Protocol for evaluating the effects of Danggui Shaoyao Powder in the treatment of DPN with qi deficiency and blood stasis pattern based on routine therapy:a randomized controlled trial
Shi-Guang Qiu,Kai Li,Hong Gao,Wen-Bin He,Chang Liu,Xian Wang,Yi-Wei Gui,Jing-Yu Zhao,Gao-An Wang,Yi-Qiang Xie*
1Second affiliated Hospital of Hainan Medical University,Haikou 570311,China.2College of Traditional Chinese Medicine,Hainan Medical University,Haikou 571199,China.3Affiliated Hospital of Chengdu University of Traditional Chinese Medicine,Chengdu 610081,China.4Affiliated Hospital of Shanxi University of Traditional Chinese Medicine,Taiyuan 030024,China.
Abstract Background: Diabetic peripheral neuropathy (DPN) has high incidence.At present,the treatment for DPN mainly focuses on controlling of blood sugar,nourishment of nerves and symptomatic treatment of pain which face some limitations,such as poor effects and adverse reactions of drugs and so on.Traditional Chinese Medicine (TCM) has certain curative effect in the prevention and treatment of DPN.Danggui Shaoyao powder,one of the famous classic prescriptions in TCM,has been often used clinically in the treatment of DPN in China,which without any relevant evidence-based medical research data.The protocol was designed to evaluate the effects of Danggui Shaoyao Powder in the treatment of DPN with qi deficiency and blood stasis pattern based on routine therapy.Methods: This protocol is designed as a three-arms parallel-group,outcome-assessor blinded RCT.303 Patients with DPN,from outpatient and inpatient departments of four clinical centers in China,will be randomly allocated into 3 groups (routine group,treatment group and control group).The course of treatment was designed as 1 month and followed up for 3 months.The TCSS scale,TCM syndrome scale,nerve conduction function,serum indexes will be tested to provide more clinical evidences for the effect of Danggui Shaoyao Powder on prevention and treatment of DPN.Finally,we will make statistics and analyse the data to draw a conclusion.Discussion: Danggui Shaoyao Powder is the ideal drug which is an agent acting on multiple targets related to DPN’s pathogenesis.We hope to find more clinical evidences for the effect of Danggui Shaoyao Powder on prevention and treatment of DPN through this RCT experiment.
Keywords: protocol;Danggui Shaoyao Powder;diabetic peripheral neuropathy;RCT
Background
The global prevalence of diabetes and impaired glucose tolerance are increasing year by year.It was estimated that 425 million people worldwide are suffering from diabetes in 2017 which means that the morbidity is 8.4%.By 2045,the number of diabetics worldwide is expected to increase to 629 million with the 9.9% morbidity [1].The incidence of diabetic peripheral neuropathy (DPN) is also increasing year by year,which is closely related to the course of diabetes.Some studies have shown that the incidence of DPN is about 30%,60% and 90% in 5,10 and 20 years after the diagnosis of diabetes,and even 8%~30% of prediabetic patients already have multiple neuropathy[2-4].
DPN manifests in several forms,including sensory,motor,and autonomic neuropathies,and has a significant negative impact on health-related quality of life [5].The pathogenesis of DPN is not yet fully understood.At present,western medicine mainly focuses on controlling of blood sugar,nourishment of nerves and symptomatic treatment of pain which also face some problems,such as poor effect and adverse reactions of drugs and so on[6,7].
An ideal drug should be an agent acting on multiple targets related to DPN’s pathogenesis,that is the superiority of Traditional Chinese Medicine(TCM) [8].TCM has certain curative effect in the prevention and treatment of DPN.Both single herbs and herbal compounds could improve glucose and lipid metabolism,regulate the expression of inflammatory factors,improve nerve conduction to relieve the discomfort symptoms of the patients[9].
Danggui Shaoyao powder,one of the famous classic prescriptions of the Han Dynasty in China,recorded inthe synopsis of the Golden Chamber.TCM believes that DPN belongs to the syndrome of blood stasis inconsumptive thirst and is one of the common complications of diabetes.It is caused by diabetes for a long time,and prolonged illness causes blood stasis and disorders of water and fluid metabolism,muscle and vein formation [10].Therefore,the treatment should be based on invigorating qi and promoting blood circulation,eliminating phlegm and removing blood stasis.Danggui Shaoyao powder comes fromSynopsis of the Golden Chamberwritten by Zhang Zhongjing in the Eastern Han Dynasty.It consists ofAngelica,Paeonia lactiflora,Poria cocos,Atractylodis macrocephalae rhizoma,Rhizoma alismaandLigusticum chuanxiong(In this study,non-decocted granules of corresponding drugs were used).The whole prescription has the effects of regulating liver and spleen,promoting blood circulation and removing dampness.In Danggui Shaoyao Powder,Poria cocos,Atractylodes atractylodes rhizomaandRhizoma alismacan regulate water metabolism and clear damp,whileLigusticum chuanxiong,Angelica and Paeonia lactiflora can promote blood circulation and remove blood stasis without damaging positive factors.Modern studies have found that it has a good therapeutic effect on diseases such as Alzheimer,Parkinson,nephrotic syndrome and so on,whose pathogenesis is "liver deficiency and blood stagnation,spleen deficiency and dampness stop,liver and spleen disharmony" [11,12].Study has shown that Danggui Shaoyao Powder can protect hippocampal neuron injury and effectively inhibit AB-induced neuronal apoptosis in Rats with Alzheimer.The mechanism may be that Danggui Shaoyao Powder inhibits the activation of inflammasome and inhibits neuroinflammatory response by regulating NLRP3/caspase-1 signaling pathway,thus playing a neuroprotective role [9].In their research,Zhang Wei and others found that in the Danggui Shaoyao Powder,doubling the amount of blood-activating drugs can promote angiogenesis and blood supply,and thus promote the recovery and regeneration of nerve function [13].Because of its clinical effect,our team has got the support of the fund to carry out this RCT trial for Danggui Shaoyao powder.We designed a parallel-group,noninferiority randomized trial protocol to assess the clinical effects of Danggui Shaoyao Powder on treatment of DPN.The trial protocol follows relevant recommendations established by SPIRIT.A checklist of Standard Protocol Items:Recommendations for Interventional Trials (SPIRIT) is provided in Figure 1.SPIRIT 2013 Checklist:Recommended items to address in a clinical trial protocol and related documents are provided in Supplementary file 1.
Methods
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Second affiliated Hospital of Hainan Medical University on 9 October 2018 (2018 R008).Any protocol modifications will be submitted to the Ethics Committee for review and participants will be informed.After eligibility screening we will request signed consent from participants.The participants can withdraw from the study without any reason at any time.The privacy of all participants will be strictly protected.
Trial status
The anticipated starting date of recruitment is 1 October 2019.Recruitment is expected to be completed in 30 June 2022.
Study aims and design
This protocol is designed as a three-arms parallel-group,outcome-assessor blinded RCT that is already registered in Chinese Clinical Trial Registry (ChiCTR1900024374).The trial follows all recommendations established by SPIRIT [14].The protocol will be conducted at outpatient and inpatient departments of Second Affiliated Hospital of Hainan Medical University,Affiliated Hospital of Chengdu University of Traditional Chinese Medicine and Affiliated Hospital of Shanxi College of Traditional Chinese Medicine.Endpoint adjudication committee is set at the Second Affiliated Hospital of Hainan Medical University College of Traditional Chinese Medicine of Hainan Medical University is the coordinating centre and steering committee.
A brief flow diagram of the trial protocol is summarized in Figure 2.
In this study,patients were randomly divided into three groups by multi-center,randomized double-blind method:
(1) Routine group (RG):The patients in this group will be given basic treatment and placebo.The routine basic treatments will be maintained in both groups.Placebo is produced by pharmaceutical company and is consistent with the Danggui Shaoyao Powder in appearance and dosage.
(2) Treatment groupm (TG):In addition to these routine basic treatments,the subjects of the TG will be treated with TCM prescription Danggui Shaoyao powder.
(3) Control group (CG):On the basis of these routine basic treatments,the subjects in CG will be treated with western medicine Methycobal,Weisai(China) Pharmaceutical Co.,Ltd.,0.5mg/oral,tid.
Object
Evaluating the effects of Danggui Shaoyao Powder in the treatment of DPN with qi deficiency and blood stasis pattern based on routine therapy.
Eligibility criteria
This study is going to recruit patients from October 2019 (Due to the epidemic,patient recruitment has not yet started).
The inclusion criteria are:
◆Either gender
◆From 16 years old to 65 years old
◆In accordance with the diagnosis of DPN by western medicine(The diagnostic criteria are as follows)[15]
(1) Typical symptoms of diabetes (polydipsia,polyuria,polyphagia,and unexplained weight loss).And adding one of the following
(2) random blood glucose levels ≥11.1 mmol/L.
(3) Fasting plasma glucose levels ≥7.0 mmol/L
◆Accord with TCM diagnosis of DPN syndrome of qi deficiency and blood stasis[16]
◆After 2 to 4 weeks of basic treatment,blood glucose,Glycosylated serum protein,blood lipid,blood pressure and other baseline levels were controlled to reach the standard.
◆No other clinical trials in 2 months The exclusion criteria are:
◆The person with serious diseases such as cardiovascular,hepatic,pneumonic,nephric and hematopoietic system disorder,etc.;
◆Patients with severe primary neurological and mental diseases.
◆A person with a history of exposure to toxic substances
◆Unstable blood glucose control:Blood sugar fluctuates greatly.
◆Pregnant or lactating women
◆Receiving any drugs would affect neurologic function
◆Receiving any physiotherapy during the intervention period.
Interventions
The patients in routine group will be given basic treatment and placebo.Routine basic treatments contain diet control and exercise,regulation of hypoglycemic,hypotension and hyperlipoidemia(according to the patient's condition).The routine basic treatments will be maintained in both groups.Placebo is produced by pharmaceutical company and is consistent with the Danggui Shaoyao Powder in appearance and dosage.
In addition to these routine basic treatments,the subjects of the treatment group will be treated with TCM prescription Danggui Shaoyao powder (10 g of angelica sinensis,20 g ofRadices paeoniae alba,12 g ofPoria cocos,12 g ofBighead atractylodes rhizome,15 g ofRhizoma alismatis,15g ofLigusticum wallichii).1 agent per day,2 times per day,1 hour before the meal or 2 hours after the meal.The formulation particles of the Danggui Shaoyao powder will be uniformly produced by Jiangyin Tianjiang Pharmaceutical Co.,Ltd.
The patients in control group will be treated with western medicine Methycobal,Weisai (China) Pharmaceutical Co.,Ltd.,0.5 mg/oral,tid.
The course of treatment was designed as 1 month (4 weeks) and follow-up for 3 months.
Outcomes
Primary outcome
The primary outcome is The TCSS scale,TCM syndrome scale and nerve conduction function TCSS scale includes three parts:nerve reflex,neurological symptoms and sensory function score.The total score of TCSS scale is 19 and the diagnostic cut-off score of DPN is 6.The score 6-8 indicates mild DPN,9-11 is moderate one and 12-19 is the severe DPN.
TCM syndrome scale is divided into three grades:mild,moderate and severe condition.
Nerve conduction function is measured by electromyography,and recording metrics include velocity,amplitude,frequency,and waveform.
Secondary outcome
Expression of serum markers,including Hcy,IL-1,IL-6,IL-8,TNF-α and CRP.
Patients of both groups will be evaluated 3 times in a 1 month’s period:at baseline (T0),after 2 (T2) and 4 (T4) weeks.The TCSS scale,TCM syndrome scale and the expression of serum markers Hcy,IL-1,IL-6,IL-8,TNF-α and CRP will be detected at T0,T2,T4.Detection of nerve conduction function will be implemented at T0and T4.The detection time of other indices such as blood glucose,Glycosylated serum protein,liver and kidney function are shown in Figure 1.
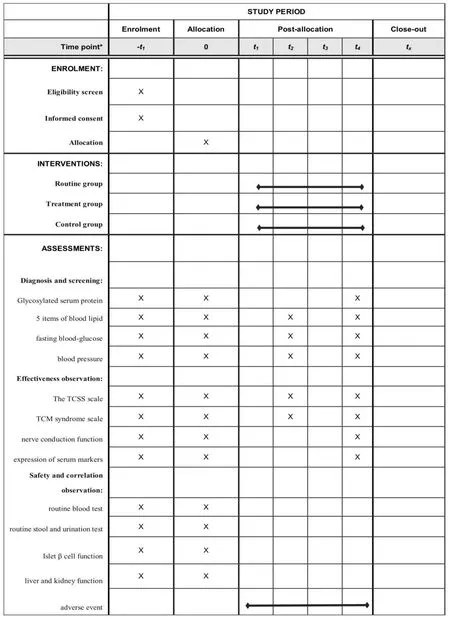
Figure 1 Recommendations for Interventional Trials(SPIRIT)
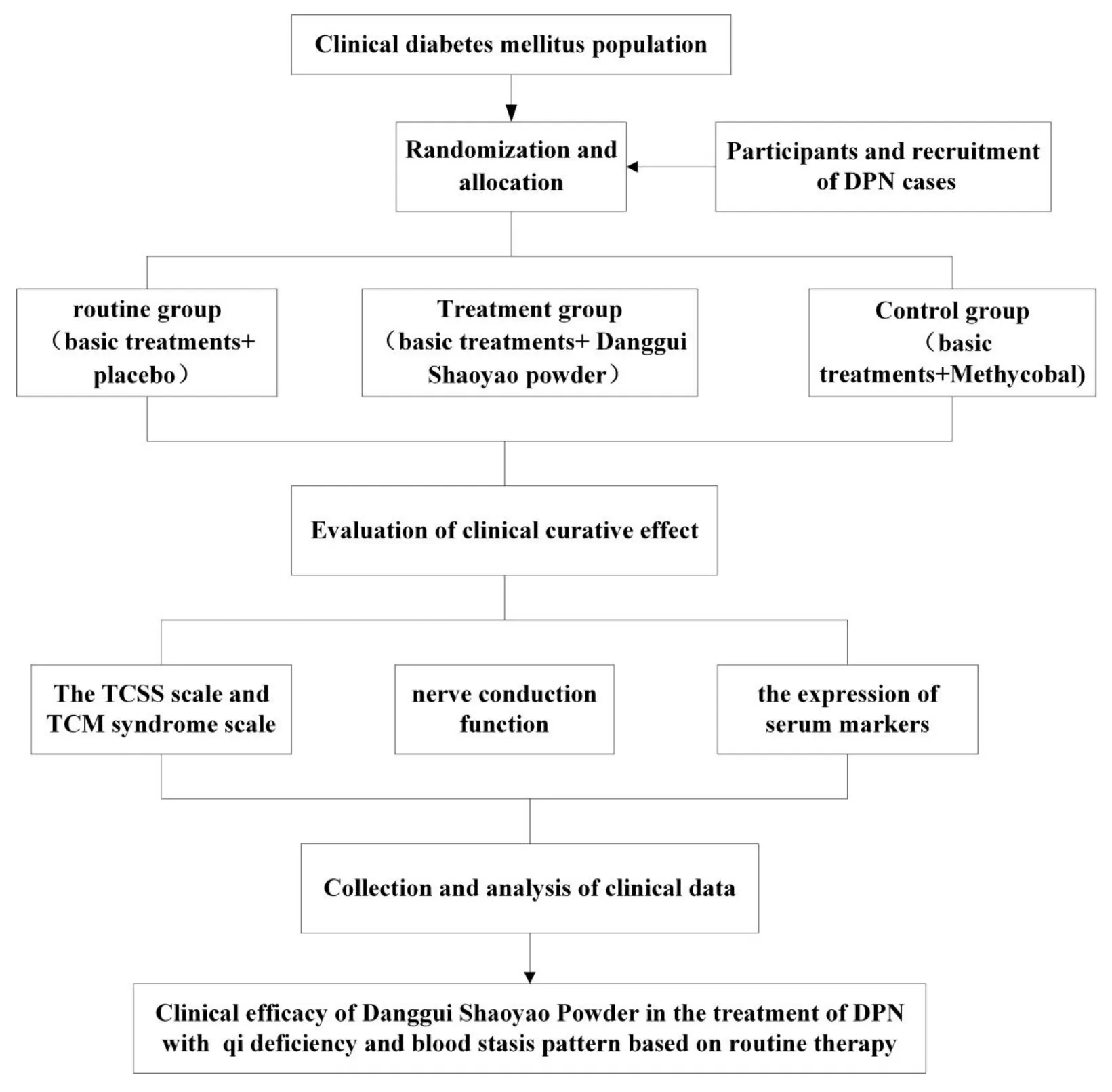
Figure 2 A brief flow diagram of the trial
The TCSS scale[17,18] TCSS scale includes three parts:nerve reflex,neurological symptoms and sensory function score.Nerve reflex includes ankle and knee reflex,a total of 8 scores.Neurological symptoms include numbness,pain,acupuncture-like feeling,fatigue,walking instability and similar symptoms of upper limbs,a total of 6 scores.Sensory function includes bilateral thumbs pain,temperature perception,tactile pressure sense,vibration perception and position perception,a total of 5 scores.The total score of TCSS scale is 19 and the diagnostic cut-off score of DPN is 6.The score 6-8 indicates mild DPN,9-11 is moderate one and 12-19 is the severe DPN.
The TCSS scale will be assessed at T0,T2,T4and after 3 m follow-up.
TCM syndrome scale[19,20] The main symptoms are numbness,pain and abnormal feeling.Secondary symptoms are fatigue,shortness of breath and laziness,sweating while moving,diarrhea or constipation.Tongue:the tongue is light and dark,or bruised,the coating is thin and white.The pulse is thin and astringent.It is divided into three grades:mild,moderate and severe condition.The higher the score,the worse the condition.
TCM syndrome scale will be assessed at T0,T2,T4and after 3 m follow-up.
Nerve conduction function[21,22] The subjects is on the supine position,make skin clean,fully exposed the limbs,relaxed the limbs,and keeps the limb temperature at 32-36°C.The parameters of Electromyography (EMG) should be set as follows:stimulation intensity 20 ≤100 mA,stimulation frequency 1 HZ,sensitivity 5,000 μV/div,stimulation pulse width 0.2 Ms,scanning speed 3 Ms ≤div to detect motor nerve conduction and sensory nerve conduction function.The recording indexes included velocity,amplitude,frequency and waveform.Motor nerves include bilateral median nerve,bilateral ulnar nerve,bilateral common peroneal nerve,bilateral tibia nerve.Sensory nerves include bilateral median nerve,bilateral ulnar nerve,bilateral peroneal nerve.
Nerve conduction function will be assessed at T0,T4and after 3 m follow-up.
The expression of serum markersHcy [23-25],IL-1,IL-6,IL-8,TNF-α and CRP [26-28] will be detected at T0,T2,T4and after 3 m follow-up.Detection of will be implemented at T0,T4and after 3 m follow-up.
Safety observation indexBefore and after the test,observe the liver function,renal function,blood routine and so on to ensure the safety and effectiveness.
Participant timeline
Randomization,allocation and blinding[29]
In this experiment,blind method will be applied to the result subjects and assessors.The blind method will be also applied to the statistical analysts,that is,after the unblinding,the statistical analysts will be told which is group A,B or C rather than treatment group,control group or routine group.
A total of three people (Person A,B,C) will be responsible for random allocation.They don’t know who will be the subjects and could not participate in the subjects.In order to ensure the effectiveness of the blind in the trial process,they do not participate in the subsequent trial.Person A will use seed number 1,2,3 to generate random sequences including 303 random numbers on SAS software.Person B will divide random numbers into three groups,1 is RG group,2 is TG group,3 is CG group.The trial drugs and placebo will be specially produced by the pharmaceutical factory (Jiangyin Tianjiang Pharmaceutical Co.,Ltd) with the same appearance without any signs.Person C will pack trial drugs and placebo in small bag respectively by the dose of a time in advance and the whole course drugs will be packed into a large bag.The corresponding serial numbers of the intervention type determined by random numbers will be marked on the large bags.The allocation tables of serial number,random number and group mark will be in triplicate and kept by the Person B,pharmacy and Person C respectively.
Clinicians are responsible for recruiting subjects according to the inclusion criteria for grouping and treatment.The serial numbers will be assigned sequentially according to the order of treatment.The names and serial numbers of the subjects will be recorded and kept by the clinicians.The nurses will be responsible for the distribution of drugs.The clinicians can not participate in the evaluation.Blind assessors will fill in TCSS scale,TCM syndrome scale and carry out nerve conduction function.Blind examiner will be responsible for the detection of blood biochemical indexes.Until the completion of the trial,the assessors and the examiners don’t know the grouping of the subjects.
Follow up
TCSS scale,TCM syndrome scale,Nerve conduction function,The expression of serum markers are will be assessed at T0,T2,T4and after 3 m follow-up.
Sample size[30] and statistical analysis
The subjects are randomly divided into routine group,treatment group and control group according to the proportion of 1:1:1.The calculation of sample size was designed according to the equal samples of two groups in a randomized controlled trial.Setting up inspection level:Zα/2=Z0.05/2=1.96,Zβ=Z0.20=0.842,P1=effective percentage of RG,P2=effective percentage of TG or CG,P=(P1+P2)/2,Putting in the formula:

The estimation refers to remission rate of DPN by the routine endocrine therapy.It was reported that the remission rate of routine treatment is 40%.The minimum effective clinical difference is increased by at least 20%,that is,from 40% to more than 70% after adding other drugs,which has statistical difference and deserve the clinical promotion.After calculation,there are 84 cases in each group.Taking into account the loss of visit,shedding and other factors,the samples size should be increased by 20%,the number of samples in each group should be 101,so the total number of original cases planned for this project is 303.
Using SPSS20.0 software for data statistics and analysis.The measurement data will be measured by t test,the counting data by χ2test,the self comparison before and after treatment by paired t test,and the result of the data will be statistically significant by mean ±standard deviation ofP<0.05.
Discussion
DPN patients have many subjective symptoms.These subjective symptoms affect the life quality of patients,so the TCSS scale containing subjective symptom score is used to evaluate the curative effect.DPN is the result of combination of multiple factors,including dysfunction of blood vessels,nerves and the immune system [31,32].Whatever the cause might be,the result eventually is the degeneration of nerve fibers and necrosis of nerve cells.Nerve conduction function examination by EMG was designed in the trial.Some serum indexes,blood glucose,blood lipid,Hcy and inflammatory factors will be observed.Blood routine,liver and kidney function as safety indicators will also be tested.We hope to find more clinical evidences for the effect of Danggui Shaoyao Powder on prevention and treatment of DPN through this RCT experiment.
 Clinical Research Communications2022年2期
Clinical Research Communications2022年2期
- Clinical Research Communications的其它文章
- Prevention of nausea,vomiting and reflux aspiration in two cases of painless hysteroscopy
- Primary tuberculosis of the left greater trochanter of femur:a case report
- An association between overweight and obesity and dietary sugar intake among adults in China
- Xi-Feng-Hua-Shi granules for diarrhea-predominant irritable bowel syndrome:protocol for a randomized,double-blind,placebo-controlled multi-center clinical trial
- The effectiveness and safety of HuangQiXiXin decoction for cough variant asthma:protocol for a systematic review
