Current progress and emerging technologies for generatingextrapancreatic functional insulin-producing cells
Md Aejaz Habeeb, Sandeep Kumar Vishwakarma, Safwaan Habeeb, Aleem Ahmed Khan
Abstract Diabetes has been one of the major concerns in recent years, due to the increasing rate of morbidity and mortality worldwide. The available treatment strategies for uncontrolled diabetes mellitus (DM) are pancreas or islet transplantation. However, these strategies are limited due to unavailability of quality pancreas/ islet donors, life-long need of immunosuppression, and associated complications. Cell therapy has emerged as a promising alternative options to achieve the clinical benefits in the management of uncontrolled DM. Since the last few years, various sources of cells have been used to convert into insulin-producing β-like cells. These extrapancreatic sources of cells may play a significant role in β-cell turnover and insulin secretion in response to environmental stimuli. Stem/progenitor cells from liver have been proposed as an alternative choice that respond well to glucose stimuli under strong transcriptional control. The liver is one of the largest organs in the human body and has a common endodermal origin with pancreatic lineages. Hence, liver has been proposed as a source of a large number of insulinproducing cells. The merging of nanotechnology and 3D tissue bioengineering has opened a new direction for producing islet-like cells suitable for in vivo transplantation in a cordial microenvironment. This review summarizes extrapancreatic sources for insulin-secreting cells with reference to emerging technologies to fulfill the future clinical need.
Key Words: Diabetes mellitus; Cell-based therapy; Insulin producing cells; Extrapancreatic sources; Biomaterials; Tissue engineering
lNTRODUCTlON
Currently diabetes mellitus (DM) and associated complications have been a major concern due to the continuous increasing rate of morbidity and mortality worldwide. DM is caused by insufficient insulin production due to autoimmune destruction of functional β-cells [insulin-dependent type 1 DM (T1DM)] and insulin resistance [non-insulin-dependent type 2 DM (T2DM)] and subsequent β-cell apoptosis within the pancreas[1]. The pancreas is an acinar gland and a critical controller of blood glucose levels. It has both exocrine and endocrine functions. The endocrine part of the pancreas consists of small islands of cells, called the islets of Langerhans, that encompass α-cells (involved in secretion of glucagon), β-cells (involved in insulin secretion), δ-cells (involved in somatostatin secretion), ε-cells (involved in release of ghrelin), and PP cells (release of pancreatic polypeptide). These hormones upregulate or downregulate depending upon the blood glucose levels and inadequate secretion of these hormones may result in uncontrolled glycemic regulation in our circulation causing T1DM or T2DM[2].
The currently available treatment approaches for uncontrolled DM are pancreas and islet transplantation[3,4]. However, these strategies are limited due to unavailability of quality pancreas or islet donors, life-long need of immunosuppression, and associated complications. Exogenous insulin administration is one of the clinically established approaches for patients with T1DM[5]; however, this strategy does not completely control blood glucose level and impaired insulin release, resulting in continuous abnormal functioning of β-cells. Hence, there is an urgent need to develop clinically relevant and scalable strategies to replenish the loss of β-cell function to provide long-term recovery. Cell therapy has emerged as one of the promising alternatives to achieve the required clinical benefits in both T1DM and T2DM.
Since the last decade, various sources other than pancreas-derived cells have been used to generate insulin-producing β-like cells[6-8]. These extrapancreatic sources of cells may play a significant role in βcell turnover and insulin secretion in response to environmental stimuli. However, the most critical limitation with such extrapancreatic source of cells is that they do not respond to glucose stimuli, and do not mimic clinical conditions. In addition, the production of insulin-producing cells (InPCs)in vitroas well asin vivorequires complicated protocols which should be glucose responsive. Among various extrapancreatic sources, the liver has been considered as one of the most appropriate options for isolating large numbers of cells with the potential to dedifferentiate into InPCs due to their similar embryonic developmental origin from the endoderm. The stem/progenitor cells from the liver respond well to glucose stimuliin vitroandin vivo. Hence, the liver could be one of the most suitable sources of large numbers of functional InPCs. This review summarizes the few crucial extrapancreatic sources that are currently proposed to generate InPCs, along with other advancements in this field that may fulfill the current clinical needs for the welfare of DM patients.
EXTRAPANCREATlC SOURCES OF β-CELLS
Hepatic stem/progenitor cells
Studies have demonstrated that liver cells can be converted into InPCs under the influence of several growth factors and a high-glucose environment[9-11]. However, several questions remain to be addressed regarding which cell type undergoes differentiation and the minimum cascade of genes essential to activate or inactivate to generate fully functional β-cells. An initial study by Zalzmanet al[12] in 2003 reported successful reversal of hyperglycemia in mice using fetal liver progenitor cells that were converted into InPCs. This study gave a boost for utilization of human fetal liver progenitors to obtain significant numbers of InPCs and develop relevant protocols to be applied in clinical settings. In support of the above study, Caoet al[13] in 2004 reported the importance of liver cells in reversal of hyperglycemia in diabetic mice, using a rat liver cell line cultured in high-glucose medium. However, cells failed to produce enough insulin when injectedin vivo, which is comparatively very less to desired insulin content (< 1%) obtained from the native β-cells. Hence, it was assumed that primary cells are of utmost important for furtherin vivostudies.
In our preliminary study, we have demonstrated the successful production of InPCs from human fetal liver-derived EpCAM+stem/progenitor cells in response to glucose stimuliin vitro[14]. Following to this study, we also reported a cascade of transcription factors that are required to convert EpCAM+human fetal liver-derived stem/progenitor cells into InPCsin vitrounder a high-glucose microenvironment (Figure 1). This study revealed that activation of master regulator Pdx-1 with β-cell-specific transcription factor Nkx-6.1 in combination with Ngn-3, Pax-4, Pax-6 and Isl-1 is required to transdifferentiate hepatic stem/progenitor cells into InPCs under hyperglycemic challenge without the need of any genetic manipulation, which is crucial for its potential clinical relevance. The study also showed that the amount of insulin production was higher compared to that with other developed protocols. This particular strategy has the additional advantage for its clinical potential of scalability and lack of genetic manipulation.
Embryonic stem cells
Embryonic stem cells (ESCs) are derived from the inner cell mass of blastocysts. ESCs are pluripotent and can produce cells present in all three germ layers, including InPCs, when placed in appropriatein vitroorin vivoconditions. Due to self-renewal and differentiation potential, ESCs can be used to produce large numbers of desired cell types for downstream applications. Different derivatives of ESCs have shown restoration of functional and structural benefits in injured organs/tissues[15]. Hence ESCs are considered a good source for the generation of large numbers of InPCs. D’Amouret al[16] in 2006 demonstrated a stepwise protocol involving five stages for the conversion of ESCs into InPCs in response to a number of secretagoguesin vitro. Recently, Vegaset al[17] in 2016 also developed a multistep protocol for converting ESCs into β-cells and achieved long-term glycemic control in immunocompetent mice using an encapsulation approach. However, these strategies are not clinically relevant due to their non-responsive nature to glucose stimuli, and ethical issues. Zhanget al[18] in 2009 developed a four-step protocol for converting ESCs into insulin/C-peptide-producing cells in response to glucose stimuli. Based on the clinical applicability of this protocol, Huaet al[19] in 2014 developed a four-step protocol to convert human ESCs into pancreatic InPCs and successfully corrected the hyperglycemia in immunodeficient mice. These studies have boosted our knowledge for the experimental conversion of ESCs into functional β-cells; however, the clinical applicability of these approaches is still questionable due to ethical concerns and complicated protocols, and importantly, these experiments were conducted in immunocompetent mice that do not mimic clinical conditions. Hence, there is still a need to discover alternative sources of human β-cells that can mimic clinical conditions and can be applied in clinical settings without the need for immunosuppression. More studies are desired to obtain homogeneous populations of functional human InPCs from ESCs and further to answer whether an inductive or selective mechanism of differentiation can be clinically relevant or not.
Bone-marrow-derived stem cells
Bone-marrow-derived stem cells (BMSCs) are considered one of the potential sources for generating functional pancreatic β-cells[20,21]. Several studies have demonstrated experimental conversion of BMSCs into InPCs[22-25]. However, the amount of insulin/C-peptide secreted by these cells was less compared to that from isolated pancreatic islets and was not enough to reverse hyperglycemia in diabetic rats. Several other studies have reported similar problems with BMSCs[26,27]. Some of the results of these studies were nonreproducible, which warrants their further investigation for clinical applicability.
Umbilical cord blood cells
Human umbilical cord blood (UCB) is known to contain stem/progenitor cell populations that can be converted into different types of organ-specific cells including InPCs. UCB is considered one of the most appropriate sources of stem/progenitor cells because of fewer ethical concerns and biological waste materials. Human UCB can also be readily available in sufficient amounts with low risk of graft rejection[28,29]. A few studies have successfully reported the conversion of human UCB-derived stem cells into functional InPCs by activating several crucial pancreatic transcription factors (Pdx-1, Isl-1, Pax-4 and Ngn-3), which is capable of correcting hyperglycemia in diabetic mice[30-33]. However, for their clinical applicability, more appropriate protocols need to be developed with suitable transplantation strategies to support long-term cell survival and function post-transplantationin vivo.
Fibroblast cells
A recent study by Zhuet al[34] in 2016 demonstrated that fibroblasts of adults or neonates can generate precursors of endodermal lineages following the cell activation and signaling directed transdifferentiation paradigm. They developed conditions for expansion of glucose responsive β-like transdifferentiated pancreatic endodermal cells into the progenitor stage. These transdifferentiated cells can control hyperglycemia in mice and provide a new approach for the production of patient-specific InPCs for studying unresolved questions in pancreatic biology, disease modeling and drug testing strategies.

Figure 1 Schematic representation showing activation of pancreatic transcription factors in human hepatic progenitor cells under hyperglycemic conditions to generate insulin producing cells similar to de novo pathway. InPCs: Insulin producing cells.
Induced pluripotent stem cells
Patient-specific cell lines can be generated using induced pluripotent stem cells (iPSCs), which can then be converted into cells of interest for disease models or cell replacement therapy. Current advances in iPSCs research have evolved several robust protocols for the production of patient specific functional βcells. These differentiation protocols resemble with the same developmental stages ultimately targeting the final differentiated β-cells[35]. To trigger the pathways required for differentiation phases, a mixture of signaling molecules and growth factors are often utilized, with the aim of mimicking embryonic stages of developmental. For converting somatic cells towards iPSCs, the Yamanaka factors (OCT4, KLF4, SOX2 and c-MYC) are commonly used[36].
Several studies have examined the role of iPSCs-derived β-cells in the context of T1DM[37,38]. Maehret al[39] in 2009, for the first time demonstrated conversion of iPSCs derived from T1DM patients into glucose-responsive functional InPCs[39]. Millmanet al[40] in 2016 compared differentiation potential of iPSCs derived from diabetic and nondiabetic individuals and concluded that both the sources of iPSCs encompass similar expression patterns of the specific surface markers, and capacity of insulin secretion bothin vitroandin vivo[40]. Despite progress towards potential applicability of iPSC-derived β-cells, there are technical difficulties and financial concerns related to iPSC therapy in clinical settings[41]. To alleviate the financial concern, iPSC biobanks with ability to match the majority of HLA types and occasional blood types are continuously being explored. However, the genomic instability involving chromosomal aberrations or mutations, as well as tumor formation remain obstacles for bench to bedside clinical translation of iPSCs.
Other sources
In addition to the above extrapancreatic sources, several other source of tissues have also been investigated that contain progenitor cell populations which can be converted into InPCs. Among these sources, spleen[42], adipose tissues[43], blood[44], amniotic membrane, and central nervous system[45] have showed β-cell-specific markers duringin vitroorin vivotransdifferentiation (Table 1). In particular, Kodamaet al[42] in 2003 demonstrated that injected splenocytes can be converted into InPCs and minimize the onset of autoimmunity. Transplantation of these cells combined with Freund’s adjuvant improves diabetes in nonobese diabetic (NOD) mice. However, subsequent reports[46,47] found no such evidence which questions their clinical applicability.
EMERGlNG TECHNOLOGlES FOR CELL TRANSPLANTATlON lN DlABETES
Microencapsulation
Cell encapsulation technology is based on the concept of immunoisolation. Because islet cells can be effectively harvested and transplanted, encapsulation technology is promising for future clinical transplantation. Therefore, during the last three decades, various encapsulation strategies have been demonstrated in different animals such as mice[48], rats[49], dogs[50] and monkeys[51]. Encapsulation technology also offers enhanced cell survival post-transplantation without the use of immunosup-pressive drugs. This technology involves an artificial compartment of semipermeable membrane as a capsule that contains cells and allows oxygen and nutrient supply. The capsule protects cells from injuries against antibodies, proteins, and potent immune cells. Hence, these capsules are also referred to as an immunoisolation device. Diffusion of insulin, glucose, nutrients and oxygen across the capsule has additional advantages that allows efficient glucose homeostasis. Moreover, additional intravascular devices have been designed that contain a small planar or tubular diffusion chamber which is directly connected with the host vascular system and also referred to as an encapsulation device[52]. This device does not require anastomosis after implantation, and encompasses better clinical application compared to the intravascular device.
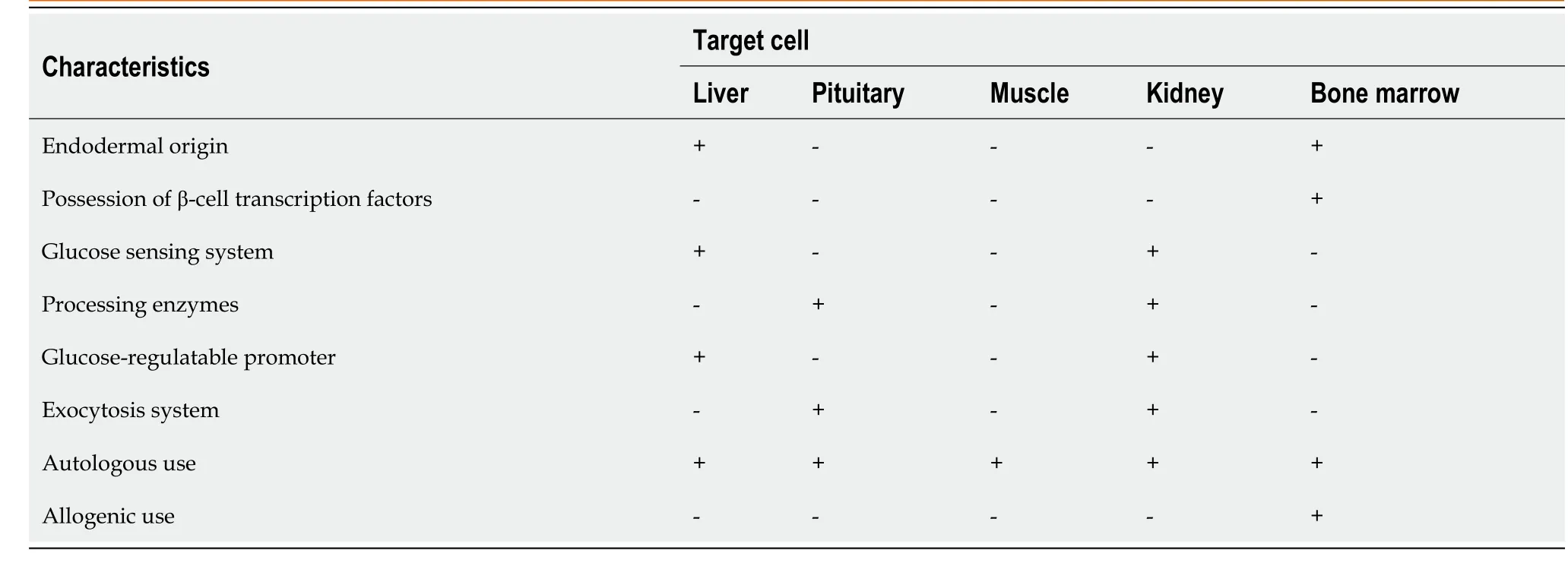
Table 1 Summary of target cells and the degree of β-cell similarity
In a recent study of Vegaset al[17] in 2016, long-term glycemic control was achieved in immunocompetent hyperglycemic mice using polymer-encapsulated human stem-cell-derived β-cells. This study highlighted the decreased obscurity in successful immunoprotection against xenogenic human cell implants in diabetic mice. This report provided groundwork for future studies in autoimmune animal models using xenogenic cells transplantation with the goal of achieving long-term glycemic control and cell survival to offer insulin independence in patients with DM. However, this study had the limitation of generating β-cells using complex long duration protocols and was conducted in immunodeficient mice. Hence, further investigations are required to prove that this technology is clinically acceptable using a wild-type diabetic model without the need of immunosuppression.
In a similar direction, our group has also been working to generate functional insulin-producing βlike cellsin vitrothrough transdifferentiation of human fetal liver-derived EpCAM+enriched progenitor cells in response to high glucose concentration (Figure 2). This protocol has been simplified and encapsulated functional β-like cells in alginate beads (with modified protocol) have been transplanted into C57BL6 hyperglycemic wild-type mice without the need of immunosuppression. The mice were able to restore the blood glucose level within 30 d after transplantation of encapsulated cells and maintained for up to 90 d (unpublished data). In our view, this is the only study where xenogenic human functional β-like cells have been transplanted into wild-type mice without immunosuppression. The encouraging results of this study have shown a path to further investigate their potential in clinical applications.
Hydrogel-based cell transplantation
Suspending viable cells in an aqueous medium of hydrogel precursors, infusing the cocktail to target areas, and stimulating crosslinking (gelation) to generate 3D gel matricesin situare the three primary phases in cell transplantation utilizing hydrogels. Cell transplantation using a hydrogel-based strategy has several crucial advantages. Hydrogels are injectable, and can be used to construct cell-encapsulated gels that have a uniform distribution of cells in the transplanted space. They have water content that can be used to construct different shapes and sizes. Hydrogels can be used for cell therapy when they are constructed with limited pore size that can facilitate diffusion and metabolic wastes but prevent leakage in the cells[53]. Hydrogel scaffolds can be coated with encapsulated InPCs and placed at the site of transplantation[54].
Despite these advances, using hydrogels to control the fate of transplanted therapeutic cells still poses significant obstacles including unsatisfactory cell survival rate due to their death in the new microenvironment post-transplantation. Cells that survive in the hydrogels have uncontrolled interactions, proliferation and differentiation. The overall therapeutic efficacy was unsatisfactory using this method. The nonautologous cells were rejected by the host immune system and the cells were strictly confined. The encapsulated cells were not able to proliferate freely, the cell viability varied for different cell types and in many cases it decreased to nearly 50% when assessed after 10 wk. Hence, the methodologies were modified to improve the drug delivery and integration with the target tissue for successful delivery of the cells.
To improve the functional response and stability of the implanted cells, dual-layer hydrogels as well as silk hydrogels are being tested for efficient transfer of islet cells to the required site. Silk hydrogels are formed using extracellular matrix (ECM) proteins such as collagen IV and laminin. These hydrogels have been tested for cell signaling and survival by immunostaining and oscillatory theometry and found to be stable. The silk hydrogels encapsulated with islet cells in laminin had high insulin response compared with nonencapsulated cells when stimulated over a 7-d period. Silk hydrogels allow coencapsulation of other ECM proteins and cells that can ultimately be used as a transplant device for the treatment of T1DM[55].
Gene therapy
The generation of functional β-cells by genetic engineering necessitates a thorough knowledge of pancreas development and a good understanding of the organ. The production of a cascade of transcriptional regulators, as well as their involvement in regulating endocrine cell fate and maturation, are important considerations for creating artificial β-cells. Furthermore, the type of vector used for gene delivery and the selection of suitable cell type candidates for differentiation into functional β-cells are key challenges.
Selecting an ideal cell type:The ultimate aim of gene therapy is to produce cells with the ability to produce insulin and maintain blood glucose levelin vivo. Different types of cells have been investigated over the last few years, in particular, pituitary cells that contain both the proinsulin processing enzymes and the secretory granules. However, these cells were non-glucose responsive and later became glucose responsive after transfection with glucose transporter 2 and glucokinase genes. However,in vivoproduction of adenocorticotropic hormone inhibits insulin function, which limits its clinical efficacy[56]. Muscle cells, liver cells, mesenchymal stem cells from UCB and bone marrow cells have also been investigated by gene transfer technology and demonstrated the reversal of hyperglycemia in immunodeficient diabetic mice. More recently, a combination of gene and cell therapy was used to produce glucose-responsive InPCs after retroviral transfection with Pdx-1. Transplantation of this combination reversed hyperglycemia in immunodeficient diabetic mice[57].
Choice of vector for gene delivery:The ideal method of gene delivery in cells relies on integrating viral vectors for sustained gene transfer into the daughter cells to provide sustained therapeutic benefits throughout the life of the patient. Four main different kinds of viral vectors have been used inin vitroandin vivomodels for gene delivery purposes: (1) Retroviral vectors[58]; (2) Adenoviral vectors[59]; (3) Adeno-associated vectors[60]; and (4) Lentiviral vectors[61]. Among these, lentiviral vectors have become a popular choice for gene delivery in animal models of diabetes. However, the clinical applicability of these strategies is limited due to virus-mediated complications and resultant pancreatic transdifferentiation.
β-cell transcription factors:Transcription factors play a significant role in determining the phenotype of β-cells. Homeobox factor Pdx-1 is considered to be the master regulator and has a crucial role in early development of the pancreas[62]. Hes-1 and Neurogen-3 are present in pancreatic progenitor cells and direct the respective compartmental fates through Notch signaling[63]. However, for subsequent differentiation into respective pancreatic cell lineages, a cascade of transcription factors are required. All endocrine cells express Neurogen-3, which activates NeuroD1, and maintains the endocrine cell differentiation program[64]. As soon as the endocrine cell differentiation program is activated, Pax-4 and Pax-6 direct the differentiation into different kinds of endocrine cells[62]. In these two transcription factors, Pax-4 is responsible for the fate of β- and γ-cells, and Pax-6 is essential for α-cell fate. More importantly, NK homeobox factors Nkx2.2 and Nkx6.1 are responsible for driving the β-cell fate. Both these transcription factors are imperative in β-cell differentiation. Nkx6.1 is also responsible for preservation of β-cell function during its interaction with Pdx-1, the master regulator, and the other transcription factor NeuroD1 that modulates insulin transcription. Alteration in the expression of these transcription factors and their subcellular localization alters the cellular processes related to β-cell differentiation, and cell cycle modulation and function.
Direct gene transfer:In relation to β-cell replacement therapy, direct gene delivery represents one of the potential techniques to obtain β-like cell phenotype in autologous tissues[65]. Although in past decades a lot of work was focused on direct transcription factor/gene transfer in hepatocytes, the emergence of stem cells has changed the way to look into the cells and needs further investigation due to their clinical suitability. However, before developing a suitable strategy, one needs to consider the appropriate type of transcription factor for direct delivery and true conversion into functional β-cells without causing future complications.
Nano-bioengineering
Nano-bioengineering is an emerging field that has the potential to revolutionize DM treatment. Merging of nanotechnology with medical biology has given a new direction to create smart delivery systems that can regulate blood glucose levels within the body and could produce the desired amount of insulin. Recent advances have been made in this field and novel blood glucose measurement and insulin delivery methods are being developed to improve the quality of life of DM patients (Figure 3).
Nano-bioengineering has been used to develop a novel smart insulin patch that can deliver glucoseresponsive insulin with the help of a painless microneedle-array patch. This device is based on the glucose-responsive enzymatic mechanism that can regulate the blood glucose level in T1DM faster than the commonly used pH-sensitive formulations. In addition, it can also avoid the risk of developing hypoglycemia. The study by Leeet al[66] in 2016 showed the diversified application of a graphenebased electrochemical device to monitor DM and efficient transcutaneous delivery of drugs to reduce blood glucose levels in hyperglycemic mice.
In our recent study, we have demonstrated the applicability of an unique strategy that enables effective transdifferentiation of human hepatic progenitor cells (hHPCs) into InPCs on 3Dnanostructured TiO2substrate developed on conducting surfaces[67]. This 3D-TiO2cellularized chip was able to reverse hyperglycemia in wild-type mice (C57BL6) when transplanted into the peritoneal cavity. We also observed enhanced cell survival and insulin production, and long-term glycemic control in hyperglycemic animals where it does not elicit significant immunological response after ectopic transplantation. Another advantage of this approach includes a sufficient amount of insulin production within a short time post-transplantation in hyperglycemic animals. Due to rapid insulin production in the bloodstream, this approach is more successful in reducing the need for exogenous insulin in T1DM. Ongoing investigation has shown that by expanding the surface area of microchips, this strategy can be used to scale up the procedure for incorporating a sufficient number of InPCs. However, more specialized 3D packaging of InPCs, as well as pancreatic exocrine and ductal cells using TiO2nanostructures supported by conducting substrates, would be required to construct a full pancreatic organotypic system to evolve a more effective approach for regulating hyperglycemia. Therefore, further exploration of this approach is required to achieve its real therapeutic possibility for the clinical management of hyperglycemia.
Neo-organ bioengineering
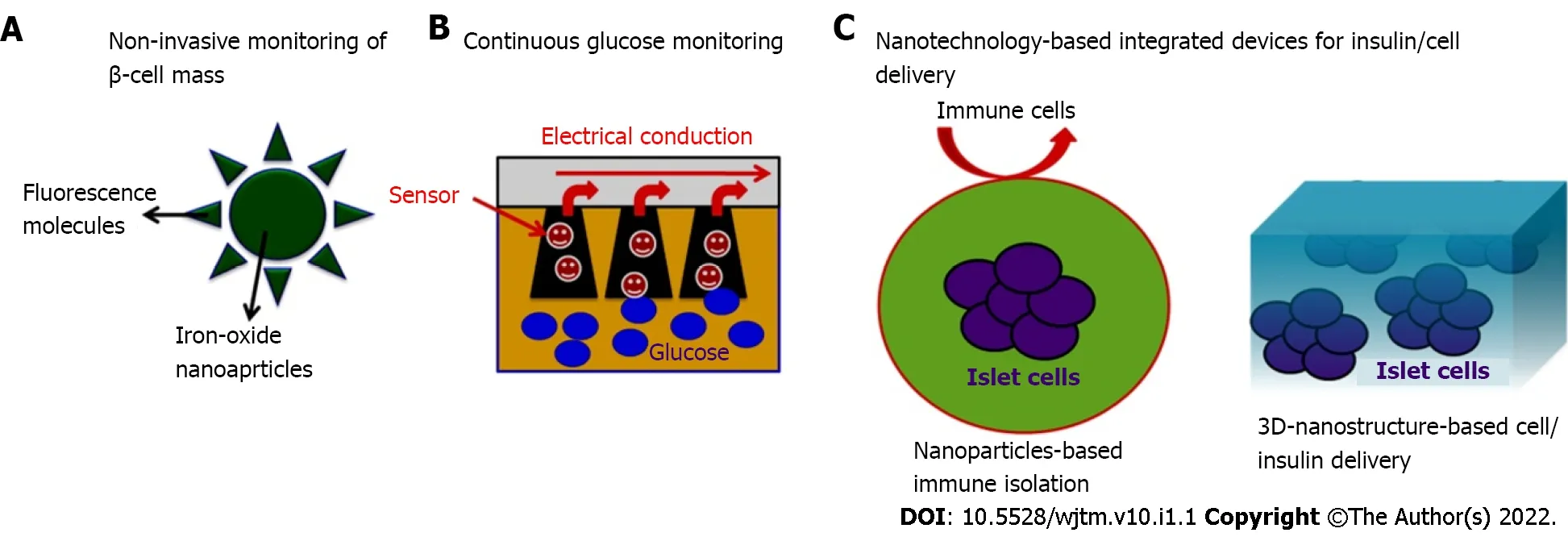
Figure 3 Nanotechnology-based emerging strategies to address the challenges in diagnosis and management of diabetes. A: Iron-oxide nanoparticles-based approach (such as enhanced contrast imaging) for non-invasive monitoring of progressive β-cell loss which could be used to address the patient needs at the various stages of disease progression; B: Nanoparticle-based glucose sensors can assist improved accuracy and patient comfort for regular monitoring of blood glucose levels; C: Nanotechnology-based devices may provide immunological protection of transplanted pancreatic cells which could help to maintain normoglycaemic conditions in patients with diabetes.
Neo-organ bioengineering is one of the most promising approaches, which includes decellularization and repopulation of whole organs harvested from human or xenogenic sources. This approach generates a whole functional neo-organ construct for future clinical applications as a bridge therapy for organ transplantation. It offers a unique strategy of using natural organ platforms to produce natural organ systems using different sources of InPCs. Therefore, this particular technology can overcome the above-mentioned critical concerns and has attracted a lot of attention to generate different organs, including the pancreas[68].
A few recent studies have reported the use of decellularized pancreas to produce functional neoorgans forex vivoinsulin secretion[69-71]. However, a critical stumbling block is the lack of sufficient pancreata for decellularization and repopulation. Furthermore, the mechanical integrity of the neopancreas has not been reported. The developing concept of using heterografts to create viable humanized neo-organ systems has opened a new avenue for meeting transplant demand[72,73]. Nevertheless, due to the small amount of research conducted, some important questions remained unanswered. To date, no effective decellularization and repopulation of xenogeneic spleen sources with endocrine cells has been documented. As a result, it is still unknown if decellularized spleen can be repopulated with human InPCs and perform similarly to islets or the entire pancreas.
Our recent study has demonstrated a heterograft approach of using whole decellularized xenogenic scaffold of spleen to generate functional constructs that are capable of producing the desired amount of insulin in response to hyperglycemic stimulus[74,75]. In our preliminary study, we standardized a unique strategy for activating pancreatic transcription factors of EpCAM+-enriched human hepatic progenitor cells repopulated within acellular spleen harvested from rats[74]. This indicates that 3D, intact acellular splenic scaffolds can provide a superior microenvironment for long-term survival of cells, activation of crucial transcription factors, and transdifferentiation of hHPCs into functional InPCs. Our subsequent study demonstrated that the heterograft approach developed in our previous study generates secondary neo-organoids during ectopic transplantation in diabetic rats and is capable of transporting insulin into the bloodstream, which is essential to manage uncontrolled blood glucose levels[75]. Moreover, this study provides the first proof-of-concept for creating bio/immunocompatible, humanized insulin-producing neo-organoids, which could evolve into more acceptable functional biological implantable devices for long-term diabetes management.
lMPORTANCE OF TRANSPLANTATlON SlTES
The ideal choice for cell transplantation offers optimum engraftment and long-term cell function. The appropriate site for cell transplantation should include: (1) Membrane drainage for the permeabilization of blood glucose and to avoid systemic hyperinsulinemia; (2) Rich arterial supply; (3) Minimal invasive infusion; (4) Access for morphological and functional follow-up of the transplant; (5) Microenvironment with maximum cell survival; and (6) Immunological tolerance. Such types of transplantation sites need to be defined. Over the last few years, various sites have been attempted for islet cell transplantation[76] to manage DM in different animal models. Among different proposed sites/routes, the portal vein has been the site of choice for clinical transplantation (Table 2). However, inflammatory reactions and low oxygen tension lead to cell loss and varied responses among patients. Peritoneal transplantation has the advantage of being an immunologically privileged site, and offers enough space for housing the cells. Hence, the peritoneum overcomes the limitations of other identified transplantation sites and could be an ideal choice for ectopic transplantation. In our recent study, we have demonstrated the usefulness of the peritoneal site in diabetic animal models and have reported that this site increases animal survival and faster recovery of normoglycemia within 30 d post-transplantation without the need for immunosuppression (unpublished data).
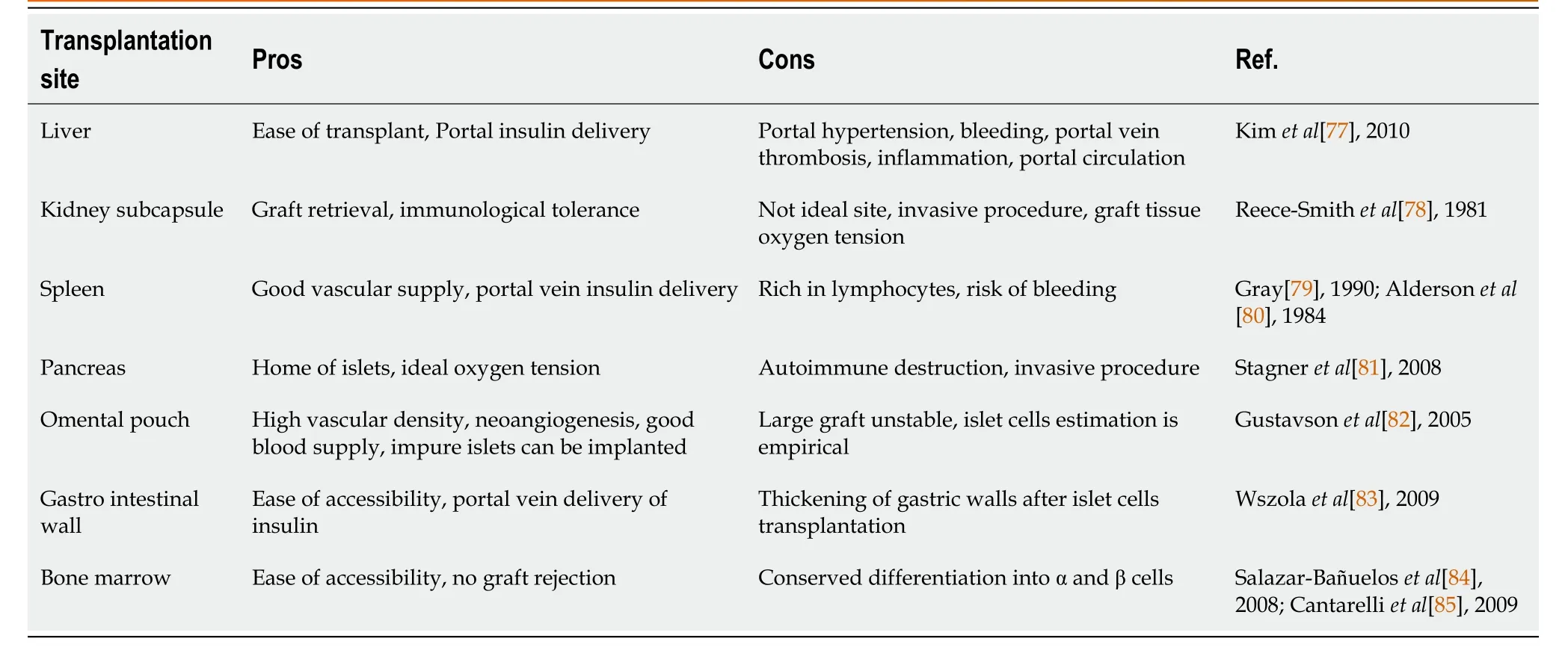
Table 2 The pros and cons of different transplantation sites used for cell delivery in diabetic condition
CLlNlCAL CHALLENGES
The major challenges in DM cell therapy are: (1) Identifying clinically acceptable sources of cells that can be used to produce homogeneous populations and therapeutic doses of insulin-secreting cells; (2) Prevention of immunological rejection post-transplantation; (3)in vivoglucose responsiveness of transplanted cells; (4) Long-term cell survival and function post-transplantation; and (5) No need for immunosuppression. Other clinical challenges include: Safety of the transplantation procedure; determining the cell delivery and engraftment efficiency using live clinical imaging systems; cell delivery at the targeted site within a clinically relevant time; identification of ways to promote regeneration of resident β-cells; ease of source tissue collection and clinical grade cell isolation; and costeffectiveness of procedures. These key considerations and challenges need to be resolved to successfully translate the stem-cell-based therapeutic possibilities for timely management of T1DM into clinical practice. Given the current debate on such issues, clinical applicability of stem-cell-based therapies for the treatment of DM is still a future goal.
CONCLUSlON
In summary, in the next decade, we expect stem-cell-based therapeutic strategies in combination with nanotechnology and other potential areas of science for improved management of DM. Recent developments in neo-organ bioengineering and United States Food and Drug Administration-approved nanotechnology-based formulations with the success of insulin-delivery are encouraging and provide newer opportunities for DM treatment. In our view, the need to develop more effective microencapsulation, neo-organ bioengineering, and nanotechnology-based diabetes therapies lies in the development of robust sensitive micro- and nanodevices for insulin delivery, using clinically acceptable platforms.
FOOTNOTES
Author contributions:All the authors participated in literature search, manuscript writing and revision; Vishwakarma SK formatted the manuscript and designed figures; Habeeb MA provided clinical inputs; Khan AA supervised overall study.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:India
ORClD number:Md Aejaz Habeeb 0000-0003-1519-3186; Sandeep Kumar Vishwakarma 0000-0001-5731-8210; Safwaan Habeeb 0000-0001-7607-6210; Aleem Ahmed Khan 0000-0001-7075-9037.
S-Editor:Fan JR
L-Editor:Kerr C
P-Editor:Fan JR
