Dual biologic therapy with ocrelizumab for multiple sclerosis and vedolizumab for Crohn’s disease:A case report and review of literature
INTRODUCTION
Biologics play an important role in treating the full spectrum of Crohn’s disease(CD).Biologics are a group of drugs derived from living biological sources that undergo complex processes,such as recombinant DNA processes,genetic isolation,or protein purification[1].Biologics can be divided into monoclonal antibodies(mAbs),receptor modulators,and enzyme modulators[2].In CD,biologics are incorporated into the treatment regimen when a response to thiopurines and glucocorticoids cannot be achieved[3].Biologics used for the treatment of CD include inhibitors of tumour necrosis factor-α(TNFα)(infliximab,adalimumab,and certolizumab),inhibitors of αβintegrins on leukocytes(vedolizumab and natalizumab),and inhibitors of the p40 subunit of interleukins(IL)-12 and IL-23(ustekinumab)[4].Patients who respond to biologic therapy show improved clinical outcomes,can avoid surgery,and have a reduced hospitalisation rate,fewer complications,and improved quality of life[5,6].
In the setting of CD,dual biologic therapy(DBT)refers to the use of two different biologic agents to achieve remission.Typically,biologics have been used alone or in combination with other immunomodulators to enhance the therapeutic response and prevent the formation of human anti-chimeric antibodies(HACAs).The use of DBT has been cautioned due to side effects,including immunosuppression,infections,and malignancy[7,8].Newer biologics such as vedolizumab and ustekinumab offer improved safety profiles that do not cause systemic immunosuppression and lower the risk of HACA formation;no cases of progressive multifocal leukoencephalopathy(PML)from John Cunningham virus(JCV)have been reported;and theoretically,there is a lower risk of malignancy due to the gut-specific activity of these biologics[9,10].
Few studies have explored the safety and efficacy of using DBT for CD,and further details regarding the safety of concurrent use of multiple biologics as indicated for different immune-mediated disorders remains to be explored[11,12].Whether a cumulative immunosuppressive effect exists with DBT remains to be clarified,resulting in hesitancy in the widespread use of DBT.In a systematic review with a pooled analysis of patients with inflammatory bowel diseases(IBDs)receiving DBT with TNF-α inhibitors,vedolizumab,or ustekinumab,clinical improvement was observed in all patients,with seven out of 18 patients experiencing mild side effects but no serious adverse events[13].The current evidence is promising,and DBT appears safe,but further controlled trials are required.Furthermore,the coronavirus disease 2019(COVID-19)pandemic presents new challenges to the clinical setting and raises concerns over the safety of biologic therapy[14].The following case report demonstrates the safety of DBT in a patient receiving two biologics for separate immune-mediated diseases and provides an update on recent developments from research into the use of DBT for CD.
CASE PRESENTATION
Chief complaints
A 45-year-old female presented with diarrhoea associated with ileal CD,which was diagnosed four months prior to presentation,without strictures or fistulising complications.
20. The ring from my finger: The young woman s offering is the last of her valuable possessions. It is a more symbolic piece of jewelry than a necklace. Rings are used to plight troths and represent formal unions, especially marriages.
History of present illness
The patient was referred for multidisciplinary care given her complex medical history.She was counselled on the potential need for surgical management.In conjunction with the patient,the decision was made to commence vedolizumab with induction and maintenance therapy.
History of past illness
The patient was diagnosed with CD with active terminal ileitis,and she safely commenced DBT given her concurrent MS.
53.A golden spinning-wheel: Spinning wheels have long been important in folklore, especially in tales like Sleeping Beauty and Rumpelstiltskin. A spinning wheel is a small domestic spinning machine with a single spindle that is driven by hand or foot (WordNet). It is used to produce flax for cloth production, a traditionally feminine domestic chore.Return to place in story.
In this case report,DBT was used for the treatment of two different immune-mediated disease entities.Limited evidence is available on the use of multiple biologics for different immune-mediated disease entities,with most studies examining the role of DBT in refractory disease.To the best of our knowledge,only one other case series,by Fumery[19],reported the use of DBT with ocrelizumab and vedolizumab,which was administered to one patient.No adverse events were observed for a period of 6 mo in this patient,who had been diagnosed with UC and MS[19].
Personal and family history
Her faecal calprotectin was elevated at presentation(>1000 μg/g).Other pathogenic causes of diarrhoea,such as bacteria,parasites,and viruses,were excluded on the basis of blood test and stool culture results.Histopathological analysis of a terminal ileum biopsy sample demonstrated patchy mild active inflammation.
Physical examination
The clinical examination findings were unremarkable.
Then John put spurs to his horse, calling with all his might Stop! stop! But the coach drove on as before, and though the little soldier rode after it for a day and a night, he never got one step nearer
Laboratory examinations
The patient had a history of multiple sclerosis(MS)and had been receiving treatment for the past five years with ocrelizumab,a humanised anti-CD20 B cell depletory drug with similar properties to rituximab,with which it shares a similar epitope.Anti-CD20 therapy has been associated with immunemediated colitis;however,it is not a recognised cause of ileitis.At the time of review,the patient was not taking any other medication or nonsteroidal anti-inflammatory drugs.The patient had been previously treated with fingolimod and natalizumab for her MS.However,natalizumab was subsequently withdrawn,as she was positive for JCV.
Imaging examinations
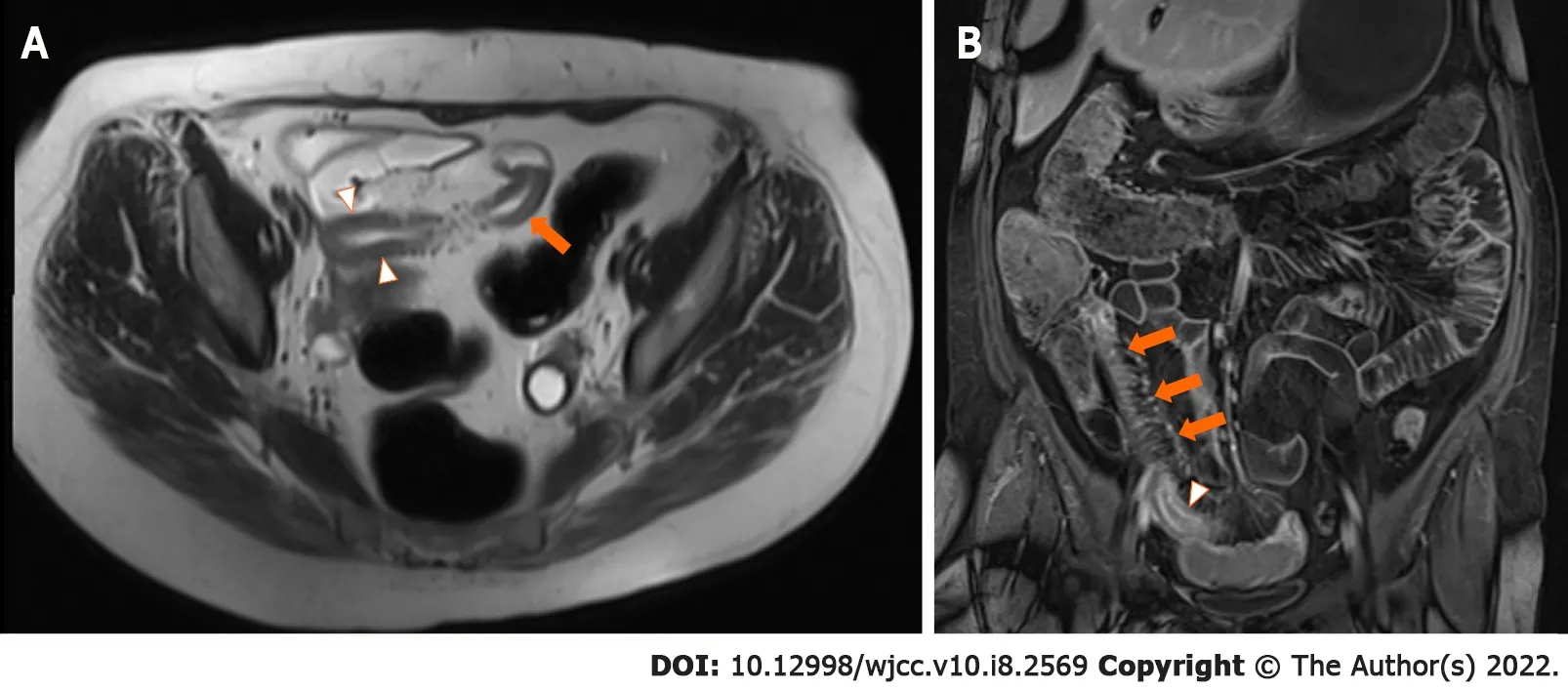
Active ileitis,with the involvement of 30 cm from the ileal-caecal junction and an increased bowel wall thickness of 5 mm without upstream small bowel dilatation,was confirmed on magnetic resonance enterography.
46. Queen had killed it: In the original version instead of this softened translation, the mother-in-law goes so far as to smear the sister s mouth with blood while she is sleeping. She accuses the woman of witchcraft and cannibalism. Return to place in story.
MULTIDISCIPLINARY EXPERT CONSULTATION
Colonoscopy demonstrated active ileitis with a Simple Endoscopic Score for Crohn’s disease of 5(2,1,2,0).
FINAL DIAGNOSIS
After a brief good clinical response to budesonide therapy and the resolution of diarrhoea,recrudescence of the symptoms occurred despite almost 10 wk of treatment.
TREATMENT
Significant gaps still exist in the understanding of the use of multiple biologic therapies in patients with CD.This case report demonstrated the short-term safety of the use of two biologics,ocrelizumab and vedolizumab,in a patient with different immune-mediated conditions.Only a few studies have attempted to elucidate the safety and efficacy of DBT for refractory CD,but some of the results were promising.In a retrospective study by Yang[15],22 patients who had CD refractory to a single biologic underwent 24 trials of DBT(consisting of either infliximab,adalimumab,vedolizumab,ustekinumab,certolizumab,or golimumab),with 50% achieving a clinical response and 41% achieving clinical remission.Adverse events occurred in three trials due to infection,malignancy,or drug-induced lupus[15].Additionally,Kwapisz[16]described 14 patients with CD and one patient with ulcerative colitis(UC)treated with combination biologics for refractory disease.Eleven patients had symptomatic improvement,with 10 patients achieving a reduction in their corticosteroid dose requirements and only three patients requiring surgery.Adverse events in this case series included three hospitalisations and four infections treated with antibiotics[16].In the only randomised controlled trial investigating DBT in CD,Sands[17]investigated 79 patients with active CD despite infliximab treatment in a multicentre double-blind,placebo-controlled trial.A similar overall incidence of adverse events between patients who were administered natalizumab and infliximab and those who were administered placebo with infliximab was observed,and positive trends towards greater efficacy were seen in the patients who received natalizumab and infliximab[17].Further evidence is needed to investigate the safety and efficacy of DBT in controlled settings.A current trial is underway to examine the use of DBT(vedolizumab and adalimumab)with methotrexate for patients with newly diagnosed CD who are at higher risk for complications[18].Additional studies are needed to gain further insight into the immunopathological mechanisms underlying DBT and to clarify whether an additive or synergistic effect occurs.
OUTCOME AND FOLLOW-UP
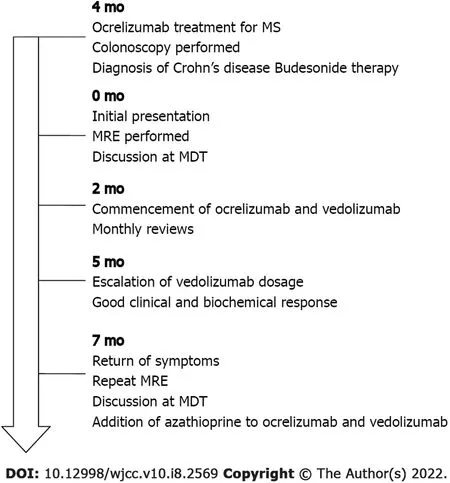
Unfortunately,after 5 mo of therapy,her diarrhoea returned,despite achieving therapeutic levels of vedolizumab at 33 μg/mL.Repeat magnetic resonance enterography demonstrated terminal ileitis with some worsening interval changes,including a bowel wall thickness increase to 7 mm in the terminal ileum but no upstream dilation or complications(Figure 1).The Simplified Modified Magnetic Resonance Index of Activity(MaRIA)score of the terminal ileum was 3,suggesting severe disease.Repeat CRP analysis showed an increase to 52 mg/L.Following multidisciplinary discussion,azathioprine was added to her DBT,as the patient wished to avoid surgery.To date,the patient has safely completed five months of DBT without any adverse side effects noted(Figure 2).
DISCUSSION
Review of the current literature
The patient received treatment with vedolizumab in conjunction with her usual treatment with ocrelizumab for her MS.Because the biochemical response after the first three months was inadequate,the dose of vedolizumab was escalated to 300 mg every 4 wk,which resulted in a good clinical and biochemical response.The patient’s diarrhoea and abdominal pain resolved,her C-reactive protein (CRP)decreased from 47 mg/L prior to dose escalation to 28 mg/L following dose escalation,and her erythrocyte sedimentation rate decreased from 17 mm/h pre-treatment to 5 mm/h post-treatment.During maintenance therapy,the patient completed monthly follow-ups,and she did not develop any adverse reactions.
See here, opening the false merchant s garment and showing the dagger; see what an enemy you have entertained! Remember, he would eat no salt with you, and what more would you have? Look at him! he is both the false oil merchant and the Captain of the Forty Thieves
Case discussion
This is a chance of a life time, I declared to my friend Stacy as I locked the door of my office and left the restaurant I managed. It s every twenty-seven-year-old woman s dream to live in New York City, and in a few months I ll know if I get the transfer.
One differential diagnosis that needs to be made for the patient presented here is ocrelizumabinduced colitis,which has been reported in case reports[20].The presentation of ocrelizumab-induced colitis may be similar to that of new-onset CD[21].Rituximab,another CD20-depleting drug,has also been reported to be possibly associated with colitis[22].This condition cannot be excluded for the patient in this case report.However,disease activity restriction to the terminal ileum,as well as the prolonged duration of ocrelizumab use in this patient prior to the onset of diarrhoea,favoured the diagnosis of CD rather than medication-induced colitis.Consideration should be given to medicationinduced colitis in patients receiving biologics who have symptoms of colitis,although the differentiation of medication-induced colitis from IBD can be difficult[23].
In the era of the COVID-19 pandemic,the safety of DBT needs to be considered,and COVID-19 concerns were a part of this patient’s pre-immunosuppression workup.In one case report,a patient receiving DBT with adalimumab and ustekinumab for CD had a positive severe acute respiratory system coronavirus 2(SARS-CoV-2)result,but the patient was asymptomatic and the infection did not affect the course of treatment,with the patient safely continuing DBT[14].To date,there is no evidence to suggest a worse prognosis in patients with SARS-CoV-2 infection receiving biologics[24].The SECURE-IBD registry demonstrated that TNF-α antagonist treatment was not associated with severe COVID-19[25].However,further evidence is required to determine whether DBT confers increased immunosuppression and increased severity of SARS-CoV-2 infection.
39.Married her: Marriage is the ultimate goal and reward in many romantic fairy tales. Despite the bridegroom s mercenary thoughts, we are intended to believe in a happily ever after for the couple.Return to place in story.
When his request was granted, he set off on his journey, and in the course of it he one day came to a large pond, on the edge of which he noticed three fishes which had got entangled14 in the reeds and were gasping15 for water
The choice of biologic agent in this case report was made in a multidisciplinary setting.Consideration was given to natalizumab,but the risk of PML from JCV reactivation has been reported[26].Furthermore,TNF-α inhibitors are contraindicated for MS due to case reports of demyelination[27].The gut-specific integrin inhibitor vedolizumab has been given preference,and in this case scenario,vedolizumab initially led to a favourable response.In the treatment of IBD,combination therapy incorporating vedolizumab is theoretically a safe approach given its specificity[9].The specificity and safety of vedolizumab provide new opportunities for its use in DBT,particularly with other systemic immunosuppressants such as ocrelizumab,which can cause profound B cell depletion.
However,in patients with extraintestinal manifestations,gut-specific vedolizumab may not be as effective as TNF-α inhibitors.In fact,in a case report by Hirten[28],a patient who had a brief overlap in infliximab and vedolizumab treatment experienced a flare of an extraintestinal manifestation of CD when infliximab was withdrawn and a flare of mucosal symptoms when vedolizumab was withdrawn,with improvements in symptoms when biologics were restarted in both instances[28].Another case report of a patient with UC and spondylarthritis described the successful control of both diseases with vedolizumab and etanercept[29].Privitera[30]also retrospectively examined sixteen patients receiving dual biologics or one biologic and one small molecule,either for refractory IBD or for IBD with extraintestinal manifestations.A clinical response was reported by all patients,with only three patients experiencing an adverse event,which included a perianal abscess,a cutaneous reaction,and drug-induced liver injury[30].Indeed,if the indications for each combination therapeutic are carefully considered,dual ‘targeted’ therapies may be an avenue of treatment in certain patients.Considering the current evidence,DBT can be considered safe in some limited circumstances.Careful selection of DBT,discussion with a multidisciplinary team,and an understanding of the patient’s history and the emerging literature are all needed prior to the commencement of treatment.
Limitations
Several limitations of this case report should be noted.Although the short-term safety of DBT has been demonstrated,the efficacy of DBT remains to be clarified.Vedolizumab is a promising and excellent therapeutic choice for many patients,as demonstrated in the GEMINI studies[31].Further research,particularly in the form of randomised controlled trials examining the efficacy of DBT with vedolizumab,is needed.
CONCLUSION
DBT offers a new treatment strategy to patients with CD and those with different immune-mediated conditions.In addition,the combination of biologics may be an avenue of treatment for patients who have been refractory to existing treatment regimens or those with multiple immune-mediated diseases.Vedolizumab and ocrelizumab in combination may be safe for the treatment of patients with different immune-mediated diseases,such as CD and MS.However,careful selection of biologics with respect to patient characteristics in multidisciplinary settings is required.Further evidence is needed to guide clinical decision-making and the selection of biologics,particularly in the form of randomised controlled trials.
ACKNOWLEDGEMENTS
Images courtesy of Dr.Jessica Yang,Radiologist,Macquarie University Hospital and Concord Repatriation General Hospital.
FOOTNOTES
Au M compiled data,reviewed records,performed literature review,and wrote first draft;Mitrev N and Kariyawasam V reviewed records,reviewed literature,and reviewed draft;Leong RW reviewed records.
All involved persons(subjects or legally authorised representative)gave their informed consent prior to study inclusion.
Dr Rupert W Leong is currently serving on the advisory boards of AbbVie,Aspen,BMS,Celgene,Chiesi,Ferring,Glutagen,Hospira,Janssen,MSD,Novartis,Pfizer,and Takeda.The authors declare no other conflicts of interest.
The authors have read the CARE Checklist(2016),and the manuscript was prepared and revised according to the CARE Checklist(2016).
From then on, we both talked incessantly5. I discovered that they were heading to the same orphanage to be met by the same coordinator6. We became fast friends. I whispered a prayer of thanks to God for answering my earlier prayer.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial(CC BYNC 4.0)license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Australia
Michael Au 0000-0002-5015-701X;Nikola Mitrev 0000-0001-7771-5248;Rupert W Leong 0000-0001-5944-3488;Viraj Kariyawasam 0000-0003-2623-1336.
Fan JR
A
Fan JR
 World Journal of Clinical Cases2022年8期
World Journal of Clinical Cases2022年8期
- World Journal of Clinical Cases的其它文章
- Analysis of bacterial spectrum,activin A,and CD64 in chronic obstructive pulmonary disease patients complicated with pulmonary infections
- Developing natural marine products for treating liver diseases
- Computed tomography perfusion imaging evaluation of angiogenesis in patients with pancreatic adenocarcinoma
- Epidemiological features and dynamic changes in blood biochemical indices for COVID-19 patients in Hebi
- Identification and predictive analysis for participants at ultra-high risk of psychosis:A comparison of three psychometric diagnostic interviews
- Prognostic significance of peritoneal metastasis from colorectal cancer treated with first-line triplet chemotherapy
