Asparaginase-associated pancreatitis in chemotherapytreated pediatric patients: a five-year retrospective study
Chen-xi Liu, Yun-yu Zhang, Qiu-shi Yang, Shu-hong Shen, Jing Chen, Yan-jing Tang, Chang-cheng Chen, Zhuo Wang, Bi-ru Li, Juan Qian, Ying Wang, Wen-ting Hu, Bo-tao Ning
1Department of Pediatric Hematology and Oncology, Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China
2Department of Pediatric Intensive Care Unit, Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China
Corresponding Authors: Bo-tao Ning, Email: ningbotao@126.com; Wen-ting Hu, Email: huwenting@scmc.com.cn;
Ying Wang, Email: ywang_picu@shsmu.edu.cn
Asparaginase (Asp) represents a key agent in induction remission for acute lymphoblastic leukemia (ALL) and certain subtypes of non-Hodgkin lymphoma (NHL). By catalyzing the hydrolysis of extracellular asparagine, thus depleting the level of plasma asparagine necessary for the growth of leukemic lymphoblasts, Asp can inhibit leukemic lymphoblast protein synthesis and lead to subsequent apoptosis with little myelosuppression.This will achieve anti-leukemia efficacy, and promote the remission of leukemia.A previous study showed that an Asp-containing regimen could significantly increase the event-free survival rate (71% vs. 31%).A previous study showed that those who completed the Asp regimen had a higher 5-year eventfree survival rate (90%) than those who were unable to tolerate toxicity and quitted the treatment (73%).
However, repeated administration and hypersensitivity to-derived Asp leads to various undesired clinical events, which could either delay or suspend chemotherapy.A chemically recombinant form of Asp was developed to combat this high immunogenicity and to maintain an effective Asp activity. Polyethylene glycol-asparaginase (PEG-Asp) contains a PEG molecule conjugated to-derived L-asparaginase (L-Asp), which could alter its immunogenic properties and decrease the possibility of hypersensitivity.Moreover, with a longer half-life and better patient tolerance, PEGAsp is now approved as a first-line Asp formulation in ALL chemotherapy regimens.This treatment is also effective in patients with other malignant diseases and tumors such as lymphoma and myelosarcoma.
Adverse events during chemotherapy have always been a major concern. During leukemia treatment, events such as hyperinsulinemia, hypertriglyceridemia, hypoproteinemia, and coagulation disorders have been reported.No significant diff erences are observed in the types of adverse events between the L-Asp and PEG-Asp formulations.Asparaginase-associated pancreatitis (AAP) is one of the common adverse events. The onset of severe AAP requires permanent withdrawal of Asp from the patient’s chemotherapy regimen, which aff ects the treatment outcome.Therefore, it is important to outline the clinical characteristics of AAP not only induced by regular Asp but also by PEG-Asp.
This study aimed to investigate the clinical profile of AAP in pediatric patients. This 5-year retrospective study was conducted in a pediatric center. All baseline characteristics, underlying disease profiles, laboratory findings, and radiographic results were analyzed. The clinical manifestations of patients with AAP were summarized, and patients were classified into mild/moderate and severe groups. Clinical outcomes were compared between the two groups.
METHODS
Study participants and definitions
All clinical diagnoses and treatment procedures were carried out in Department of Hematology, or Pediatric (ultrasonography, computed tomography [CT] scan, or magnetic resonance imaging [MRI]).
Pancreatitis was defined as Asp-associated if diagnosed within 50 d of the last Asp injection, regardless of formulations. The clinical diagnosis of AAP was based on the patient’s symptoms (abdominal pain associated with nausea and vomiting) and previous medications, concurrently supported by elevated serum amylase levels and imaging evidence. Patients with trauma-related pancreatitis, biliary duct obstruction, or hyperlipidemia were excluded. Patients with abnormal pancreatic imaging findings prior to Asp administration were also excluded. The patients’ medical records were carefully assessed. For severity classification, patients with AAP were further classified into mild/moderate (severe pain, vomiting, and medical intervention required) and severe AAP groups (life-threatening incidents or urgent intervention required) according to the Common Terminology Criteria for Adverse Events (CTCAE, version 4.03), which was used in a previous study.
Acute complications due to AAP were defined as acute insulin need, acute systemic or local symptoms after AAP, and pancreatic pseudocysts. Persisting complications due to AAP were defined as a persistent need for insulin therapy, elevated pancreatic enzymes or imaging showing pancreatic inflammation/edema, pancreatic atrophy, pseudocysts, hemorrhage, or obstruction of the main pancreatic duct at the last followup, and recurrent abdominal pain.
Data collection
The cytogenetic profiles of patients were determined once the underlying disease was diagnosed. The karyotype and immunophenotype were determined using the cytometric bead array system, while the targeted gene mutation was detected using fluorescence in situ hybridization assay. All Asp-treated hospitalized patients underwent routine blood sample collection for laboratory testing to monitor Asp-related adverse events. For emergency patients admitted to the hospital, laboratory tests were performed within 2 h of inpatient admission. Ultrasound was used to screen and diagnose AAP, while contrast-enhanced CT was used to confirm pancreatitis severity and local complications. For patients who were suspected of having AAP but inflammatory edema was not detected by ultrasonography, contrast-enhanced CT or MRI was performed to obtain imaging evidence further. In addition, MRI was performed to evaluate the presence of severe complications in patients with persistent pancreatic enzyme elevation and progressing Intensive Care Unit, Shanghai Children’s Medical Center (SCMC), School of Medicine, Shanghai Jiao Tong University. Once the underlying disease diagnosis and risk stratification were confirmed, patients were enrolled in the study following a standardized nationwide protocol. ALL was diagnosed and treated according to the CCCG-ALL 2015 protocol (ChiCTR-IPR-14005706), whereas patients with NHL were diagnosed and treated following the CCCG-LBL 2016 protocol (clinicaltrials.gov no: NCT02845882). This study was approved by the hospital’s ethics committee, and written informed consent was obtained from the patients’ parents or guardians.
PEG-Asp is a preferential formulation, as it only requires a few injections, has lower immunogenicity, and has better patient tolerance. All recipients were tested for hypersensitivity to Asp before administration. A single intramuscular injection of PEG-Asp at a dosage of 2,000 U/mwas equivalent to regular E. coli- Asp 6,000 U/mor Erwinia chrysanthemi-Asp (Erwinia-Asp) 10,000 U/minjection every other day for 10 times.
ALL risk stratification was based on patients’ cytogenetic profiles and baseline white blood cell (WBC) counts. The risk group was modified according to the patient’s remission status, referred to as minimal residual disease (MRD). Patients with a precursor B-cell phenotype and with one of the following features were classified as the low-risk (LR) group: age >1 year, baseline WBC count ≤50×10/L, chromosomes ≥50, and presence of TEL-AML1 gene. Patients with the MRD >1% on Day 19 were classified as the intermediate-risk group (IR). The other IR groups were Philadelphia chromosome-positive (Ph), T-cell phenotype, hypodiploidy (<44 chromosomes), and LR MLL rearrangement (MLL-r) ALL patients (indicated as WBC <300×10/L and age >6 months). Patients with a high-risk (HR) MLL-r phenotype or MRD >1% on Day 46 were classified as the HR group. Lymphoma was classified as either stage III (tumors in the lymph node areas on both sides of the diaphragm) or stage IV (central nervous system [CNS] or bone marrow involvement), according to the invasiveness of the neoplasm.
The diagnosis of pancreatitis was made based on the Ponte di Legno Toxicity Working Group (PTWG) consensus criteria,which require the fulfillment of any two of the following criteria: (1) abdominal pain strongly suggestive of pancreatitis; (2) serum lipase or amylase ≥3 times the upper normal limit (UNL); and (3) characteristic imaging findings of pancreatitis clinical symptoms.
Statistical analysis
All statistical analyses were performed using SPSS version 19.0. Continuous data were presented as median and range. Enumeration data were expressed as frequency and rate (%). The differences in categorical variables in the recorded values between the mild/moderate and severe AAP groups were tested for significance using the Fisher’s exact test. The continuous variables were analyzed using the Mann-Whitney U-test. A two-tailed P-value of <0.05 was considered significant.
RESULTS
Patients’ characteristics
Between July 2014 and July 2019, 965 patients were treated with Asp in our center. Among them, 52 patients were diagnosed with pancreatitis during the Asp treatment. Three patients with abnormal pancreatic imaging findings before Asp administration were excluded. The remaining 49 patients met the AAP criteria and were eligible for this study. The incidence of all AAP was 5.08% (49/965). All AAP cases were associated with PEG-Asp (n=45), E.coli-Asp (n=2), and Erwinia-Asp (n=2), respectively. The median age was 7.18 years (0.78-15.97 years) (Table 1).
Concerning the underlying disease, 89.8% (n=44) of the patients were diagnosed with ALL, of whom 29 experienced AAP in the remission induction phase, five during the continuation therapy, nine during the reinduction stage, and one during the preparation for HSCT. The other five cases of AAP were associated with mixed lineage leukemia, blast crisis of chronic myeloid leukemia (CML-BC), Burkitt lymphoma, T-cell lymphoblastic lymphoma (TLBL), and peripheral T-cell lymphoma (PTCL), respectively, and four of them experienced AAP in the remission induction phase. Twelve patients tested positive for fusion genes, including ETV6-RUNX1 (n=5), Ph/BCR-ABL (n=4), TCF3/PBX1 (n=2), and MLL-r (n=1) (Table 1).
The ALL IR group comprised 70.5% (n=31) of patients in our study. Approximately 20.5% (n=9) and 9.1% (n=4) of the patients were in the LR and HR groups, respectively. The IR and HR groups received the same combination of chemotherapeutic agents, as recommended in the CCCG-ALL-2015 protocol. Two patients were diagnosed with stage III disease for lymphoma, while one was diagnosed with stage IV disease (Table 1).
Asparaginase-associated pancreatitis Clinical manifestation
Symptoms related to PEG-Asp-associated pancreatitis occurred after a median of 13 (1-50) d from the last PEG-Asp administration. The median dose of PEG-Asp administered before AAP onset was two doses (range: 1-9 doses), in which the majority of AAP cases occurred during induction treatment (32/45, 71.1%).
Abdominal pain was the most common symptom reported in all patients with AAP manifesting as localized (n=29) or diffuse (n=18) abdominal pain. The duration of abdominal pain was diverse and could last 41 d. Other commonly associated symptoms included nausea (n=23), vomiting (n=26), fever (n=26), diarrhea (n=5), and gastrointestinal bleeding (n=4) (Table 2).
Approximately 67.3% (n=33) of the patients developed acute complications. Systemic complications included septic shock or hypovolemic shock (n=16), multiple organ failure (n=3), hypoproteinemia (n=9), electrolyte disorder (n=7), coagulation disorder (n=4), disseminated intravascular coagulation (DIC) (n=4), and diabetic ketoacidosis (n=1) (Table 2). Local complications included abdominal ascites (n=20), peripancreatic fluid collection (n=12), intestinal obstruction (n=3), and pancreatic pseudocyst (n=5). In contrast, seven patients developed persistent complications, and all experienced severe AAP during the acute phase. Persistent complications included obstruction of the main pancreatic duct (n=3), pancreatic inflammation/edema (n=3), and pancreatic atrophy (n=1). Patients with diabetic ketoacidosis do not require persistent insulin support. Four patients who experienced hypoproteinemia or shock were developed persistent pancreatic inflammation/edema or pancreatic atrophy. Three patients required endoscopic retrograde cholangiopancreatography (ERCP) for bile duct stent placement, while two required drainage of ascites.
Pancreatic enzymes and imaging results
Elevation of serum amylase levels was reported in 47 patients with AAP. Approximately 71.4% of patients (n=35) experienced an elevation three times higher than the UNL within 48 h of onset. Serum amylase reached its peak value within a median of 2 (1-42) d, with a median peak value of 1,035 (40-3,149) U/L. Serum amylase levels returned to normal within 10 (1-355) d. However, serum amylase levels in four patients did not return to normal because of poor clinical outcomes. Forty-eight patients experienced an elevation in serum lipase levels three times higher than the UNL, with a median peak value of 1,742 (899-4,545) U/L (Table 3). Serum lipase level was significantly elevated compared with serum amylase and persisted at a markedly high level (median days of elevation 21.5 d, range 4-355 d) after serum amylase level normalized (median days of elevation 10 d, range 1-355 d). In sporadic cases, lipase levels remained extraordinarily high (>2,000 U/L) for months until normalization.
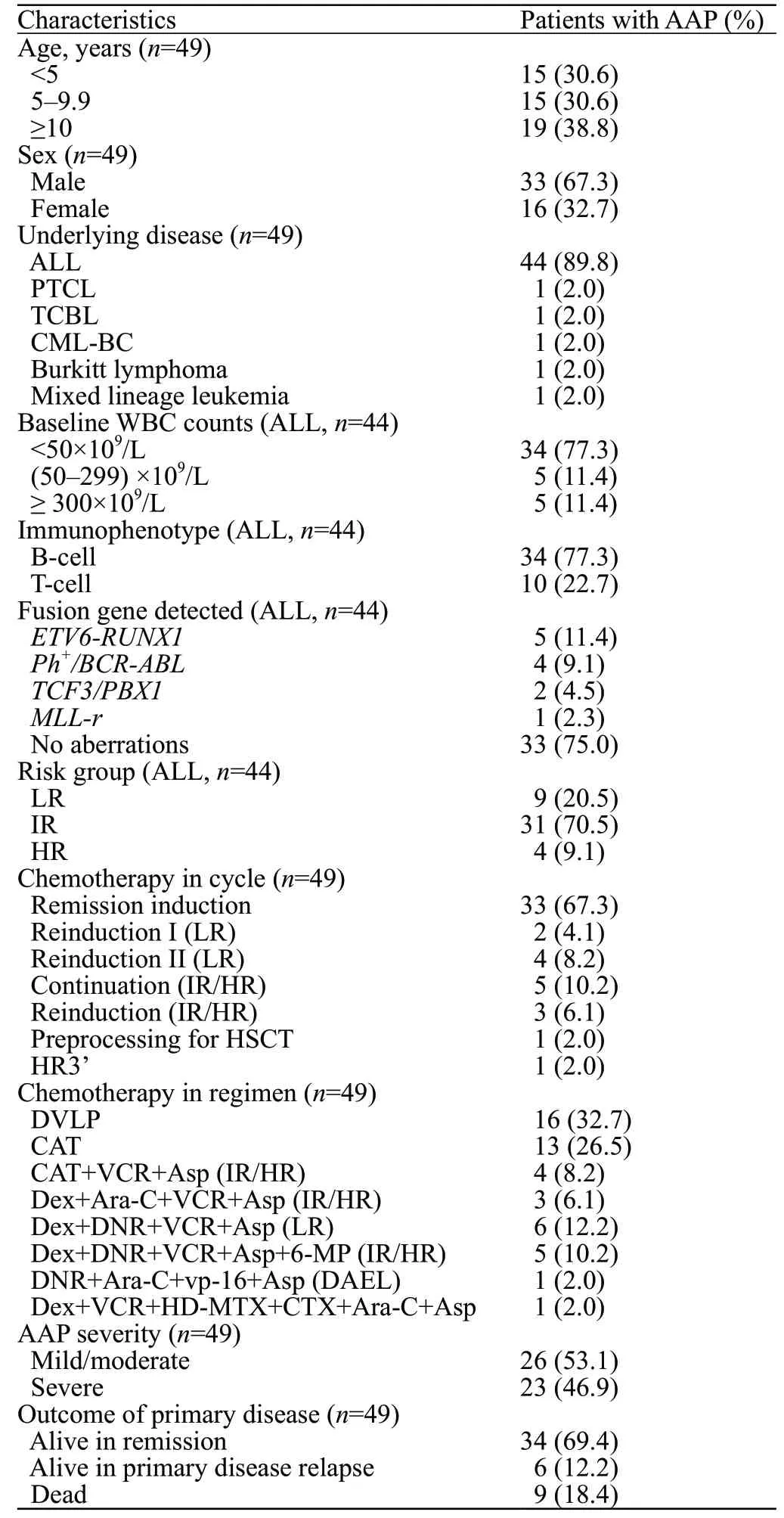
Table 1. Baseline and underlying disease characteristics
In our cohort, either abdominal ultrasound or contrast CT scan, or both depending on the case, were performed in most of our patients. The detection rates of ultrasound and CT were 77.8% (35/45) and 66.7% (24/36) (Table 3). The overall imaging detection rate was 87.5% (42/48). The remaining seven patients were diagnosed based on their clinical symptoms and elevated serum amylase or lipase levels. Both imaging methods provided an acceptable detection efficacy. Ultrasound can be applied in earlier clinical settings and performed at bedside. Hence, it is preferentially used under critical conditions. Direct ultrasound findings included enlargement, edema, and echo-heterogeneity of the pancreas. Contrastenhanced CT, which had a higher sensitivity in detecting abdominal effusion and was less disturbed by intestinal gas, was better for assessing the severity. In addition, hepatic and renal function status should be assessed before injecting the contrast medium.
AAP severity
Regarding the AAP severity, 53.1% (=26) of the patients had mild/moderate AAP, whereas 49.6% (=23) had severe AAP (Table 1). For 44 patients with ALL, a comparison of the baseline characteristics of mild/moderate and severe AAP is shown in Table 4. Patients with ALL aged ≥10 years were more likely to develop severe AAP than those younger than 10 years (=0.008). In all 23 patients with severe AAP, 16 patients experienced septic shock or hypovolemic shock, accompanied by organ dysfunction within 48 h of onset; others simultaneously experienced more than one systemic complication. Seven patients with severe AAP were developed to persisting complications, compared with none in mild/moderate AAP group (=0.003).
Treatment and clinical outcomes
The major treatment measures for pancreatitis include fasting, nutritional support (total parenteral nutrition or nasojejunal tube feeding), intravenous fluid resuscitation, and pancreatic secretion inhibition. According to the CCCG-ALL 2015 protocol, patients were advised to temporarily suspend Asp once diagnosed with AAP and receive pancreatic secretion inhibitors as a major treatment. Somatostatin or its synthetic analog, octreotide, is widely used to inhibit both pancreatic exocrine and endocrine secretions. In some cases, ulinastatin, a protease inhibitor, was administered to stabilize the pancreatic enzymatic activity and alleviate local tissue inflammation. Minor treatment measures include antibiotics against infection, proton pump inhibitors (i.e., omeprazole) to inhibit gastric acid secretion, and continuous renal replacement therapy (CRRT) to alleviate systemic inflammation and remove toxic substances. All major measures were provided once the diagnosis was confirmed. Octreotide was administered at a dose of 0.1 mg, 1-3 times/d (intradermal), and ulinastatin was administered at a dose of 100,000 U, 1-3 times/d (intravenous). Complications such as obstruction of the main pancreatic duct and pancreatolithiasis were resolved by performing an ERCP, while pseudocyst, peri-pancreatic fluid, and abdominal ascites were resolved by performing percutaneous puncture drainage.
Twenty-two patients (44.9%) were immediately transferred to or continued to stay in the pediatric intensive care unit (PICU) to receive advanced supportive care for either AAP or other co-existing life-threatening conditions, with a median length of PICU stay of 7.5 d (2-20 d). Among the 22 patients, five were in the mild/moderate AAP group. They were admitted to the PICU for non-AAP-related reasons, such as systemic inflammatory response syndrome before AAP (n=2), severe pneumonia (n=2), and hepatic encephalopathy before AAP (n=1). In the severe AAP group, five of them continued to be treated in the general ward because the shock was quickly corrected. One of them received palliative care directly owing to family and financial factors and finally died because of multiple organ failure. All patients who developed a severe infection owing to myelosuppression were administered antibiotics accordingly, as infection may increase the risk of developing severe AAP. Finally, 89.8% (n=44) of the AAP patients survived after the occurrence of AAP, and 11.4% (n=5) died due to septic shock or MODS after receiving appropriate medication and measures. No fatalities were observed in the mild/moderate AAP group.

Table 2. Clinical manifestations of AAP patients
A total of 81.6% (40/49) of patients continued the chemotherapy, with 12 patients (30%) re-exposed to Asp after recovery from AAP. Most of the re-challenged patients with AAP received an adjusted dose of either PEG-Asp or E.coli-Asp during chemotherapy. Five patients (41.7%) with Asp re-exposure experienced recurrent AAP, while seven patients (58.3%) completed all of the courses containing Asp. Most recurrent AAP followed by re-challenged Asp were mild or moderate cases, while one re-challenged patient after two courses of Asp suspension developed severe AAP. Five patients experienced ≥2 recurrence AAP. Nine patients discontinued the chemotherapy protocol completely because of death, hematopoietic stem cell transplantation, or switching to another regimen, while 28 patients continued chemotherapy without Asp. Regarding the outcome of the primary disease, 13.5% (5/37) of patients without Asp re-challenged or discontinued the chemotherapy protocol completely experienced primary disease relapse. In comparison, 8.3% (1/12) of patients with re-challenged Asp experienced primary disease relapse. For the final outcome of primary disease, nine patients died, six patients experienced primary disease relapse, and the remaining 34 were still alive (Table 1).

Table 3. Laboratory and radiographic ratio of patients with AAP
Compared with mild/moderate patients, severe AAP patients were associated with a longer PICU stay (P=0.048) and higher mortality rate (P=0.019) after the first AAP. Although there was no significant diff erence in the outcome of the primary disease (P=0.905) and impact on chemotherapy (P=0.070) between mild/moderate and severe AAP, there was still a potential risk factor for survival. Therefore, the prognostic value of AAP severity remains to be confirmed in a larger cohort.
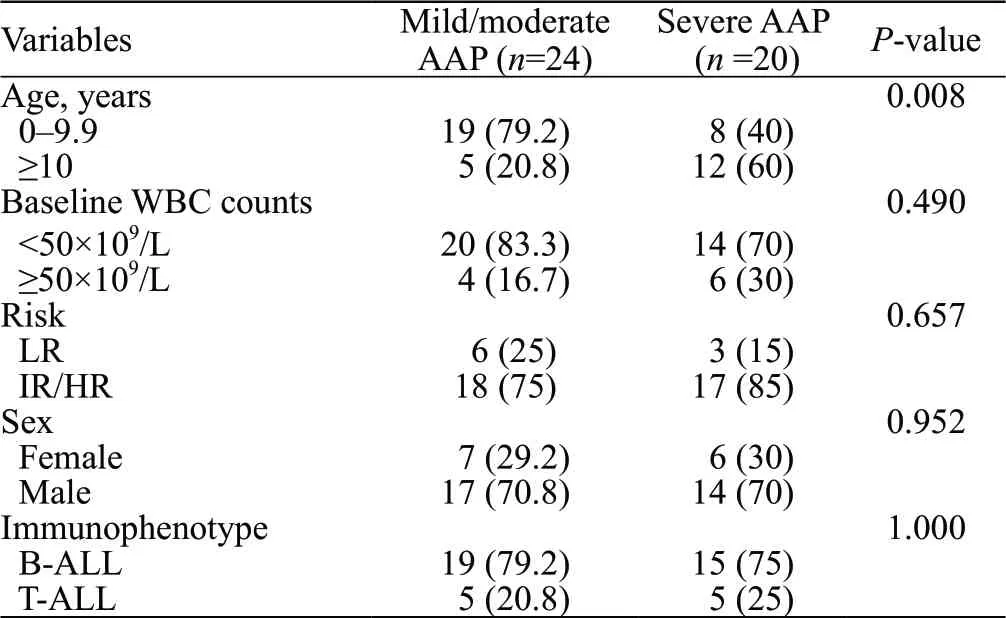
Table 4. Comparation of baseline characteristics of mild/moderate and severe AAP in patients with ALL
DISCUSSION
Currently, there is an observational studyincluding 26 trials conducted by 18 trial groups internationally, mainly focused on the characteristics of patients with AAP. And there are many collaborative studies on AAP, such as the Nordic Society of Paediatric Haematology and Oncology ALL2008 protocol (NOPHO ALL2008) and PTWG, mainly to explore the clinical and genetic risk factors of AAP.However, all of these studies were primarily based on the European and American populations. Ethnic differences may also play a role in the development of AAP. Till now, studies on AAP in China are rare.
In this study, most of the AAP were PEG-Aspassociated. AAP occurred at a median of 13 d (1-50 d) after PEG-Asp administration, which agrees with previous studies (11-15 d).One previous study reported a longer duration (26 d) between Asp administration and AAP.However, these results suggest that the occurrence of pancreatitis after 2-3 weeks of PEG-Asp administration should be alert.
The manifestations of AAP are closely related to the pharmacokinetic characteristics of PEG-Asp. The halflife of PEG-Asp (5.7 d) was significantly longer than that of the other Asp formulations.The enzymatic activity could last for 18-21 d or longer after administering a single dose of PEG-Asp at 2,500 U/m.Serum asparagine remained depleted for 26-34 d.Thus, PEGAsp should be administered at intervals of no less than 20 d during the induction period. Imbalance in serum amino acids is associated with pancreatic injury;prolonged activity could pose a delayed or sustained toxic effect in certain clinical settings, which presents as delayed symptoms weeks after administration, leading to an impression of “sudden severe onset.” AAP can be characterized by a delayed onset of abdominal symptoms, dynamic changes in radiographic evidence, and a longer time for serum amylase to normalize.

Table 5. Clinical outcome and prognosis of patients with AAP
In our study, AAP was more prevalent in the early induction stage (67.3%), and the median number of PEG-Asp doses before the onset of AAP was two doses, which suggested that the episodes of AAP might not be related to the cumulative doses but to individual differences such as genetic predisposition. A previous study on the genotype and discovery of AAP-related genetic variants provided insights into potential risk factors for developing AAP. CPA2, RGS6, and ULK2 are AAP-related variants reported previously.Further validation is required by conducting large cohort studies and investigating its pathophysiological mechanism.
As suggested in recent ALL guidelines, patients with severe AAP (severe pancreatitis with amylase elevation >3 times the UNL, accompanied with pseudocyst within 48 h) were not allowed to receive any Asp as a prospective chemotherapy regimen.This definition was based on the revised Atlanta criteria (2012), a classification system for adults. However, previous studies have used inconsistent criteria, such as the CT severity index, CTCAE, PTWG, or Children Cancer Group criteria, to classify pediatric AAP severity. There are certain limitations to different standards. For example, in PTWG, patients who experienced shock due to AAP but corrected within 72 h were also classified into the mild group. Moreover, persistent elevation of serum lipase or amylase may not be consistent. Excessive emphasis on time is not conducive to classifying pancreatitis severity. In contrast, the CTCAE standard used in our study is more practical for clinical application. The reported incidence rate of severe AAP varies from 7% to 86%.In our study, the incidence of severe AAP was 46.9% (23/49). Diff erent criteria for judging pancreatitis severity, chemotherapy regimens selected by diff erent studies, and diff erent chemotherapy intensities may have affected the results. Moreover, the propensity difference in etiological factors between children and adults with pancreatitis determines their distinct clinical manifestations. Similar to previous findings, among our patients with ALL, the older they were, the more likely they would develop severe pancreatitis.Although the sample size of our study was limited, it also suggested that for older patients, especially those aged ≥10 years old, attention should be focused on whether sudden changes occurred in the condition and the possibility of developing severe AAP. However, it is necessary to establish a well-defined classification for pediatric AAP. Evidence-based studies and long-term follow-up are required to determine the appropriate AAP definition and practical AAP severity classification.
With regard to the imaging approach used for diagnosis, both ultrasound and contrast CT scans are the preferred screening tools for AAP. In some mild/moderate patients, ultrasound might be unable to detect inflammatory edema or signs of pancreatitis. This has been observed in a previous study.A large amount of abdominal gas and incompliance during the procedure (mostly in younger children) might impact the ultrasound results. Thus, an alternative imaging approach, such as contrast-enhanced CT, should be used to assess pancreatitis. Although both MRI and CT scans demonstrated equal strength in detecting pancreatitis, CT scans are less time-consuming and require fewer imaging sequence procedures; thus, clinicians can obtain instant imaging results and provide prompt management.
For the treatment of most patients without primary diseases, the mainstay for acute pancreatitis is supportive therapy. However, considering that the basic status of ALL patients after chemotherapy was relatively poor, and the occurrence of pancreatitis may cause a delay or interruption of chemotherapy, the treatment was relatively active, and pancreatic enzyme inhibitors (octreotide or somatostatin) were administered. Most of our patients received scheduled chemotherapy in the wards during the onset of AAP. Chemotherapy-related toxicities were carefully monitored, and appropriate immediate treatments were provided when AAP was suspected. Therefore, pancreatic enzyme inhibitors were administered immediately after the diagnosis to prevent the progression to severe AAP. As all patients received the same standardized treatment simultaneously, we cannot determine whether such medication led to a more rapid recovery.
In some patients, inappropriate administration of Asp, such as improper re-exposure dosage and choice of formulation, may contribute to AAP relapse. These patients might be more susceptible to the eff ects of Asp and require further examination to determine risk factors. Nevertheless, most patients with AAP relapse achieved sustained complete remission of leukemia, except for one who had ALL relapse. In summary, clinicians should carefully consider Asp use in patients at a higher risk of AAP relapse. Unfortunately, our study could not include larger samples of patients with relapse; hence, it was difficult to confirm potential risk factors. The management of AAP should be more pragmatically, and suggested treatments should be explained further to avoid AAP during re-challenge to improve the clinical outcome.
Our study reported a higher mortality rate than a previous study (12.2% vs. 2%).Regarding the potential impact of Asp re-exposure on AAP clinical outcome, all patients with Asp re-exposure remained alive during follow-up, although one patient experienced primary disease relapse (ALL). It should be noted that all severe AAP patients in our cohort required intensive supportive care. Some patients previously developed severe infections due to myelosuppression and persistent fever during Asp treatment. Moreover, adverse events such as coagulation disorders, pleural effusion, and substantial ascites have been widely observed. All these events posed increasing difficulties in the management of AAP. Therefore, we presumed that these patients experienced irreversible multiple organ failure during AAP, resulting in increased mortality in our study.
Moreover, prophylactic measures, early recognition, and AAP management effectively reversed severe conditions. Our center serves as a standardized treatment center for pediatric hematological diseases; thus, the healthcare personnel in our center have high levels of awareness and clinical experience regarding chemotherapy-related toxicities. Most of our patients were admitted to the hospital throughout chemotherapy. They received intensive supportive care once diagnosed with AAP, thus their clinical outcomes were improved. In an expert guideline for adolescent and adult patients, the panel suggests using AAP grading system based on the dynamic changes observed during monitoring, and anticipates that HR patients may develop severe AAP.We agree with the urgency and importance of establishing such treatment protocols for pediatric patients.
It is important to stress that the complications of AAP were just the integrated outcomes resulting from the use of Asp-included therapy, but not the direct consequences of Asp medication alone. Using concomitant chemotherapeutic agents such as Ara-C and 6-MP can potentially cause pancreatitis. Other HR medications have also been used in certain clinical settings. In addition, patients’ nutritional status and history of Asp use should be considered. Long periods of fasting may contribute to the development of cholelithiasis, a common etiological factor of pancreatitis. Patients with a hypersensitive reaction and a history of AAP should also adjust their dosage.
Limitations
However, as a retrospective study, some limitations cannot be ignored. (1) It involved a highly selected patient cohort. (2) Because this was a single cohort study, the samples of these studies were small. (3) Mainly focused on the clinical characteristics of AAP, this study did not analyze the risk factors of AAP and the event free survival rate and overall survival rate of patients with and without AAP, which are also important for a complete understanding of AAP.
CONCLUSIONS
Early recognition and management are essential in reversing the severity of AAP. Unfortunately, the existing criteria had a low strength in determining the severity of pediatric AAP. Hence, a well-defined AAP definition could help distinguish patients with high risk in redeveloping AAP and ALL relapse, and prevent unnecessary withdrawal of Asp. This study summarized AAP’s clinical characteristics, especially with PEG-Asp, which provided clinical evidence for large cohort studies.
Funding: Shanghai Natural Science Foundation (No. 19ZR1432900) supported this study.
Ethical approval: Our study was approved by the Ethics Committee of Shanghai Children’s Medical Center (SCMC), affiliated hospital of Shanghai Jiao Tong University School of Medicine, and written informed consent was obtained from patients’ parents or guardians.
Conflicts of interests: The authors have no competing interests.
Contributors: All authors made individual contributions to the writing of the article, including design, literature search, data acquisition, data analysis, statistical analyses, manuscript preparation, and manuscript editing. CXL, YYZ and QSY are the co-first authors.
