N-hydroxyphthalimide anchored on hexagonal boron nitride as a metal-free heterogeneous catalyst for deep oxidative desulfurization
Lin-Jie Lu ,Pei-Wen Wu ,*,Jing He ,Ming-Qing Hu ,Yn-Hong Cho ,Ning Yng ,Lin-Lin Chen ,Wei Jing ,Lei Fn ,Hong-Bing Ji ,Wen-Shui Zhu ,***
a School of Chemistry and Chemical Engineering,Institute for Energy Research,Jiangsu University,Zhenjiang,Jiangsu,212013,PR China
b School of Pharmacy,Jiangsu University,Zhenjiang,Jiangsu,212013,PR China
c Yangzhou University,Yangzhou,Jiangsu,225002,PR China
d Fine Chemical Industry Research Institute,School of Chemistry,Sun Yat-sen University,Guangzhou,Guangdong,510275,PR China
Keywords:
ABSTRACT
1.Introduction
Recently,environmental pollution has emerged to be a major global problem because of the massive use of fossil fuels.The combustion of sulfur-containing compounds in fuels is the major cause of air pollution(Khan et al.,2020).Thusly,the clean utilization of fuel oils has turned to be an urgent demand.Currently,hydrodesulfurization(HDS)is the predominant technology for the remove of sulfides in the industry which requires high temperature(>300˚C)and pressure(>3.0 MPa)(Astle et al.,2019;Hajjar et al.,2018;Wang et al.,2020).Inevitably,the harsh conditions will result in large energy consumption.Meanwhile,the remove of aromatic sulfides,especially 4,6-dimethylbenzothiophenes(4,6-DMDBT),by HDS moderately is still a challenge.So far,several alternative desulfurization methods have been studied as a supplement to HDS for the removal of aromatic sulfides under moderate condition such as extractive desulfurization(EDS)(Lee et al.,2020;Li et al.,2013;Zhu et al.,2020),adsorptive desulfurization(ADS)(Kabtamu et al.,2020;Luo et al.,2021;Shi et al.,2020),biodesulfurization(BDS)(Silva et al.,2020;Sousa et al.,2020;Tatangelo et al.,2016)and oxidative desulfurization(ODS)(Ghubayra et al.,2019;Kampouraki et al.,2019;Yao et al.,2019).
Among them,ODS has drawn much attentions because of its low capital investment and high catalytic efficiency to the refractory aromatic compounds.In the ODS technology,the sulfides will be oxidized to the sulfoxides or sulfones with increased polarity under the assistance of oxidant and catalyst,and the oxidation product can be easily separated from the fuels by extraction,and adsorption(Luo et al.,2019;Zou et al.,2021).More recently,Kang et al.(2018)had reported using the filtration method to separate the sulfone,which was simpler and more effective.
Nowadays,various kinds of oxidants have been applied into the ODS process,for example,ozone(O3)(Ma et al.,2014),hydrogen peroxide(H2O2)(Jiang et al.,2019),organic hydroperoxides(Chang et al.,2020;Smolders et al.,2019)and molecular oxygen(O2)(Dong et al.,2019;Song et al.,2019).And the O2is an ideal one because of its low price and environmentally friendly characteristics.Nevertheless,it is difficult to be activated due to its high activation barrier(Zhang et al.,2012).Therefore,the seeking of an appropriate catalyst to activate O2efficiently is the central topic of this work.
N-hydroxyphthalimide(NHPI)has lately shown notable performances in the aerobic catalysis of multiple organic compounds under moderate conditions by a free-radical chain mechanism,among them the NHPI is converted to phthalimido-N-oxyl(PINO)radical in situ under the aerobic condition to advance the reaction.The process can be accelerated in the presence of an initiator,such as transition metals salts,especially Co2+ions salts(Arzumanyan et al.,2018;Hruszkewycz et al.,2017;Ma et al.,2019;Mahmood et al.,2018).Despite the high efficiency of NHPI,its further application in an industrial scale is hindered due to the difficulty in recovering from the reaction system.What's more,the generated PINO radical is unstable and will decompose under aerobic conditions(Rajabi and Karimi,2005).
To overcome this issue,many researchers have reported the strategy of immobilize NHPI on certain supports.Blandez et al.(2018)reported a heterogeneous catalyst in which NHPI anchored on diamond nanoparticles(DH)and was applied to the selective oxidation of benzylic hydrocarbons and cyclic alkenes.The NHPI/DH catalyst can be recycled three times with the minimum turnover number of 20600.Dhakshinamoorthy et al.(2012)loaded the NHPI onto the metal-organic framework(MOF)material for catalyzing cycloalkenes aerobic oxidation.It wasfound that there wasonlya slight decrease in theconversionof cyclooctene oxidationafter tworeuses.Hence,itcan be efficient to find a proper support to heterogenization the NHPI for boosting catalytic activity and regeneration.
Up to now,various materials including titanium dioxide(TiO2),mesoporous silica(SiO2),magnesium oxide(MgO)and MOFs(Chen et al.,2020;Khan et al.,2014;Wang et al.,2021;Xun et al.,2016;Zeng et al.,2020)etc.have been widely investigated as supports.Although it promotes the separation,the introduction of these supports also brings a lot of shortages.For example,the MgO with low specific surface areas(SSAs)is unable to achieve the effective dispersion of active centers.The pore structure of SiO2with high SSAs would be clogged and aggregated during the reaction process,resulting in a decrease in catalytic activity.Additionally,the synthesis of MOFs is relatively complicated,which is an obstacle for their further applications.Recently,the newly developed twodimensional hexagonal boron nitride(h-BN)material,an analogues of graphene,has achieved great attention because of its high SSAs,high thermal stability and good chemical inertness,which is suitable for use as support under aerobic oxidative conditions(Wu et al.2017,2020;Zhu et al.,2014).
Herein,we explored an NHPI/h-BN-catalyzed oxidative desulfurization system.The catalyst was synthesized via the facile impregnation method.The heterogenization process of loading NHPI on h-BN can achieve an effective dispersion of active centers,thereby improving the desulfurization performance of 95% within 6 h with a proper loading amount.During the reaction,the azodiisobutyronitrile(AIBN)was used as an initiator instead of the traditional metal salts to prevent secondary metal pollution to fuel oils.Additionally,the prepared heterogeneous catalyst is facilitated for the regeneration procedure after ODS reaction.
2.Experimental
2.1.Materials
N-hydroxyphthalimide(NHPI,98%)and dodecane(C12H26,98%)were obtained from Shanghai Macklin Biochemical Co.,Ltd.Azodiisobutyronitrile(AIBN,99%)and hexadecane(C16H34,AR)were obtained from Aladdin industrial corporation.Methylene chloride(CH2Cl2,AR)was obtained from Shanghai Sinopharm Chemical Reagent Co.,Ltd.4-Methyldibenzothiophene(4-MDBT,97%),dibenzothiophene(DBT,98%),4,6-dimethydibenzothiophene(4,6-DMDBT,97%),and hexagonal boron nitride(h-BN,98%)were obtained from Sigma-Aldrich.
2.2.Characterizations
Scanning electron microscopy(SEM)was recorded on a JSM-6010PLUS/LA electron microscope.X-ray powder diffraction(XRD)patterns were measured by a XRD-6100 Lab(Shimadzu,Japan)with Cu-Kαradiation(λ=1.5406Å)at a scanning rate of 7˚min-1.Fourier transform infrared(FT-IR)spectra were operated by a Nicolet iS50 FT-IR spectrometer using KBr as diluents.The solid state1H MAS NMR spectra were acquired from a AVANCE III 400 MHz nuclear magnetic resonance spectrometer(Bruker,Germany).
2.3.Synthesis of samples
A certain mass of commercial-grade NHPI was first dispersed in 10 mL of ethanol under 60˚C.Subsequently,the commercial-grade h-BN was added in at a certain mass ratio,and then condensed and refluxed at 60˚C for 7 h.After removing most of the solvent by rotary evaporation,it was dried at 60˚C to obtain a catalyst with a different NHPI loading.The samples were denoted as NHPI/h-BN-x,where x(25,30,35)represented the mass fraction of NHPI in the catalysts.
2.4.Examination of ODS performance
The model fuels with an initial S-content of 200 ppm were prepared by dissolving different aromatic sulfides in dodecane,respectively,in which 4000 ppm of hexadecane was added as internal standard.
In the catalytic oxidative reaction,catalyst(50 mg)and model fuel(20 mL)were dumped into a 50 mL three-necked flask.Simultaneously,10 mg of AIBN was added as an initiator and carried out under 120˚C with vigorously stirred,and the air from the atmosphere was continuously blew into the system with 100 mL min-1.After reaction for a certain time,the upper oil phase was taken out and analyzed by a gas chromatograph(GC,Agilent-7890A),which was equipped with a flame ionization detector(FID)and a HP-5 type column(30 m×0.32 mm×0.25μm).The sulfur conversion was used to evaluate the efficiency of sulfide being oxidized to the corresponding sulfone and calculated through the following formula:where the C0referred to the initial S-concentration and the Ctreferred to the S-concentration after reacting for t h.
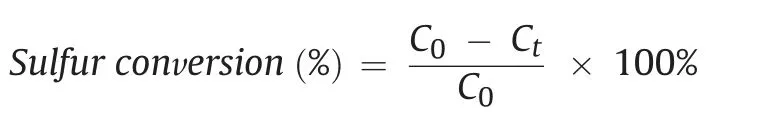
After the reaction,the oxidation products were determined via a gas chromatography-mass spectrometry(GC-MS,Agilent 7890A-5975)with the following program.GC injector temperature:200˚C.Oven:heating from 100˚C to 200˚C with a rate of 15˚C min-1,holding for 1 min,and then raising to 240˚C with 10˚C min-1.Column type:HP-5(30 m×0.32 mm×0.25μm).MS adopts EI mode with the ion source temperature of 230˚C.
During the real oil oxidation reaction,100 mg of NHPI/h-BN-30 and 20 mg of AIBN were added to the reaction equipped with 40 mL of real oil with the air rate of 100 mL min-1.The sulfur content was detected by the ultraviolet fluorescence sulfur meter(GCTS-3000).
3.Results and discussion
Firstly,SEM was carried out to directly investigate the morphology of sample(Fig.1),in which the NHPI/h-BN-30 catalyst exhibited a typical two-dimensional graphene-like structure of h-BN(Wu et al.,2020),indicating that the morphology of the catalyst has no change after immobilizing NHPI.Meanwhile,the corresponding elemental mapping images showed that all of the elements of N,O,C were uniformly dispersed on the h-BN surface(Fig.1b),suggesting the fine dispersion of NHPI after the heterogenization process.
The detailed structure of the synthesized catalyst was further determined by XRD(Fig.2a).The diffraction peaks in the XRD pattern of NHPI/h-BN-30 centered at 25.6˚and 41.6˚were attributed to the(002)and(100)planes,respectively,which consistent with the hexagonal arrangement of h-BN(Zhu et al.,2016).Besides,the characteristic peaks of NHPI can also be detected in NHPI/h-BN-30,indicating that NHPI has been successfully loaded onto h-BN.
The strong absorption peaks at 818 cm-1and 1397 cm-1in FT-IR spectra(Fig.2b)were attributed to the out-of-plane bending vibration and in-plane stretching vibration of B-N bond,respectively(Chen et al.,2018).Importantly,the weaker peaks at 1703 cm-1and 699 cm-1can be assigned to the characteristic peaks of benzene ring in NHPI and no shift of the peaks occurred.All of these characterizations confirmed the successful preparation of the heterogeneous NHPI/h-BN catalyst and the structure of h-BN were largely maintained.
In the1H MAS NMR patterns(Fig.3),the chemical shift at 6.006 ppm of NHPI shifted to the high field(6.288 ppm)after being immobilized onto h-BN,indicating the change of chemical environment of H atom in NHPI.Additionally,a new split peak can be observed at 0.610 ppm,owing to the deviation of the H atom in NHPI,suggesting the formation of a chemical interaction between NHPI and h-BN,which will be beneficial for the stability of the catalyst.
4.Investigation of catalytic activity of catalysts
The effect of different NHPI loading amounts on ODS was firstly examined in Fig.4.The catalytic activity with different loading amounts of 25%,30%,and 35%was investigated respectively.It can be observed that the catalyst with a 30% loading amount had the best catalytic activity of 95% DBT conversion within 6 h under 120˚C.When the loading amount increased to 35%,the desulfurization performance dropped obviously to 56%,which was owing to the agglomeration of the active center.Therefore,the catalyst of NHPI/h-BN-30 with a loading amount of 30% was selected as the optimum one for the subsequent studies.
Subsequently,different desulfurization experiments were carried out to assess the effect of the heterogenization process.Interestingly,the catalytic performance of NHPI/h-BN-30 was improved to 95%after heterogenization,compared to the 55%DBT conversion of pristine NHPI(Fig.5,curves c and d),and the 20%DBT conversion of commercial bulk h-BN(curve b).Similarly,the catalytic activity of the physical mixture of NHPI and h-BN was only 57%(curve e).This indicated that the loading of NHPI was favorable for dispersing the active site,thereby improving the desulfurization performance.What's more,the performance of NHPI/h-BN-30 was also studied without the addition of AIBN(curve a)and the DBT conversion decreased sharply to 48%,suggesting the important role of AIBN as an initiator to promote the generation of radicals.
To confirm the superior ODS catalytic performance of this work,the comparison of catalytic activity on different catalysts with h-BN as support was listed in Table 1.Most of the catalytic systems used metal-base material as the active center,which to a certain extend increased the investment during the practical industry application.Meanwhile,the metal-free system can effectively prevent secondary metal pollution in oil.Furthermore,compared to the Pt/h-BN catalyst(entries 1 and 2),the reaction temperature of this work was lower,which was beneficial to reduce the energy consumption.And in terms of the catalyst usage amount,the amount of this system was also relatively small.In summary,this work shows better catalytic performance than previously reported ones.
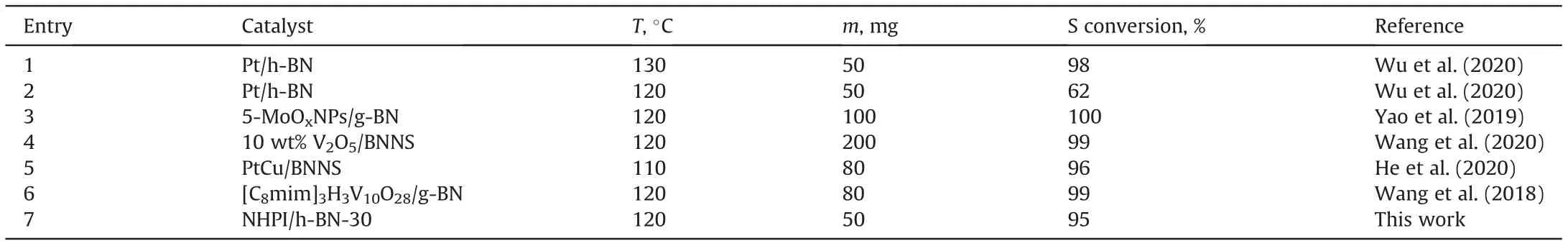
Table1 Comparison of ODS performance on different h-BN based catalysts*.
Moreover,the desulfurization performance of different substrates was investigated in Fig.6a and the conversion of DBT,4,6-DMDBT,and 4-MDBT were 97%,95%,and 62%,respectively,after 8 h.The difference in the catalytic activity was mainly related to the f-(r)Fukui function,which indicated the sensitivity of sulfur atoms to the electrophilic attacks(Li et al.,2016).The f-(r)values of different sulfides were 0.331(DBT),0.292(4-MDBT),and 0.304(4,6-DMDBT),respectively.Generally,the value of f-(r)is positively correlated with catalytic activity,so the 4-MDBT exhibits the lowest reactivity.Besides,it is worth noting that a deep desulfurization(residual S-content<10 ppm)of 4,6-DMDBT,which is considered to be the refractory sulfide in the HDS technology,can be achieved,suggesting the superiority of this catalyst.
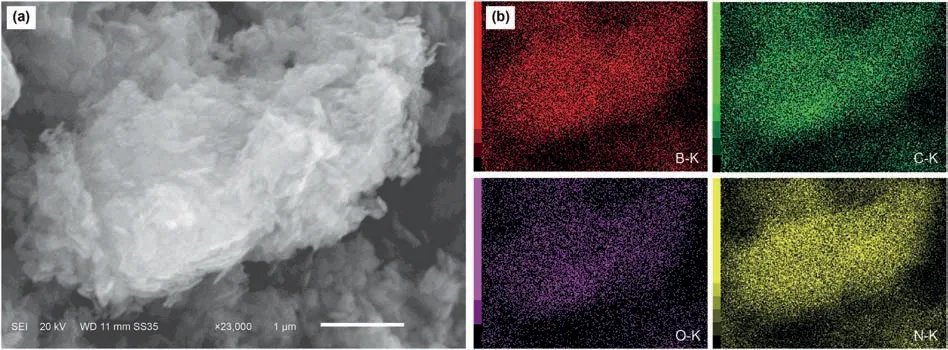
Fig.1.(a)SEM image of NHPI/h-BN-30;(b)Elemental mapping images of NHPI/h-BN-30.

Fig.2.(a)XRD patterns and(b)FT-IR spectra of NHPI,h-BN and NHPI/h-BN-30.
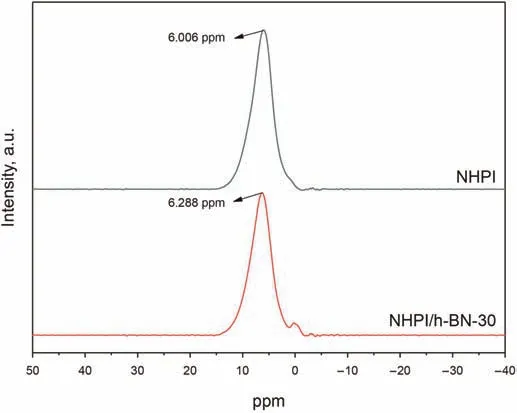
Fig.3.1H MAS NMR patterns of NHPI and NHPI/h-BN-30.
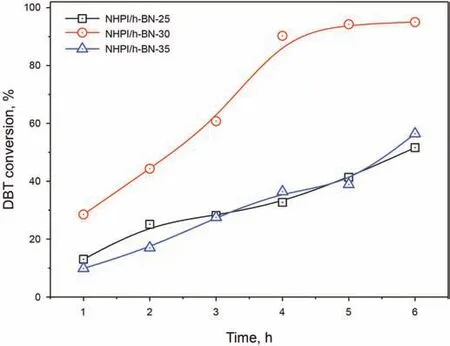
Fig.4.Effect of the NHPI loading amount on the DBT conversion.
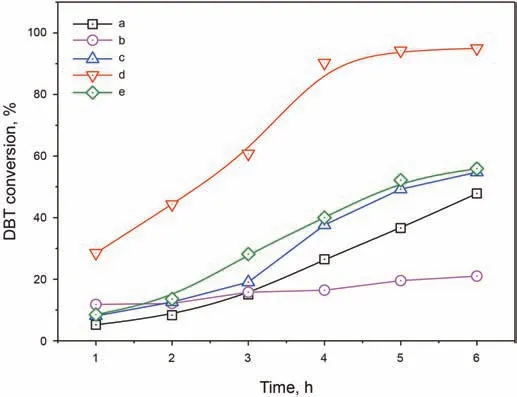
Fig.5.Catalytic performance of different desulfurization systems.Experiment conditions:m(catalyst)=50 mg,m(AIBN)=10 mg,V(model fuel)=20 mL,T=120˚C.
In addition,fluid catalytic cracking(FCC)diesel was selected as the representative real oil to examine the efficiency of NHPI/h-BN-30 in real oil.The initial sulfur content of real oil was 554 ppm and decreased to 446 ppm after extraction once by acetonitrile.Similarly,the reacted oil was extracted by acetonitrile for once to remove the produced sulfone.The result was showed in Fig.6b.After reaction for 8 h,the residual S-content in real oil dropped sharply to 123 ppm with the sulfur removal of 78%.The presence of aromatic hydrocarbons and olefins in real oil may affect the catalytic performance to some extent(He et al.,2021).
To deeply understand the oxidation process,the GC-MS was performed to determine the oxidation product(Fig.7).The upper oil phase was measured by GC-MS directly.The solid catalyst was extracted by CH2Cl2and the extraction phase was collected for detection.The main species in the oil phase was DBT(m/z=184.0),and in the solid catalyst phase,only the DBTO2(m/z=216.0)signal can be observed,which suggested that the DBTO2was the only oxidation product and will be absorbed onto the catalyst surface(Xun et al.,2020).It is conducive to the removal of sulfides with the separation of catalysts.
The ESR characterization was then carried out to further investigate the active intermediate during the reaction.As shown in Fig.8 that there was no ESR signal in the absence of NHPI/h-BN-30 or O2,which proved that the catalyst and oxidant wereindispensable for the generation of free radicals.With the introduction of O2,there was a signal which attributed to the superoxide radical(O).Meanwhile,with the addition of AIBN,the intensity of the signal increased significantly,indicating that the presence of AIBN can indeed promote the generation of free radicals which was consistent with the experimental results of Fig.5.At the same time,the selective quenching experiments were performed to further determine the active oxygen species,in which BQ or TBA was used as the quenchers of Oor hydroxyl radicals(HO·),respectively.The catalytic performance did not changed after the addition of TBA,whereas the injection of BQ inhibited the ODS performance greatly,demonstrating the presence of Oradicals.

Fig.6.(a)Catalytic performance of different substrates;(b)Desulfurization efficiency of NHPI/h-BN-30 to real oil.Experiment conditions:(a)m(NHPI/h-BN-30)=50 mg,m(AIBN)=10 mg,V(model fuel)=20 mL,T=120˚C;(b)m(NHPI/h-BN-30)=100 mg,m(AIBN)=20 mg,V(FCC diesel)=40 mL,T=120˚C.
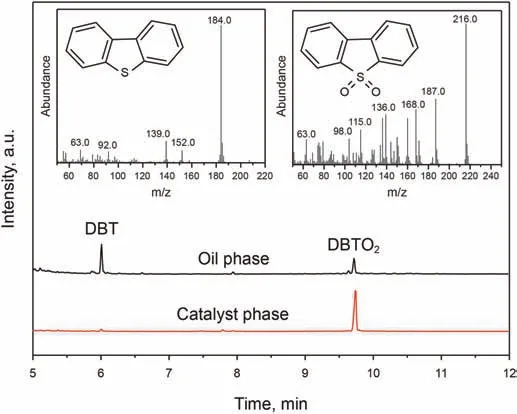
Fig.7.Detection of oxidation product by GC-MS.
Hence,a mechanism can be raised based on the above results.Firstly,the NHPI was converted to PINO in situ under the oxidative condition and subsequently activated the O2to Oradicals.At the same time,the DBT turned to sulfur-centered cation radical(Gu et al.,2017)and reacted with the Oradicals to generate the corresponding sulfones species(Fig.9).After that,the oxidation products will adsorb onto the catalyst surface,thus can realize the removal of sulfides with the separation of the catalyst.During this process,the presence of AIBN can accelerate the generation of PINO radicals and promote the activation of O2.
5.Regeneration performance
The regeneration of the catalyst after the ODS process was performed as follows:Firstly,after standing a while,most of the model fuel was removed from the reaction system through decantation,followed by centrifugation to remove the remaining model fuel.The catalyst was then dried for the next reaction.After being recycled three times,the desulfurization efficiency slightly dropped to 92%,which resulted from the accumulation of sulfone on the catalyst surface and it was raised to 95% by washing with CH2Cl2to remove the sulfone.And a deep DBT conversion of 94%can still be achieved after being recycled seven times(Fig.10).
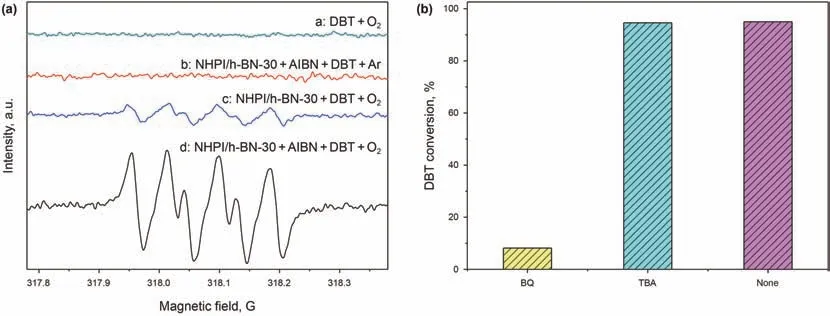
Fig.8.(a)ESR spectra of the NHPI/h-BN-30 system;(b)Selective quenching experiments.

Fig.9.The reaction mechanism of catalytic oxidation of DBT.
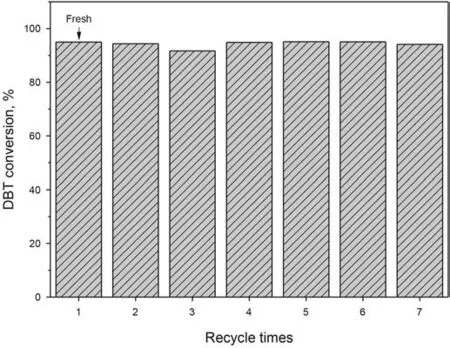
Fig.10.Regeneration performance of NHPI/h-BN-30.
The FT-IR and XRD characterizations of the used catalyst were performed in Fig.11 to confirm the structure change before and after the reaction.In the FT-IR(Fig.11a),the characteristic absorption peaks of NHPI/h-BN-30 can still be detected,indicating the structural stability of the sample.And new adsorption peaks around 1164 cm-1and 1284 cm-1can be obtained in the used catalyst,which resulted from the S=O symmetric and asymmetric vibration,respectively,confirming the presence of DBTO2adsorbed on the catalyst surface(Nisar et al.,2011).Similarly,no change can be observed in the diffraction peaks of NHPI/h-BN-30 before and after the reaction.And the new diffraction peaks in the used catalyst can be ascribed to the DBTO2(Fig.11b).In general,the catalyst was stable during the ODS reaction with oxidation product adsorbed on the catalyst.
6.Conclusions
In summary,a heterogeneous metal-free catalyst was synthesized by a simple impregnation method successfully with commercially available NHPI anchored on h-BN.The NHPI/h-BN catalyst is highly effective for the oxidation of aromatic sulfides with 95%conversion of DBT using metal-free AIBN as the initiator at 120˚C.GC-MS proved the fully selective conversion of DBT to the sulfone of DBTO2.Based on the serious characterizations,a reasonable mechanism can be proposed that the O2was activated to the Oradicals by NHPI after it converted to PINO radicals with the assistance of AIBN,subsequently,oxidized the sulfides to the sulfones and adsorbed onto the catalyst surface.Additionally,the regenerability of the catalyst was confirmed with a 94% DBT conversion after being recycled seven times.
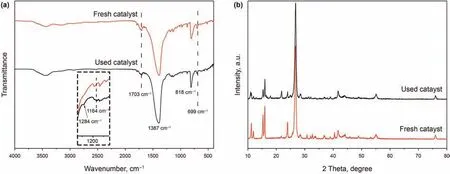
Fig.11.FT-IR spectra and XRD patterns of NHPI/h-BN-30 before and after reaction.
Acknowledgements
All authors appreciate the financial support from the National Key R&D Program of China(No.2017YFB0306504),National Natural Science Foundation of China(No.22008094,22178154 and 21878133),Chinese Postdoctoral Science Foundation(No.2019M651743,2020M671364 and 2020M673039),Natural Science Foundation of Jiangsu Province(No.BK20190852),Natural Science Foundation for Jiangsu Colleges and Universities(No.19KJB530005).
- Petroleum Science的其它文章
- Sedimentary characteristics and implications for hydrocarbon exploration in a retrograding shallow-water delta:An example from the fourth member of the Cretaceous Quantou Formation in the Sanzhao depression,Songliao Basin,NE China
- Study of the gas sources of the Ordovician gas reservoir in the Central-Eastern Ordos Basin
- A novel hybrid thermodynamic model for pore size distribution characterisation for shale
- Microstructural analysis of organic matter in shale by SAXS and WAXS methods
- Investigation of oil and water migrations in lacustrine oil shales using 20 MHz 2D NMR relaxometry techniques
- Fast least-squares prestack time migration via accelerating the explicit calculation of Hessian matrix with dip-angle Fresnel zone

