Efficacy and safety of a sequential treatment with clearing heat and eliminating phlegm and tonifying Qi and activating blood circulation in treating acute ischemic stroke:study protocol for a randomized controlled trial
ZHOU Ziyi,WAN Can,ZHAO Yuanqi,LIU Xiangzhe,GAO Ying,AN Hongwei,LI Lejun,ZHANG Huili,YU Xiaofei,ZHANG Xinchun,WANG Huijuan,SHI Qing,WEI Chunhua,CHEN Jie,HUANG Wenguo,CHEN Junbin,GAO Ying,HU Mingzhe,CAI Yefeng
ZHOU Ziyi,Department of Neurology,Guangdong Provincial Hospital of Chinese Medicine,Guangzhou 510120,China;The Second Clinical College of Guangzhou University of Chinese Medicine,Guangzhou 510120,China
WAN Can,ZHAO Yuanqi,ZHANG Xinchun,ZHOU Ziyi,CAI Yefeng,Department of Neurology,Guangdong Provincial Hospital of Chinese Medicine,Guangzhou 510120,China;the Second Clinical College of Guangzhou University of Chinese Medicine,Guangzhou 510120,China
LIU Xiangzhe,Department of Neurology,The First Affiliated Hospital of Henan University of Traditional Chinese Medicine,Zhengzhou 450000,China
GAO Ying,Department of Neurology,Shenyang Second Hospital of Traditional Chinese Medicine,Shenyang 110100,China
AN Hongwei,Department of Neurology,Liuzhou Traditional Chinese Medical Hospital,Liuzhou 545001,China
LI Lejun,Department of Neurology,Wuxi Traditional Chinese Medicine Hospital,Wuxi 214071,China
ZHANG Huili,Department of Neurology,Qinhuangdao City Hospital of Traditional Chinese Medicine,Qinhuangdao 066000,China
YU Xiaofei,Department of Neurology,Shanghai Shuguang Hospital,Shanghai 200120,China
WANG Huijuan,Department of Neurology,the Second Hospital of Hebei Medical University,Shijiazhuang 050000,China
SHI Qing,Department of Neurology,Jiangmen Wuyi Hospital of Traditional Chinese Medicine,Jiangmen 529000,China
WEI Chunhua,Department of Neurology,Nanshi Hospital Affiliated to Henan University,Nanyang 473065,China
CHEN Jie,Department of Neurology,Shanxi Province Hospital of Traditional Chinese Medicine,Xi'an 710003,China
HUANG Wenguo,Department of Neurology,Maoming Hospital of Traditional Chinese Medicine,Maoming 525000,China
CHEN Junbin,Department of Neurology,the Northern Guangdong People's Hospital,Shaoguan 512025,China
GAO Ying,Department of Neurology and Stroke Center,Dongzhimen Hospital affiliated to Beijing University of Chinese Medicine,Beijing 100700,China
Abstract OBJECTIVE:To investigate the short-term efficacy and safety outcomes following a sequential treatment with clearing heat and eliminating phlegm (CHEP) formula and tonifying Qi and activating blood circulation (TQABC)formula in patients with acute ischemic stroke (AIS) within a 72 h time window.METHODS:In this randomized,multicenter,doubleblinded,placebo-controlled trial,500 participants will be randomly assigned in a ratio of 1∶1 to the CHEP+TQABC group or control group.In addition to guidelinebased standard medical care,participants in the treatment group will receive the CHEP formula for the first 5 consecutive days followed by the TQABC formula for another 10 consecutive days,while those in the control group will receive CHEP formula placebo and TQABC formula placebo consecutively.The primary outcome measure will be the comparison of the change in the National Institutes of Health Stroke Scale score from baseline to 15 days after randomization.The secondary outcome measures will include the scores on the modified Rankin Scale,Barthel Index,Patient-Reported Outcomes,TCM symptom pattern (Zheng-hou) evaluation Scale,and the incidence of in-hospital complications.Safety assessment will include the physical examination,laboratory detection,any adverse events or serious adverse events,and the proportion of any complications during hospitalization.DISCUSSION:The results of this study will provide objective and scientific data with which to assess the efficacy and safety of a sequential treatment based on“integrating disease and symptom pattern” for patients with AIS.
Keywords:stroke;clearing heat and eliminating phlegm;tonifying Qi and activating blood circulation;randomized controlled trial;clinical protocols
1.INTRODUCTION
Globally,stroke is one of the leading causes of death and long‑term disability,and China accounts for roughly one-third of worldwide stroke mortality,which consequently results in a huge financial burden.1The most common subtype of stroke in China is ischemic stroke (IS),accounting for about 69.6% of all strokes.2Traditional Chinese medicine (TCM) plays an important role in the prevention and treatment of acute ischemic stroke (AIS) in China.The general practice of TCM treatments depends on the rule of “syndrome identification and treatment”,and the core elements are constantly evolving in different stages which can be altered after treatment.3Currently,the “integrating disease and symptom pattern” model is widely accepted for clinical efficacy evaluation of stroke by TCM scholars.The clinical syndrome elements of IS can be summarized as six factors including internal-wind,internal-fire,phlegm-dampness,blood-stasis,Qideficiency,andYin-deficiency,4and the nature of these symptom patterns has been explained by the application of laboratory technology.5For example,recent studies have demonstrated the association between some novel biological markers in phlegm-heat symptom pattern of AIS and have explored their value in evaluating the clinical efficacy.6
The critical period for the evolution of symptom pattern of AIS is the first week,especially 5-7 d after onset.7,8Besides,the vast majority of AIS patients regularly developed phlegm-heat symptom pattern and bowelexcess symptom pattern on the very early stage (3-5 d after onset),and the severity of these two symptom patterns can affect the prognosis significantly.9Phlegmheat is the primary pathogenic factor of stroke at the very early stage,which can be resolved by clearing heat and eliminating phlegm (CHEP) formula. Animal experiments have proven that CHEP formula can resist the pathologic injury caused by cerebral ischemia and reperfusion through various mechanisms.10Clinical trials have also shown that CHEP formula can shorten the duration of unconsciousness,11decrease the in-hospital complications such as pneumonia,12and reduce the serum concentration of inflammation indicators.13After the early stage,a substantial number of patients with phlegm-heat symptom pattern evolve into blood stasis andQideficiency symptom pattern,which can be eliminated by tonifyingQiand activating blood circulation (TQABC) formula.14,15The possible mechanism may be associate with improving the ischemia-reperfusion injury,promoting the formation of the collateral and inhibiting inflammatory reaction.16
Nevertheless,no clinical trials have evaluated the efficacy and safety of a sequential therapy with CHEP formula followed by TQABC formula in AIS based on the aforementioned theory of the evolution rules.Thus,we designed a prospective,multicenter,double-blinded,randomized controlled trial to confirm the hypothesis.
2.METHODS
2.1.Study design
This trial was designed in accordance with the Standard Protocol Items:Recommendations for Interventional Trials (SPIRIT) checklist and we will rigorously follow the Consolidated Standards of Reporting Trials(CONSORT) Extension for Chinese Herbal Medicine Formulas 2017 recommendations in reporting the results.17We registered the study at clinical trials.gov(Unique identifier:NCT04199455) in December 2019.The study will be conducted in fourteen centers in China,and a total of 500 participants will be recruited and allocated randomly to either the CHEP+TQABC treatment group or the control group.Both groups will undergo a treatment period of (15 ± 2) d and a 3-month follow-up.A flow diagram of the study is illustrated in Figure 1.Recruitment to the study started in December 2019.The trial is currently on going.
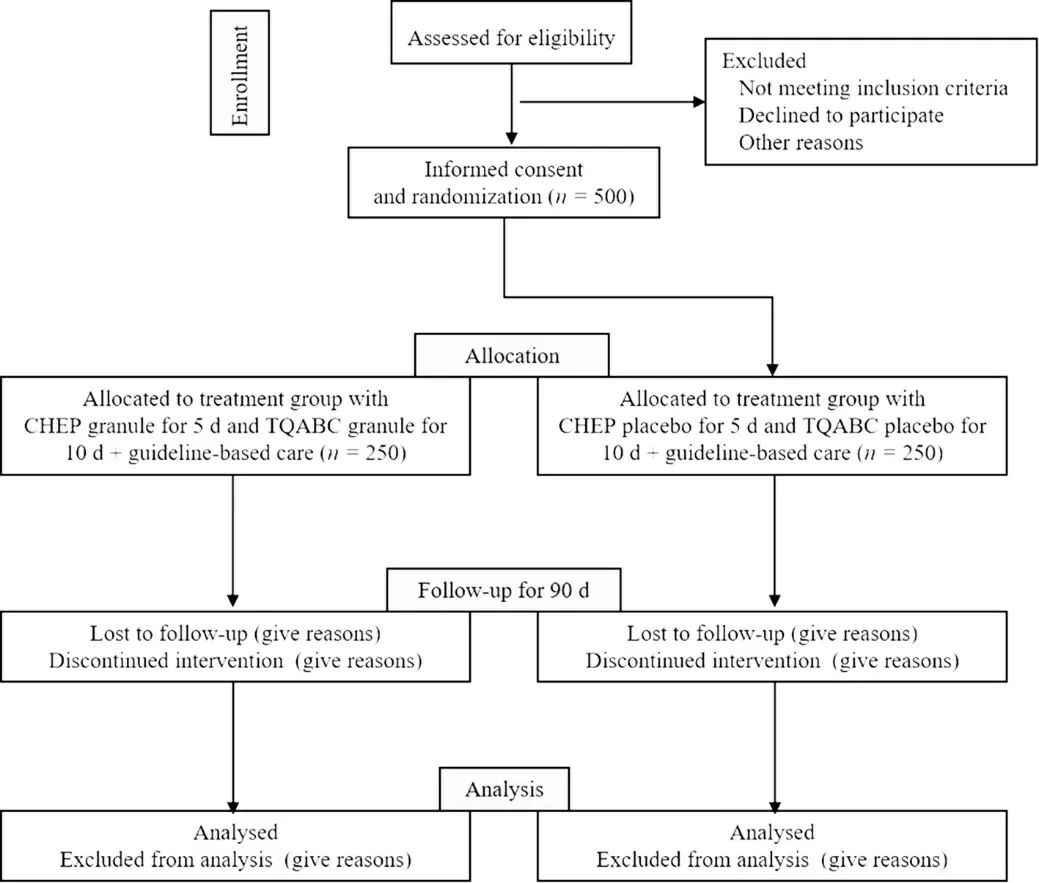
Figure 1 Trial flow chart
2.2.Ethics
Ethical approval was granted by the research ethics committee of Guangdong provincial hospital of Chinese medicine (No.BF 2019-183-01) and the other thirteen involved hospitals.Any severe adverse events (SAEs)will be reported to the committees immediately and any revisions to the study design can only be made with the permission of the committees.
2.3.Participant recruitment
Participants will be recruited from 14 tertiary public hospitals throughout China with emergency departments and neurology wards that receive patients with AIS.Every new patient with moderate-to-severe AIS within 72 h after symptom onset will be screened for eligibility by specific researchers who are responsible for enrolling the participants.
2.4.Informed consent
Written informed consent must be obtained from all participants or their legally authorized representatives before enrolment.Prior to enrollment,researchers will explain the detailed procedures of the study to the participants,and answer questions raised at length.
2.5.Diagnostic criteria for AIS
The diagnostic criteria for AIS are based on the Chinese guidelines for diagnosis and treatment of AIS in 2018.18
2.6.Diagnostic criteria for phlegm-heat pattern in TCM practice
Ischemic Stroke TCM Syndrome Factor Diagnostic Scale (ISTSFDS) can help to classify and diagnose the TCM symptom patterns objectively with application of syndrome factors.19Six syndrome factors,internal-wind,internal-fire,phlegm-dampness,blood-stasis,Qideficiency,andYin-deficiency,were included.Patients with phlegm-heat pattern are considered to be those who meet the diagnosis of both “phlegm-dampness” (phlegmdampness pattern scores ≥ 10 points) and “internal-fire”(internal-fire pattern scores ≥ 2 points) according to the ISTSFDS.
2.7.Inclusion criteria
Participants who meet all of the following criteria will be included:(a) meet the diagnostic criteria of AIS;(b) meet the diagnosis of both “phlegm-dampness” and “internalfire”;(c) enrolled within 72 h (including 72 h) from onset of present episode of stroke;(d) aged between 18 to 80 years (inclusive);(e) an initial National Institutes of Health Stroke Scale (NIHSS) score between 4 and 25 points at randomization and (f) volunteer to take part with provision of written informed consent.
2.8.Exclusion criteria
Participants who meet one or more of the following criteria will be excluded:(a) plan to or being already to receive intravenous thrombolysis or endovascular treatment;(b) have received other TCM decoctions,granules and Chinese patent medicines for stroke treatment;(c) diagnosed with stroke caused by tumors,brain trauma,hematological diseases,infectious diseases,etc.;(d) already dependent in activities of daily living before the present acute stroke (defined as modified Rankin Scale score ≥ 2 at randomization);(e) other conditions that lead to motor dysfunction;(f) significant abnormalities of liver or renal function ≥ 2 times the upper limit of the reference range;(g) severe comorbidity and life expectancy <3 months;(h) combine with other diseases that impact the evaluation of neurological function or affect compliance with completing the whole study process;(i) pregnant or breastfeeding women;(j)participate in another clinical trial and currently receiving an investigational drug.
2.9.Randomization
After providing written formed consent,all participants will be randomly allocated to the CHEP+TQABC treatment group or the control group in a 1∶1 ratio.The randomization procedure will be computer-and webbased,using permuted blocks,and will be stratified for medical center.It will not be possible to know the treatment allocation before randomization. An independent clinical statistician will record the method,process,a result of the entire production,and keep the random sequence.
2.10.Blinding
Information of primary and secondary outcomes will be assessed by independent assessors who are blind to the assignment and treatment.The research team will agree to view the blinding allocation only after completion of the trial.
2.11.Interventions
Participants in both groups will receive standard stroke care in accordance with the current national guidelines for AIS,including antiplatelet therapy,control of vascular risk factors,and appropriate rehabilitation.The following therapies will also be used.
2.12.Test group (CHEP granule +TQABC granule group)
The primary ingredients of CHEP formula include Tianma (Rhizoma Gastrodiae) Danshen (Radix Salviae Miltiorrhizae),Dahuang (Radix Et Rhizoma Rhei Palmati),Zhizi (Fructus Gardeniae),Shichangpu(Rhizoma Acori Tatarinowii),Dannanxing (Rhizoma Arisaematis Cum Bile) and Gualou (Fructus et Semen Trichosanthis).The main ingredients of TQABC formula include Chuanxiong (Rhizoma Chuanxiong),Chishao (Radix Paeoniae Rubra),Honghua (Flos Carthami),Shichangpu (Rhizoma Acori Tatarinowii),Huangqi (Radix Astragali Mongolici),Danggui (Radix Angelicae Sinensis),Taoren (Semen Persicae) and Chaihu (Radix Bupleuri Chinensis).The herbs for CHEP and TQABC will be extracted and made into granules that are soluble in hot water.Beijing Kangrentang Pharmaceutical Co.,Ltd.(Beijing,China) will manufacture,package,and labeled the granules in this study.
Participants randomized to the test group will be instructed to dissolve CHEP granules (7.35 g/bag) in 200 mL hot water and take the solution orally twice a day for the first 5 d (Days 1 to 5) after randomization.From Days 6 to 15 after randomization,they will receive TQABC granules(6.85 g/bag) dissolved in 200 mL of hot water and orally take the solution twice a day.
2.13.Control group (placebo group)
For the control group,CHEP placebo is no decocting granules contain 20% bitter gourd extract,75% dextrin and caramel pigment and also 5% original formula granule to achieve the similar appearance,color,smell,taste,and weight as CHEP formula granule.TQABC placebo contains 15% bitterant,85% dextrin and 0.1%pigment (such as lemon yellow and caramel pigment) to achieve the similar appearance,shape,color,smell,taste,and weight as TQABC formula granule.
2.14.Concomitant treatment:allowed and forbidden drugs
During the trial period [(15 ± 2) d after randomization],guideline-based standard stroke care is allowed.Any Chinese herbal decoctions,granules,or injections with therapeutic effects on stroke as well as acupuncture are forbidden.The name,dosage,route of administration,and duration of any concomitantly prescribed drugs will be carefully recorded in the Electronic Data Capture(EDC) System.
2.15.Outcome measures
Primary outcome:the primary outcome measure will be the comparison of the change in the NIHSS score from baseline to (15 ± 2) d after randomization.20,21
Secondary outcomes:the secondary outcomes focus mainly on the functional independence and the TCM symptom pattern evolution.Besides,the incidence of complications (including hemorrhagic transformation,pulmonary infection,urinary tract infection,etc) during hospitalization will also be included.
2.16.modified Rankin Scale (mRS)
The mRS is the most prevalent functional outcome measure in contemporary stroke trials.22The proportion of patients’ independent at (30 ± 2) and (90 ± 7) d after randomization defined by a mRS score of 0,1,or 2 will be analyzed.23
2.17.Barthel Index (BI)
BI is a scale that evaluates the activities of daily living,with higher scores indicating more independence.24The proportion of patients with a BI score of ≥ 90 at (30 ±2) and (90 ± 7) d after randomization will be analyzed.
2.18.Evaluation of TCM symptom pattern
Ischemic Stroke TCM Syndrome Factor Evaluation Scale (ISTSFES) has been proved to be reliable in the assessment of syndrome severity during the acute stage and early recovery stage of stroke.19ISTSFES will be evaluated at 5 d and (15 ± 2) d after randomization to determine the evolution of syndromes by trained investigators.
2.19.Patient-reported outcome (PRO) Scale of Stroke
PRO scale of stroke has been demonstrated to be reliable valid,responsible for capturing more comprehensive effects of stroke on patients participating in clinical trials of new drugs.25The PRO scale of stroke will be collected(15 ± 2) d after randomization.
2.20.Safety outcomes
The routine tests listed below will be conducted at baseline and after treatment (Day 15 ± 2).(a) routine examination of blood,urine,and stools;(b) liver and kidney function tests;(c) blood coagulation test;(d)electrocardiography;(e) ambulatory blood pressure monitoring.
2.21.Adverse events (AEs)
Any AEs or SAEs and unexpected symptoms or signs during the treatment period or at follow-up will be recorded in the EDC system,including the time of occurrence,symptoms and signs,degree,duration,laboratory findings,treatment,outcomes,and causal relationship with the treatment.SAEs will be reported to the ethics committee within 24 h,which will determine whether any additional measures should be taken.
2.22.Quality control
A series of training sessions will be conducted to ensure that investigators fully understand the protocol and master the standard operating procedures to guarantee accuracy and completeness of clinical data.Besides,Data management will be monitored by Zhonglianliding Pharmaceutical Technology Co.,Ltd.(Beijing,China)and a contract research organization (CRO) will cooperate with trial monitoring.The electronic Case Report Forms (eCRFs) will be regularly checked online by the supervisors who will be independent of the research team,and the researchers will be notified in time if any problems are found.
2.23.Data collection and management
An overview of study visits and data collection schedule of this study is shown in Table 1.Data management will be conducted according to the Technical Guidance for Clinical Trial Data Management.26All clinical data will be recorded into the EDC system conforming to the Clinical Data Interchange Standards Consortium(CDISC) standard.27Data safety and monitoring board will be established to provide independent review and evaluation of the scientific,rationality,effectiveness,and safety of the trial.
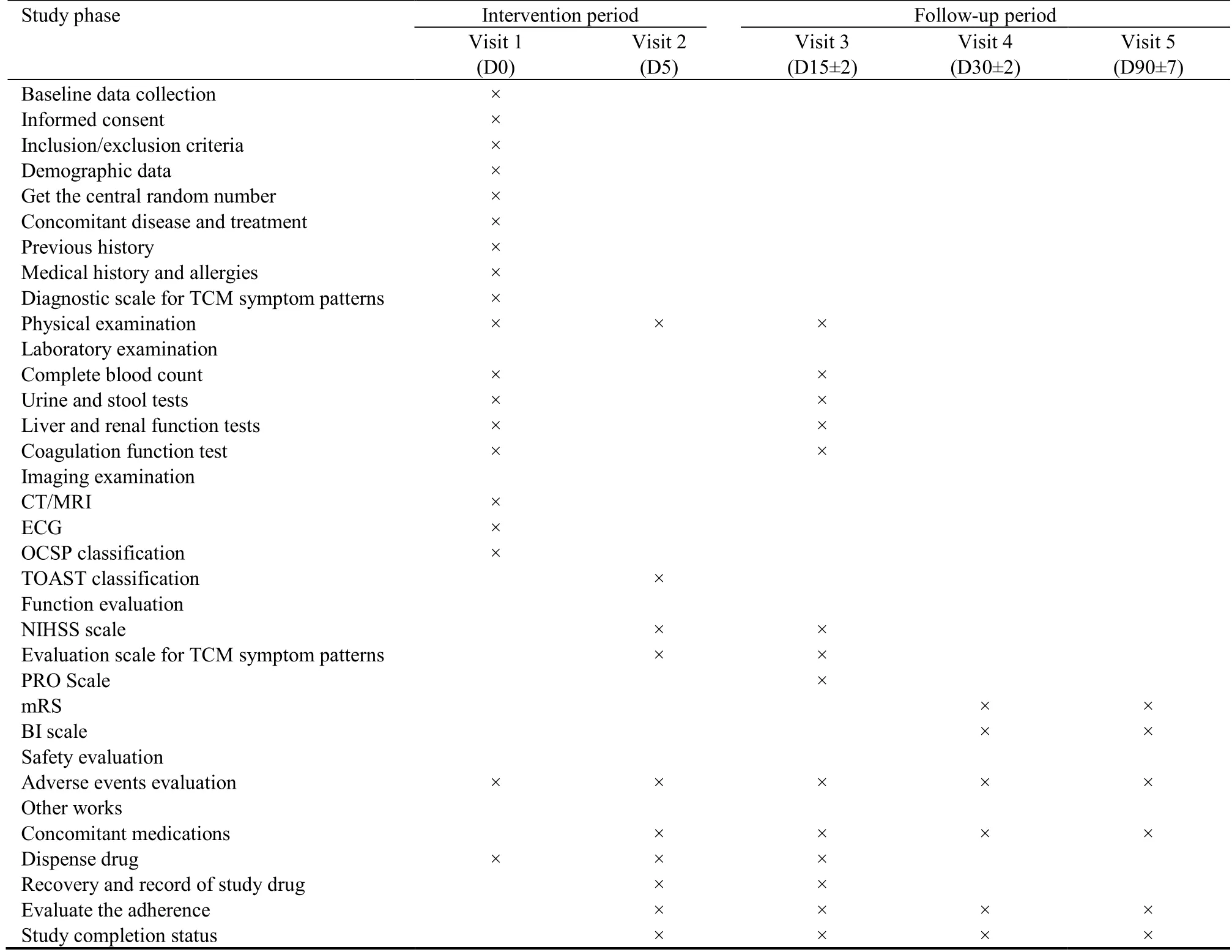
Table 1 Data collection schedule and measures
2.24.Sample size calculation
According to the pre-experiment (yet unpublished),we hypothesized that the average reduction value of the NIHSS score from the baseline was 3.22 points in the experimental group,and 2.53 points in the control group.The enrollment ratio between the CHEP+TQABC treatment group and the control group are 1∶1 with α=0.05 (two-tailed) and (1-β)=0.8.Each group needs to include at least 197 patients.Accounting for a 20%dropout rate,a total of 500 cases are required,with 250 cases in each group.
2.25.Statistical analysis
Statistical analysis will be executed on the intention-totreat principle.All statistical tests will be performed at the 0.05 level of significance unless otherwise specified.The statistical analysis system (Version 9.4,SAS Institute Inc.,Cary,NC,USA) will be used.Continuous variables will be presented as the mean ± standard deviation,while categorical variables will be presented as frequency or percentage.
Baseline characteristics will be summarized by means of simple descriptive statistics.The data collected at baseline,at the end of treatment,and at the 90-day follow-up will be compared within each group to evaluate the efficacy of each therapy.Between-group comparisons will be performed to compare the efficacy of the two therapies.The t-test (if normally distributed)or nonparametric test (if not normally distributed) will be used for continuous variables,and χ2or nonparametric tests analysis will be applied for categorical variables.APvalue of <0.05 will be considered statistically significant.Missing values will be imputed by the last observation carried forward method.
3.DISCUSSION
During the practice of TCM diagnosis and treatment,identification of symptom pattern plays an important role in the prediction of short-term prognosis of AIS.15Previous studies have found that a considerable number of patients with AIS present with phlegm-heat pattern in the early stage of symptom onset,and a week later withQi-deficiency and blood stasis pattern.As a corresponding treatment prescription,CHEP or TQABC formula has been commonly accepted and applied with favorable efficacy.However,no clinical trials have proposed a sequential treatment principle according to the evolution rules of TCM symptom pattern of AIS.
The aim of the present study is to investigate the efficacy and safety of the sequential treatment with CHEP formula followed by TQABC formula in AIS patients with phlegm-heat pattern.It is rigorously designed RCT to evaluate the compatibility between the evolution of symptom pattern and the sequential treatment formula of AIS which has important reference value for improving the appraising system of TCM clinical study.
However,the design has several limitations.Firstly,this trial lacks accurate and objective laboratory parameters to evaluate the therapeutic mechanism,and the outcome measures are all scale scores,although the objectivity of clinical efficacy evaluation can be improved by the combination of these scales.Another limitation is that our study design is based on the general rules of TCM syndrome evolution in stroke,the medication prescript in the present trial may not be appropriate for patients with specific syndromic characteristics.Notwithstanding the limitations,the results of this study will hopefully provide a reference to the clinical practice of stroke.
4.ACKNOWLEDGEMENTS
We appreciate the efforts of all the collaborators,research staffs and patients participating in this trial.And we gratefully acknowledge Prof.GAO Ying for her great contribution to the establishment and launch of this project.
5.REFERENCES
1.Wang WZ,Jiang B,Sun HX,et al.Prevalence,incidence,and mortality of stroke in China:results from a nationwide populationbased survey of 480 687 adults.Circulation 2017;135:759-71.
2.Li ZX,Jiang Y,Li H,Xian Y,Wang YJ.China's response to the rising stroke burden.BMJ 2019;364:1897.
3.Jiang M,Lu C,Zhang C,et al.Syndrome differentiation in modern research of traditional Chinese medicine.J Ethnopharmacol 2012;140:634-42.
4.Yang W,Li MQ,Li Y,et al.Exploring Chinese medicine and Western Medicine group modules in acute phase of ischemic stroke disease.Zhong Guo Zhong Yao Za Zhi 2018;43:618-26.
5.Yeh ML,Chiu WL,Wang YJ,Lo C.An investigation of the use of Traditional Chinese Medicine and complementary and alternative medicine in stroke patients.Holist Nurs Pract 2017;31:400-7.
6.Han XX,Gao YH,Ma B,et al.The clinical relevance of serum NDKA,NMDA,PARK7,and UFDP levels with phlegm-heat syndrome and treatment efficacy evaluation of Traditional Chinese Medicine in acute ischemic stroke.Evid Based Complement Alternat Med 2015;2015:270498.
7.Xin XY,Chang JL,Cao KG,Gao Y,Li S.Investigation on the relationship between each period of syndrome characteristics and short-term prognosis in early ischemic stroke based on decision tree analysis.Zhong Hua Zhong Yi Yao Za Zhi 2014;29:2647-50.
8.Xin XY,Chang JL,Gao Y.Use of generalized rule induction to study the evolution rules of syndrome of acute ischemic stroke.Zhong Hua Zhong Yi Yao Za Zhi 2016;31:3985-8.
9.Chen P,Zhang Y,Ling LL,et al.Efficacy and safety of Xinglouchengqi decoction for acute ischemic stroke with constipation:study protocol for a randomized controlled trial.J Tradit Chin Med 2017;37:810-8.
10.Wang YY,Xie YZ.Origin and development of therapy of resolving phlegm and relaxing bowels for treating syndrome of phlegm heat and bowel excess of stroke (III):spring-up features of syndrome of phlegm heat and bowel excess of stroke under imagery therapeutic mode.Beijing Zhong Yi Yao Da Xue Xue Bao 2013;20:1-4.
11.Xie YZ,Ren JT,Wang ZY,Wang YY.Influence of therapy of resolving phlegm and relaxing bowels on consciousness in patients with stroke.Beijing Zhong Yi Yao Da Xue Xue Bao 2009;16:17-9.
12.Cheng YS,Wang PL,Zhao YC.Clinical effect of retention enema with Jiaweixinglouchengqi decoction for acute ischemic stroke sthenia.Zhong Guo Zhong Yi Ji Zheng 2014;23:444-64.
13.Yang XY,Zhang GM.Curative effect of Xinglouchengqi decoction on ischemic stroke.Beijing Zhong Yi Yao Da Xue Xue Bao 2009;16:14-6.
14.Gao Q,Zhang DD,Wang YH.Preliminary study on the theory ofQideficiency and blood stasis syndrome during the recovery period of stroke.Zhong Guo Zhong Xi Yi Jie He Xin Nao Xue Guan Bing Za Zhi 2019;17:1106-7.
15.Cheng SC,Lin CH,Chang YJ,et al.Fire-heat andQideficiency syndromes as predictors of short-term prognosis of acute ischemic stroke.J Altern Complement Med 2013;19:721-8.
16.Li NF,Chen YB,Liu QH,Zou L,Huang XW.Research development on the treatment of ischemic stroke with supplementingQiand activating blood circulation.Shi Jie Zhong Xi Yi Jie He Za Zhi 2020;15:974-80.
17.Cheng CW,Wu TX,Shang HC,et al.CONSORT extension for Chinese herbal medicine formulas 2017:recommendations,explanation,and elaboration.Ann Intern Med 2017;167:112-21.
18.Peng B,Liu M,Cui LY.Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018.Zhong Hua Shen Jing Ke Za Zhi 2018;51:666-82.
19.Gao Y,Ma B,Liu Q,Wang YY.Methodological study and establishment of the diagnostic scale for TCM syndromes of ischemic stroke.J Tradit Chin Med 2011;52:2097-101.
20.Meyer BC,Lyden PD.The modified National Institutes of Health Stroke Scale:its time has come.Int J Stroke 2009;4:267-73.
21.Ghabaee M,Zandieh A,Mohebbi S,et al.Predictive ability of Creactive protein for early mortality after ischemic stroke:comparison with NIHSS score.Acta Neurol Belg 2014;114:41-5.
22.Weisscher N,Vermeulen M,Roos YB,et al.What should be defined as good outcome in stroke trials;a modified Rankin score of 0-1 or 0-2? J Neurol 2008;255:867-74.
23.Quinn TJ,Dawson J,Walters MR,et al.Exploring the reliability of the modified rankin scale.Stroke 2009;40:762-6.
24.Cabañero-Martínez MJ,Cabrero-García J,Richart-Martínez M,et al.The Spanish versions of the Barthel index (BI) and the Katz index (KI) of activities of daily living (ADL):a structured review. Arch Gerontol Geriatr 2009;49:e77-84.
25.Zhao L,Chen JQ.Patient-reported outcomes (PROs):an approach to evaluate treatment efficacy of Chinese medicine or integrative medicine.Chin J Integr Med 2005;11:151-3.
26.Santoro E,Tinazzi A.Clinical trials data management.Atlanta:American Cancer Society,2010:101-6.
27.Bruland P,Breil B,Fritz F,Dugas M.Interoperability in clinical research:from metadata registries to semantically annotated CDISC ODM.Stud Health Technol Inform 2012;180:8.
 Journal of Traditional Chinese Medicine2022年4期
Journal of Traditional Chinese Medicine2022年4期
- Journal of Traditional Chinese Medicine的其它文章
- Effectiveness of redcore lotion in patients with vulvovaginal candidiasis:a systematic review and Meta-analysis
- Efficacy and safety of external application of Chinese herbal medicine for psoriasis vulgaris:a systematic review of randomized controlled trials
- Effectiveness and safety of electroacupuncture for the treatment of pain after laparoscopic surgery:a systematic review
- Effect of astragaloside IV on the immunoregulatory function of adipose-derived mesenchymal stem cells from patients with psoriasis vulgaris
- Shenqihuatan formula (参七化痰方) reduces inflammation by inhibiting transforming growth factor-beta-stimulated signaling pathway in airway smooth muscle cells
- Drug response biomarkers of Pien Tze Huang (片仔癀) treatment for hepatic fibrosis induced by carbon tetrachloride
