-induced hyperlipidemia in rats
- Hyperpolarization-activated cyclic nucleotide-gated 2 contributes to electroacupuncture analgesia on lumbar disc herniation-induced radicular pain through activation of microglia in spinal dorsal horn
