Efficacy and safety of adalimumab in comparison to infliximab for Crohn's disease: A systematic review and meta-analysis
lNTRODUCTlON
Our current study has some limitations. First, we only included observational studies and failed to control adequately confounders, such as disease severity, disease phenotype, steroid use,
. In addition to clinical benefits, we should consider other factors, such as patients’ preferences and costs. Future studies are needed to address these questions.
IFX, a chimeric monoclonal antibody against TNF-α, is the first approved anti-TNF-α for moderate to severe CD. ADA is a humanized monoclonal antibody against TNF-α. IFX is given by intravenous infusion every 8 wk, whereas ADA is administered subcutaneously every 4 wk. Differences in immunogenicity and route of administration among them allow for potential variability in therapeutic properties and efficacy. However, clear recommendations have been limited due to a lack of head-tohead treatment comparison. A network meta-analysis published in 2014 found that ADA may be the most efficacious agent for maintenance of remission in CD in biologic-naïve patients[3], while many new clinical practice experience studies have shown their effectiveness and safety data were comparable. Furthermore, even though there has been cumulative research, few studies have focused on secondary loss of response, anti-TNF naïve or non-naïve patients, and the benefits of treatment optimization, such as dose intensification, shortening interval, and combination with immunomodulators. We performed a meta-analysis to synthesize these results and compared the efficacy and safety of ADA and IFX.
MATERlALS AND METHODS
Our protocol was registered with PROSPERO (CRD: 42021191655). We followed the Preferred Reporting Items for the Systemic Review and Meta-Analysis guidelines.
Search retrieval
We performed literature search of electronic sources, including PubMed, Cochrane Library, Web of Science, and Embase, from initiation until October 31, 2020. No language restrictions were applied. The search terms included “Crohn disease,” “adalimumab”, and “infliximab” as Medical Subject Headings terms and their entry terms (Crohn disease: Crohn*; ileitis. Adalimumab: Humira; Exemptia. Infliximab:Remicade) to improve search outcomes. We also screened references of relevant articles to avoid omissions.
Inclusion and exclusion criteria
We included cohort studies comparing ADA and IFX for treating adults with CD. Comparisons of induction of remission and response rates, maintenance of remission and response rates, secondary loss of response rates, and the incidence of adverse events were among the outcomes of included studies.Excluded studies included those conducted in the pediatric population, those that did not investigate patients with inflammatory bowel disease, and those that did not report any outcomes of interest.
Study selection
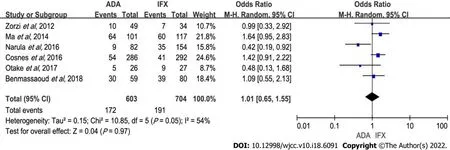
Six studies with 1307 patients were included (603 receiving ADA and 704 IFX therapy)[5-7,9,12,17]. There was no statistical difference between the two treatments (OR: 1.01,95%CI: 0.65-1.55,
= 0.97). Heterogeneity was notable (
= 0.05,
= 54%) (Figure 4). Heterogeneity was linked to the study by Narula
[9], which found that IFX had more rate of loss of response than ADA.On sensitivity analyses, the results remained the same after excluding any one study(Supplementary Table 1). There was also no significant difference between ADA and IFX when subgroup analysis was done (Table 2).
Data extraction
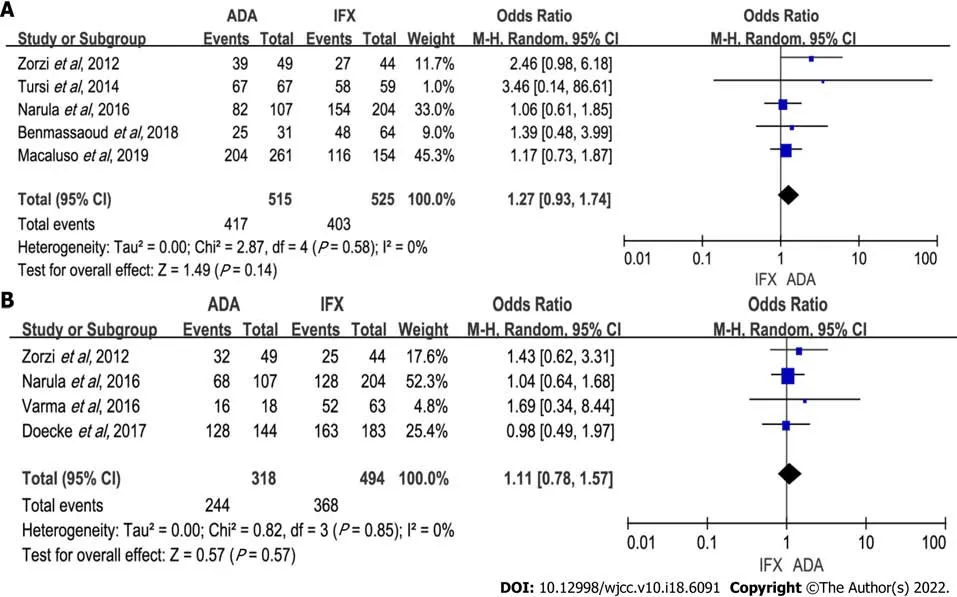
Five studies (1040 patients) recorded induction of response[6,7,9,10,15]. No difference was shown between groups in response rates (OR: 1.27, 95%CI: 0.93-1.74,
= 0.14). Of the 1040 patients in five studies, 515 received ADA therapy. The heterogeneity of those studies was insignificant (
= 0.58,
= 0%) (Figure 2A). Sensitivity analysis showed no significant changes to the exclusion of any one of the studies (Supplementary Table 1). Subgroup analysis revealed no remarkable difference between groups (Table 2).
在折页机构中引入弹簧后,折页机构的增加了8个局部自由度,4个为轴承的径向动态刚度引起的变形量lki,表现为弹簧受到压缩变形;4个为轴承径向压缩的角度γi,表现为弹簧压缩方向的变化.将运动副间隙假设成无质量刚性杆,将轴承滚子假设为弹簧.用刚性杆与弹簧组合模拟折页机构各运动副接触情况,即为刚性杆弹簧组合假设.
The outcomes of interest included: (1) Induction of clinical remission defined as Crohn’s disease activity index (CDAI) < 150, Harvey Bradshaw Index (HBI) ≤ 4, or by physician's global assessment after≤ 14 wk; (2) Induction of clinical response was defined as ΔCDAI ≥ 70, ΔHBI ≥ 2, or by physician's global assessment after ≤ 14 wk; (3) Maintenance of remission referred to clinical remission after ≤ 54 wk; (4) Maintenance of response referred to clinical response after ≤ 54 wk; (5) Secondary loss of response was defined as a reappearance of disease activity after achieving induction response, coupled with the need to change treatment, including dose intensification, the addition of an immunomodulator,or need to discontinue treatment; and (6) Secondary outcomes included a comparison of the incidence of overall adverse events, severe adverse events, and opportunistic infections in trials of maintenance therapy.
“哼哼叽叽”、“唱唱咧咧”这两个词中的“哼”和“唱”是可以单独成词的,而“叽”和“咧”不可以。重叠后也是一样,AA式“哼哼”和“唱唱”是可以独立使用的,而BB式“叽叽”和“咧咧”一般不能单独使用。但“叽叽”作为叠音词时改变声调,音为“jìji”时变成动词是可以单独成词的,当然,这种情况是比较少见的。
综上所述,在临床护理教学中采用CPBL教学模式,力求提高临床护理教学质量,为CPBL教学法在临床护理学中广泛应用提供参考。
Quality assessment
One author assessed the quality of included studies through the Newcastle-Ottawa Quality Assessment Scale (NOS). High-quality studies were defined by a total score of ≥ 6.
Statistical analysis
RevMan 5.3 and Stata 16.0 software were used for statistical analysis. Odds ratio (OR) and concomitant 95% confidence interval (CI) were evaluated for the quantitative analyses. The random-effect model was used. Heterogeneity was explored by calculating
and employing the Q test. An
estimate > 50% and a
< 0.05 were regarded markers of significant heterogeneity, and its causes were investigated. We performed sensitivity analyses and subgroup analyses to detect the source of heterogeneity.
< 0.05 was considered to indicate a significant difference. Subgroup analyses were conducted based on the following grouping criteria: (1) Studies evaluating outcomes on anti-TNF naïve patients
studies on non-naïve patients; (2) Studies evaluating outcomes on more perianal diseases in IFX group
equal perianal disease in IFX and ADA group; (3) Studies evaluating primary outcomes given with treatment optimization,
shortening the administration intervals, increasing the dose, and/or combination with immunomodulator therapy; and (4) Studies evaluating secondary outcomes at ≤ 48 wk
> 48 wk.Funnel plots and Egger’s test was used to test for publication bias.
开展探究式实验的性别、年级和GPA并不影响学生对Q2和Q3的回答,但是女生比男生更相信探究式实验有助于强化她们对环境基本概念和应用的理解。对于Q4,三学年132位所调查的学生中,压倒性的92.4%学生是第一次接触探究式实验。仅有20个学生(占7.6%)以前接触过类似的探究式实验,这些不足8%的学生可能是由于外部课程(基础学科实验室或高中[12])而接触探究式实验。
RESULTS
Literature search
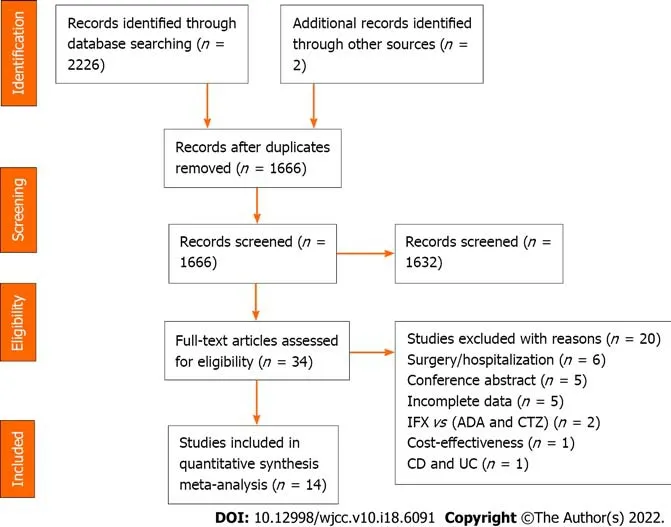
A preliminary search of the above database identified 2228 documents. Of these, we removed 562 duplicates, discarded 1632 studies after screening the titles and abstracts, and assessed the full text of 34 studies for eligibility. Finally, 14 cohort studies were included, and 20 were excluded. The flow diagram describes this process in detail (Figure 1).
Study characteristics
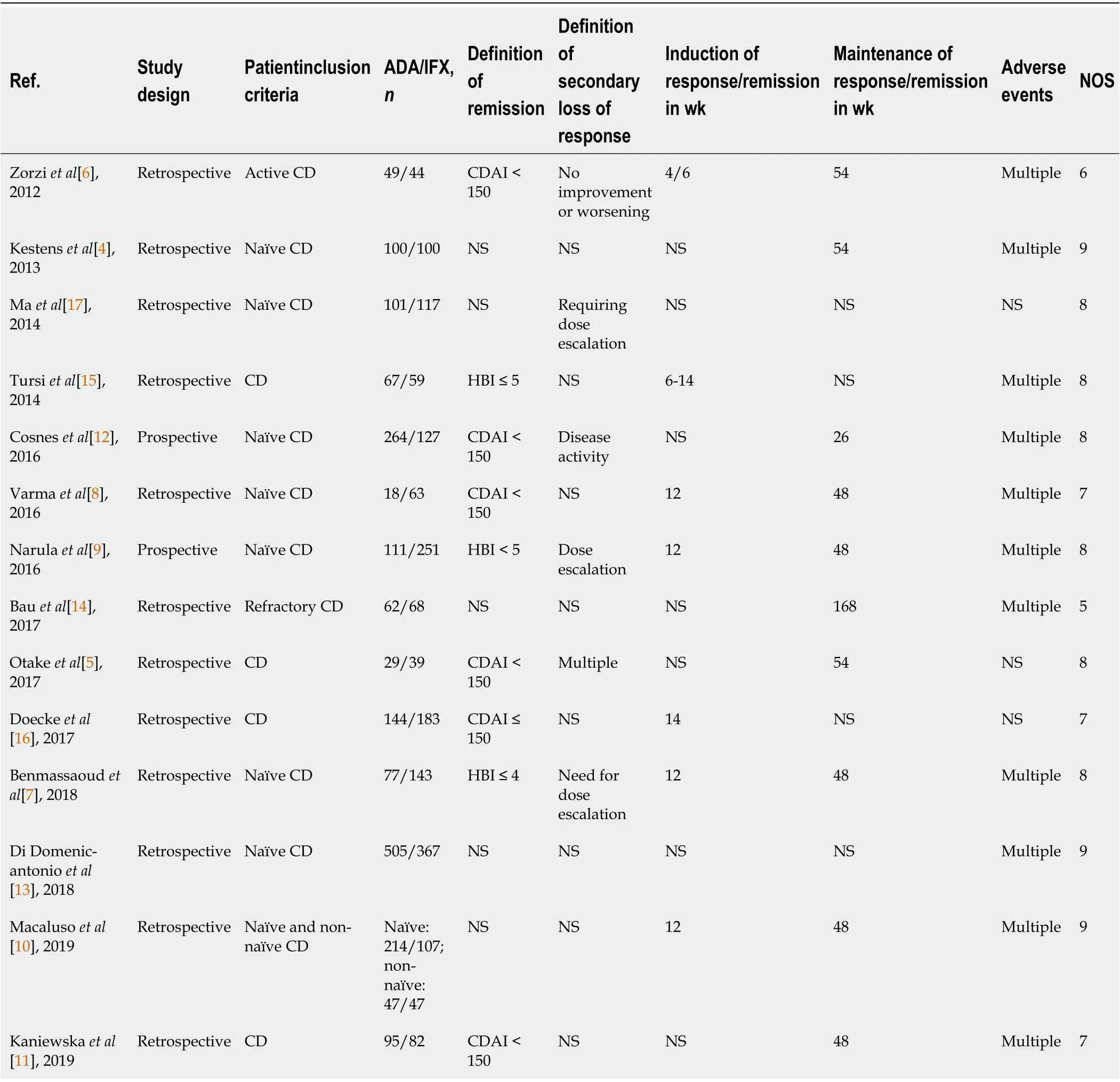
Study design, outcomes, the definition of outcomes, inclusion criteria, and follow-up time differed among the included studies. Our meta-analysis consisted of two prospective cohort studies and 12 retrospective cohort studies. Three pieces of research evaluated maintenance response or remission at 54 wk[4-6], five at 48 wk[7-11], and one at 26 wk[12]. Regarding the definition of outcomes, most incorporated studies evaluated clinical response or remission by CDAI or HBI except for the study by Macaluso
[10]. In addition, seven studies only included anti-TNF-naïve patients[4,7-9,12,13], and no study only included patients who failed anti-TNF treatment. Follow-up intervals across studies varied,ranging from 4 to 14 wk for induction period and 26 to 168 wk for maintenance period. The high NOS scores reflected the high quality of the enrolled studies. Thirteen studies got a score of ≥ 6, except for the study by Bau
[14], which scored 5. Table 1 showed the overall characteristics of the selected studies.
1.1 对象 将上海市按地域划分为9大区域,于2010年8月—2011年6月抽取每个区域1所社区医院,每所社区医院抽出1名社区护士,其中符合条件的为16名,随机分为实验组和对照组各8名,均为女性;年龄28~48岁;从事护理工作时间为4~28年;均未接触过相关培训。
Primary outcomes
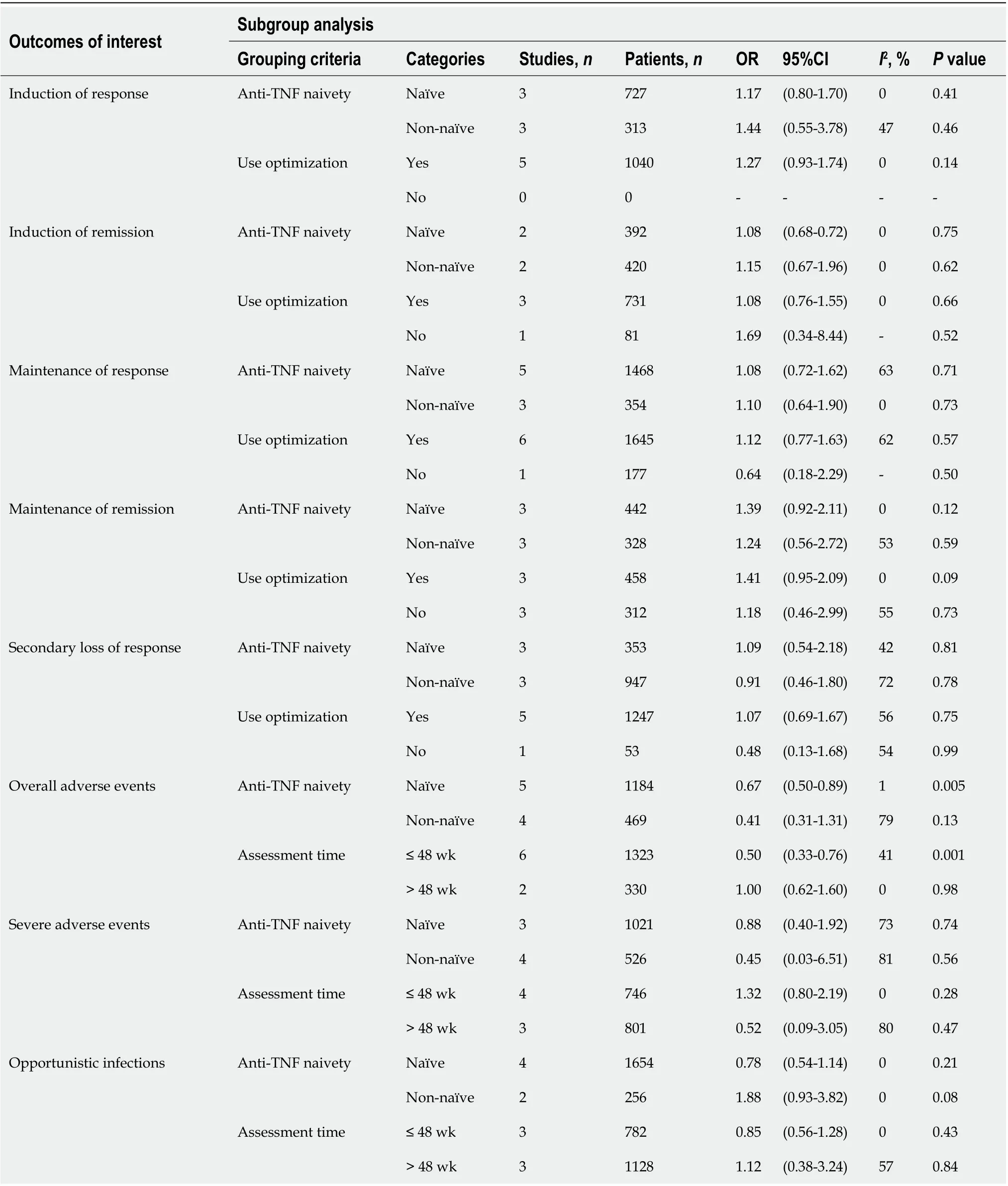
We collected the following variables: First author’s name, year of publication, country or area, study design, number of patients, gender, median age, Montreal classification, duration of follow-up, previous treatment, and outcomes of interest. The endpoint of this meta-analysis mainly included the induction response and remission, maintenance response and remission, overall adverse events rate, severe adverse events rate, and the rate of opportunistic infections.
When combining all four studies[6,8,9,16] reporting induction of remission data(318 on ADA therapy and 494 on IFX therapy), we found no difference between the two groups of patients (OR: 1.11, 95%CI: 0.78-1.57,
= 0.57). Heterogeneity was low (
= 0.85,
= 0%) (Figure 2B).Subsequent subgroup analysis showed similar results (Table 2). In sensitivity analyses, excluding any one of the studies did not significantly impact the results (Supplementary Table 1).
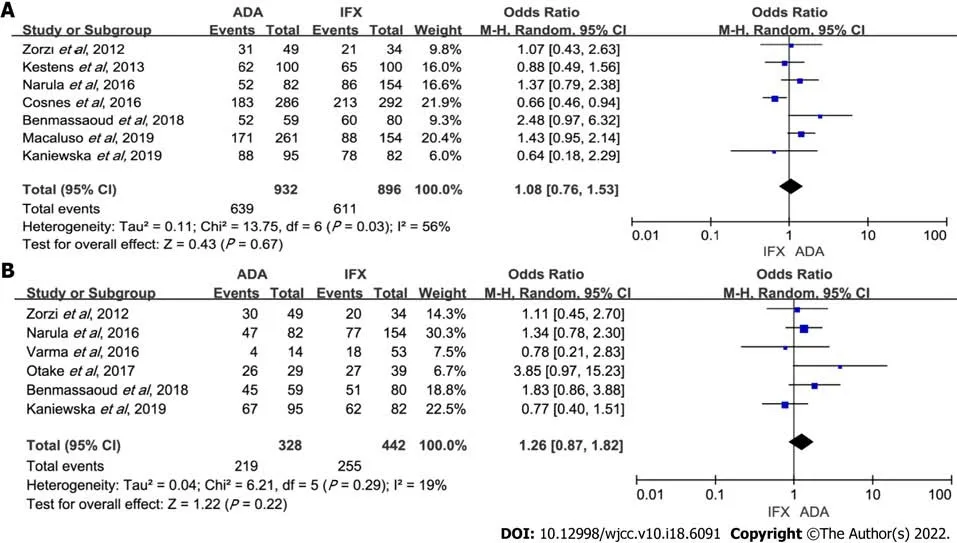
Of the 14 studies, seven reported the response rate in maintenance therapy[4,6,7,9-12]. A number of 1828 patients were included: 896 IFX-treated
932 ADA-treated. Data analysis showed that ADA and IFX had a similar rate of maintenance of response (OR: 1.08, 95%CI: 0.76-1.53,
= 0.67). Heterogeneity was significant (
= 0.03,
= 56%) (Figure 3A). Cosnes
[12] evaluating response at 26 wk increased heterogeneity. In the sensitivity analysis, the result remained unchanged with the exclusion of any study (Supplementary Table 1). Subgroup analyses also showed no difference between the two groups (Table 2).
There were 770 patients (328 on ADA therapy) available for analysis from six studies[5-9,11]. Data analysis showed that ADA and IFX had a similar rate of maintenance of remission (OR: 1.26, 95%CI: 0.87-1.82,
= 0.22). Heterogeneity was low (
= 0.29,
= 19%) (Figure 3B).Subgroup analyses also showed no statistical differences (Table 2). Sensitivity analysis indicated that the results were stable (Supplementary Table 1).
Two investigators (Yang HH and Huang Y) independently screened the titles, abstracts, and full texts of all papers to determine trial eligibility for inclusion. Investigators used a consensual approach to determine the inclusion or exclusion of selected studies after full-text assessment. Any disagreement was resolved through discussion or with a third researcher. The study characteristics were extracted independently by two authors using a standardized datasheet.
Secondary outcomes
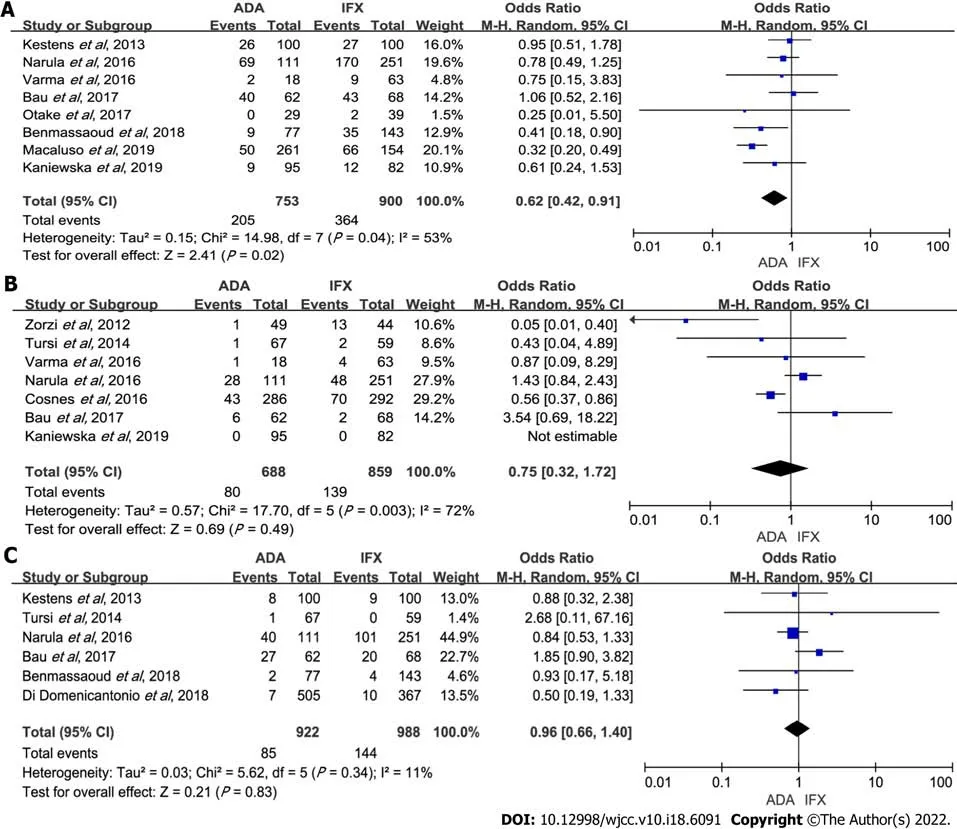
The incidence of overall adverse events was recorded in a total of eight cohort studies[4,5,7-11,14] that included 1653 patients, of which ADA was less than IFX (OR: 0.62, 95%CI:0.42-0.91,
= 0.02). There was high heterogeneity (
= 0.04,
= 53%) (Figure 5A). Subgroup analysis revealed that ADA had fewer overall adverse events than IFX in ≤ 48 wk follow-up time (OR: 0.50,95%CI: 0.33-0.76,
= 0.001); and in anti-TNF-α-naïve patients, IFX had more adverse events (OR: 0.67,95%CI: 0.50-0.89,
= 0.005) (Table 2). Sensitivity analysis indicated that the results were slightly unstable (Supplementary Table 1).
Our analysis of seven studies[6,8,9,11,12,14,15] with a total of 1547 patients showed ADA had a similar rate of severe adverse events with IFX (OR: 0.75, 95%CI: 0.32-1.72,
= 0.49).Sensitivity analysis was performed due to notable heterogeneity (
= 0.003,
= 72%) (Figure 5B).Heterogeneity mainly originated from Zorzi
[6] with more severe adverse events occurring in IFX therapy. The result remained unchanged with the exclusion of any study (Supplementary Table 1).Subgroup analysis also showed similar results (Table 2).
Six studies[4,7,9,13-15] reported side effects, with a total number of 1910 cases(ADA: IFX = 922:988). Opportunistic infections rates in the IFX and ADA groups were similar (OR: 0.96,95%CI: 0.66-1.40,
= 0.83), and no apparent heterogeneity was detected (Figure 5C). There was no significant difference when subgroup analysis was done (Table 2). Sensitivity analysis showed no significant changes when any one of the studies was excluded (Supplementary Table 1).
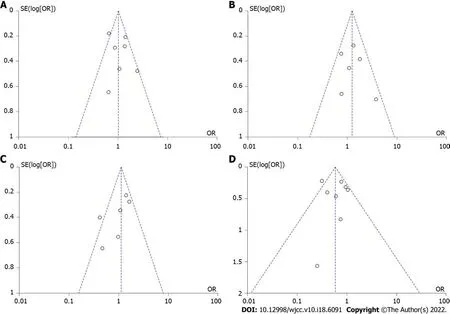
Publication bias and GRADE evaluation
The symmetry of the funnel plot indicated there was no publication bias (Figure 6). The Egger’s test showed no significant publication bias for maintenance of response (
= 0.7024 > 0.05), maintenance of remission (
= 0.1003 > 0.05), secondary loss of response (
= 0.0510 > 0.05), and overall adverse events (
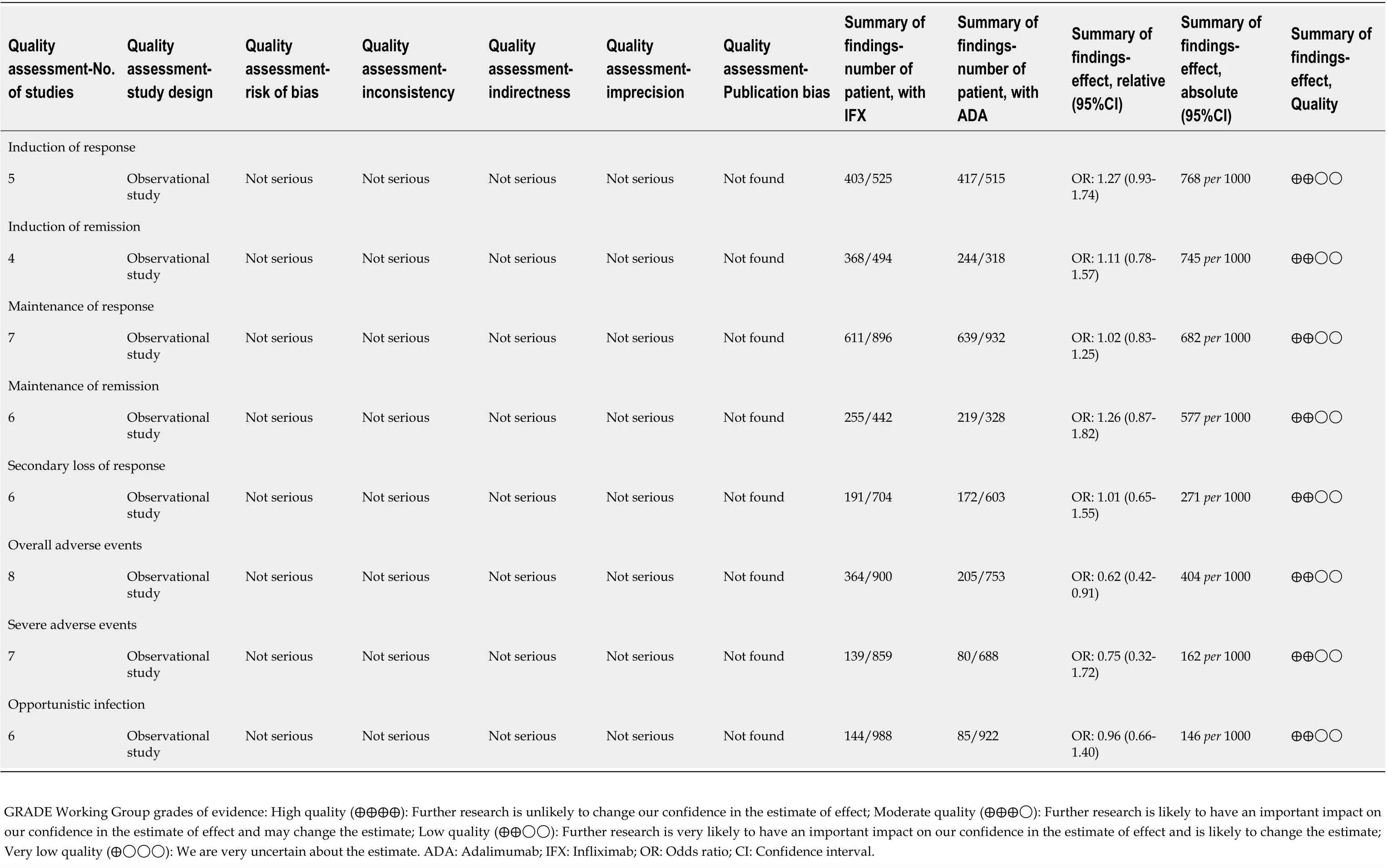
= 0.6717 > 0.05). GRADE evidence of all outcomes was judged as “low”. The results are shown inTable 3.
DlSCUSSlON
The immunogenicity of anti-TNF-α agents triggered the formation of anti-drug antibodies (ADAbs)specific to the agent administered. ADAbs of IFX or ADA and reduced serum concentrations in association with ADAbs together lead to decreased clinical benefit and increased adverse events.Although the immunogenicity of IFX is usually higher than that for ADA, we found both of them have similar response characteristics in CD patients. In our meta-analyses, no significant differences in primary outcomes were found between groups treated with IFX and ADA. These results were consistent with the results of most published studies[5-12,15-17]. One unexpected finding was the extent to which the overall adverse events rate of IFX was higher than that of ADA. Our meta-analysis indicated that physicians may choose on an individual basis, according to a possible history of adverse events to either IFX or ADA and to patient compliance, to give either an intravenous infusion or a selfadministered subcutaneous injection.
CD is a heterogeneous disease, and the therapeutic efficacy differs between the types of disease,
,location of disease, the existence of stenosis and/or fistula, or perianal involvement. There was no significant difference between IFX and ADA groups in the location of disease and existence of stenosis and/or fistula of included studies. However, IFX patients had more perianal diseases in the studies of Benmassaoud
[7], Varma
[8], Narula
[9], and Cosnes
[12]. Clinicians tended to choose IFX over ADA in patients with more severe disease activity or phenotypes (perianal disease) due to its intravenous administration and weight-based dosing schedule. We attempted to adjust for these differences through subgroup analysis, which led to the same conclusions (Supplementary Tables 2 and 3). Additionally, Ji
[18] found the cumulative rate of nonrecurrence or aggravation of fistula at 24 mo was not significantly different between IFX and ADA groups (62.5%
83.9%,
= 0.09).Current evidence suggested that IFX and ADA had similar effects in patients with perianal disease.
2.2 对选用的复合(混)肥或作物专用肥,不含有机质或含量在15%以下的肥料,要增加充分腐熟的畜禽纯干粪不少于20公斤/亩,可有效的杜绝或缓解玉米苗期肥害。
(4)位置。肺癌肿块分布可分为左肺叶、右肺叶、上叶、下叶几个部分。在36例周围型小肺癌患者中,肿块在左肺的上叶有8例,其比例为22.22%;中叶的有4例,其比例为11.11%;下叶的有8例,其比例为22.22%。患者中,肿块在右肺的上叶有4例,其比例为11.11%;在中叶的有2例,其比例为5.56%;在下叶的有10例,其比例为27.78%。
Co-immunosuppression affected the results of the analysis. The finding that combination therapy with an immunomodulator is superior with IFX but not with ADA was reported in Kestens
[4],Benmassaoud
[7], and Doecke
[16]. The possible reason is that IFX combined with immunomodulator treatment reduces its immunogenicity. However, clinical efficacy of ADA combination therapy did not differ from that of ADA monotherapy (71.8%
68.1% at week 26,
= 0.63)[21]. Therefore, more patients in the IFX group were combined with immunomodulator treatment than in the ADA group in the Narula
[9] study. No change was found in results after sensitivity analysis was conducted.Patients were on concomitant immunomodulation at anti-TNF induction to improve the efficacy of the induction of the remission and discontinued co-therapy due to adverse effects or intolerability (from the beginning). When loss of response occurred, concomitant therapy was resumed (later add on). Only the Cosnes
[12] study used immunomodulators later. No different results were found after sensitivity analysis was performed. Furthermore, CD patients who lost response were allowed to shorten intervals and double dosage. These optimization strategies also impacted the results. We conducted subgroup analyses comparing the outcomes between using dose optimization and not and found the clinical effect of ADA was similar to IFX.
Similar to the findings of many studies[4,10,17], the significantly higher rate of overall adverse events can be seen in patients using IFX, which could be attributed to infusion or allergic reactions.Benmassaoud
[7] reported that IFX group patients were more likely to have infusion or injection reactions than ADA. A higher rate of allergic reactions in the IFX was observed in a study by Narula
[9]. However, we noted that the difference did not exist in anti-TNF-α non-naïve patients and with long follow-up time. We were unable to evaluate long-term safety due to the different follow-up times of each study. Larger and long-term comparison studies will be necessary. In addition, the instability of the results also require further studies to establish these findings.
Biologic-naïve or non-naïve patients were important factors to influence the results. It is controversial whether ADA had similar efficacy to IFX in previous anti-TNF exposure CD patients. Macaluso
[10]compared clinical benefits between IFX and ADA only in biologic non-naïve CD patients and reported that there was no difference in clinical benefits at 12 wk and after 1 year (
= 0.600 and
= 0.620,respectively). A retrospective case-control study[19] found that the risk for ADAbs to IFX was higher than ADAbs to ADA when patients had prior antibodies to anti-TNF. They did not investigate clinical efficacy. However, Sasson and Ananthakrishnan[20] found that patients with high ADAbs titers exhibited similar rates of clinical efficacy to ADA therapy compared to those with low titers (at 3 mo and 12 mo
= 0.81 and 0.62 respectively). This may mean IFX and ADA have similar efficacy in previous anti-TNF exposed CD patients. Our findings indicated that either in naïve or non-naïve patients ADA and IFX had similar clinical response and remission. More studies conducted on previous anti-TNF exposure CD patients will be necessary.
Additionally, we failed to evaluate long-term results due to the different follow-up times of each study. Inokuchi
[22] performed a retrospective study to evaluate long-term prognosis. They observed that the rates of cumulative steroid-free remission rates and surgery-free did not differ significantly between the two groups after a median observation period of 64.2 mo (
= 0.42 and
=0.74, respectively). The goal of CD treatment requires more than clinical healing. Mucosal healing and tissue healing are expected to stop disease progression and reduce recurrence. Tursi
[15] found that mucosal healing and histological healing were comparable between the two groups (
= 0.946 and
=0.895, respectively).
罗璨璨(1994—),女,福建仙游人,硕士研究生,研究方向为岩土工程数值模拟、大坝与基础工程等方面的研究工作。
通过BIM技术完成的建筑模型是建筑的真实反映,可以对建筑施工进行总体计划,提高工作效率,保证工程进度,使高水平的施工技术运用到复杂的项目中,同时增强设计与施工之间的联系。
Although biologic agents targeting TNF-α have achieved remarkable progress in treating CD, some patients do not respond to the induction therapy or lose response over time (secondary loss of response). The anti-drug antibodies or low serum drug concentrations play a critical part in the loss of response[23]. If ADA is superior to IFX for remission, ADA should have a lower rate of secondary loss of response than IFX. However, we failed to find a difference in the secondary loss of response between the two groups, which contradicted our hypothesis. It was further demonstrated that both have similar effects.
This work is the first direct comparison meta-analysis to evaluate the comparative effectiveness and safety of ADA and IFX in CD. Previous network meta-analyses addressed similar outcomes in the Bayesian setting indirect comparison. In our study, we enrolled comparative trial data resulting in more credible results. Furthermore, head-to-head clinical trials comparing ADA and IFX would not be feasible in the future; therefore, our studies will help guide optimal therapies.
是啊,渺小感!想必很多登山的人都有过这种渺小感,登上高峰,觉得天空苍茫浩渺,大地宽广博大,人在天地之间,感觉自己慢慢缩小,小成一只蚁,小成一根草。人生渺茫,世事沧桑,诸多感受一拥而上,忽然间人的眼神里就流露出崇敬之光——相比大自然,人算得了什么,又有什么理由狂妄自大呢?
Crohn's disease (CD) is an incurable chronic progressive condition characterized by abdominal pain,diarrhea, and weight loss. Aminosalicylic acid preparations, glucocorticoids, immunosuppressants, and biological agents have been used for treatment. Of these, biological agents are most widely used,especially anti-tumor necrosis factor-α (anti-TNF-α) blockers, including infliximab (IFX) and adalimumab (ADA). They all have been proven effective in inducing and maintaining remission and are routinely used in the treatment of CD[1,2]. We do not know, however, which treatment should be considered the priority?
CONCLUSlON
IFX and ADA have similar response characteristics either in anti-TNF naïve and non-naïve CD patients,and ADA therapy has fewer overall adverse events. Our study indicates that IFX or ADA can be freely chosen as treatment based on physician and patient agreement. Eventually, the decision of which treatment to start may depend on factors such as patient preference and cost.
ARTlCLE HlGHLlGHTS

ACKNOWLEDGEMENTS
We would like to thank all authors of the included primary studies.
众所周知,授人以鱼不如授人以渔,课堂教学更是如此。作为教师的我们不可能手把手地教学生读每一本课外书,因而阅读方法的指导就显得尤为重要。在阅读指导课上,我首先就对学生进行了阅读整本书的方法指导。
FOOTNOTES
Yang HH and Zhou XC contributed to study design; Yang HH, Huang Y, and Wang RN contributed to the literature search; Yang HH, Huang Y, and Zhou XC participated in data extraction and analysis;Yang HH and Huang Y wrote the paper.
Nothing to disclosed.
The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
在手术医师授权方面,实现了对所有临床手术病种及所有外科医生(含介入医生)的再授权,并根据医务人员职称、工作能力授予相应手术等级资质,规范了手术操作授权,实行了手术分级动态管理。多年来,医院手术并发症发生率一直低于国家的基准值1.01%。
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
China
Hua-Hua Yang 0000-0002-0065-2741; Yi Huang 0000-0001-8049-1900; Xu-Chun Zhou 0000-0002-5128-3149; Ruo-Nan Wang 0000-0003-2049-6827.
Fan JR
Filipodia
Fan JR
1 Cushing K, Higgins PDR. Management of Crohn Disease: A Review. JAMA 2021 ; 325 : 69 -80 [PMID: 33399844 DOI:10 .1001 /jama.2020 .18936 ]
2 Danese S, Vuitton L, Peyrin-Biroulet L. Biologic agents for IBD: practical insights. Nat Rev Gastroenterol Hepatol 2015 ;12 : 537 -545 [PMID: 26284562 DOI: 10 .1038 /nrgastro.2015 .135 ]
3 Singh S, Garg SK, Pardi DS, Wang Z, Murad MH, Loftus EV Jr. Comparative efficacy of biologic therapy in biologicnaïve patients with Crohn disease: a systematic review and network meta-analysis.
2014 ; 89 : 1621 -1635[PMID: 25441399 DOI: 10 .1016 /j.mayocp.2014 .08 .019 ]
4 Kestens C, van Oijen MG, Mulder CL, van Bodegraven AA, Dijkstra G, de Jong D, Ponsioen C, van Tuyl BA, Siersema PD, Fidder HH, Oldenburg B; Dutch Initiative on Crohn and Colitis (ICC). Adalimumab and infliximab are equally effective for Crohn's disease in patients not previously treated with anti-tumor necrosis factor-α agents.
2013 ; 11 : 826 -831 [PMID: 23376000 DOI: 10 .1016 /j.cgh.2013 .01 .012 ]
5 Otake H, Matsumoto S, Mashima H. Does long-term efficacy differ between infliximab and adalimumab after 1 year of continuous administration?
2017 ; 96 : e6635 [PMID: 28422861 DOI:10 .1097 /MD.0000000000006635 ]
6 Zorzi F, Zuzzi S, Onali S, Calabrese E, Condino G, Petruzziello C, Ascolani M, Pallone F, Biancone L. Efficacy and safety of infliximab and adalimumab in Crohn's disease: a single centre study.
2012 ; 35 : 1397 -1407[PMID: 22519466 DOI: 10 .1111 /j.1365 -2036 .2012 .05100 .x]
7 Benmassaoud A, Al-Taweel T, Sasson MS, Moza D, Strohl M, Kopylov U, Paradis-Surprenant L, Almaimani M, Bitton A,Afif W, Lakatos PL, Bessissow T. Comparative Effectiveness of Infliximab Versus Adalimumab in Patients with Biologic-Naïve Crohn's Disease.
2018 ; 63 : 1302 -1310 [PMID: 29243105 DOI: 10 .1007 /s10620 -017 -4874 -6 ]
8 Varma P, Paul E, Huang C, Headon B, Sparrow MP. A retrospective comparison of infliximab
adalimumab as induction and maintenance therapy for Crohn disease.
2016 ; 46 : 798 -804 [PMID: 26865349 DOI: 10 .1111 /imj.13040 ]
9 Narula N, Kainz S, Petritsch W, Haas T, Feichtenschlager T, Novacek G, Eser A, Vogelsang H, Reinisch W, Papay P. The efficacy and safety of either infliximab or adalimumab in 362 patients with anti-TNF-α naïve Crohn's disease.
2016 ; 44 : 170 -180 [PMID: 27226407 DOI: 10 .1111 /apt.13671 ]
10 Macaluso FS, Fries W, Privitera AC, Cappello M, Siringo S, Inserra G, Magnano A, Di Mitri R, Mocciaro F, Belluardo N,Scarpulla G, Magrì G, Trovatello A, Carroccio A, Genova S, Bertolami C, Vassallo R, Romano C, Citrano M, Accomando S, Ventimiglia M, Renna S, Orlando R, Rizzuto G, Porcari S, Ferracane C, Cottone M, Orlando A; Sicilian Network for Inflammatory Bowel Diseases [SN-IBD]. A Propensity Score-matched Comparison of Infliximab and Adalimumab in Tumour Necrosis Factor-α Inhibitor-naïve and Non-naïve Patients With Crohn's Disease: Real-Life Data From the Sicilian Network for Inflammatory Bowel Disease.
2019 ; 13 : 209 -217 [PMID: 30295785 DOI:10 .1093 /ecco-jcc/jjy156 ]
11 Kaniewska M, Rosołowski M, Rydzewska G. Efficacy, tolerability, and safety of infliximab biosimilar in comparison to originator biologic and adalimumab in patients with Crohn disease.
2019 ; 129 : 484 -489 [PMID:31316042 DOI: 10 .20452 /pamw.14901 ]
12 Cosnes J, Sokol H, Bourrier A, Nion-Larmurier I, Wisniewski A, Landman C, Marteau P, Beaugerie L, Perez K, Seksik P.Adalimumab or infliximab as monotherapy, or in combination with an immunomodulator, in the treatment of Crohn's disease.
2016 ; 44 : 1102 -1113 [PMID: 27666569 DOI: 10 .1111 /apt.13808 ]
13 Di Domenicantonio R, Trotta F, Cascini S, Agabiti N, Kohn A, Gasbarrini A, Davoli M, Addis A. Population-based cohort study on comparative effectiveness and safety of biologics in inflammatory bowel disease.
2018 ; 10 : 203 -213 [PMID: 29440933 DOI: 10 .2147 /CLEP.S150030 ]
14 Bau M, Zacharias P, Ribeiro DA, Boaron L, Steckert Filho A, Kotze PG. Safety profile of anti-TNF therapy in Crohn's disease management: A Brazilian single-center direct retrospective comparison between infliximab and adalimumab.
2017 ; 54 : 328 -332 [PMID: 28954043 DOI: 10 .1590 /S0004 -2803 .201700000 -43 ]
15 Tursi A, Elisei W, Picchio M, Penna A, Lecca PG, Forti G, Giorgetti G, Faggiani R, Zampaletta C, Pelecca G, Brandimarte G. Effectiveness and safety of infliximab and adalimumab for ambulatory Crohn's disease patients in primary gastroenterology centres.
2014 ; 25 : 485 -490 [PMID: 24631020 DOI: 10 .1016 /j.ejim.2014 .02 .010 ]
16 Doecke JD, Hartnell F, Bampton P, Bell S, Mahy G, Grover Z, Lewindon P, Jones LV, Sewell K, Krishnaprasad K, Prosser R, Marr D, Fischer J, R Thomas G, Tehan JV, Ding NS, Cooke SE, Moss K, Sechi A, De Cruz P, Grafton R, Connor SJ,Lawrance IC, Gearry RB, Andrews JM, Radford-Smith GL; Australian and New Zealand Inflammatory Bowel Disease Consortium. Infliximab vs. adalimumab in Crohn's disease: results from 327 patients in an Australian and New Zealand observational cohort study.
2017 ; 45 : 542 -552 [PMID: 27995633 DOI: 10 .1111 /apt.13880 ]
17 Ma C, Huang V, Fedorak DK, Kroeker KI, Dieleman LA, Halloran BP, Fedorak RN. Crohn's disease outpatients treated with adalimumab have an earlier secondary loss of response and requirement for dose escalation compared to infliximab: a real life cohort study.
2014 ; 8 : 1454 -1463 [PMID: 24947334 DOI: 10 .1016 /j.crohns.2014 .05 .007 ]
18 Ji CC, Takano S. Clinical efficacy of adalimumab
infliximab and the factors associated with recurrence or aggravation during treatment of anal fistulas in Crohn's disease.
2017 ; 15 : 182 -186 [PMID: 28522947 DOI:10 .5217 /ir.2017 .15 .2 .182 ]
19 Vande Casteele N, Abreu MT, Flier S, Papamichael K, Rieder F, Silverberg MS, Khanna R, Okada L, Yang L, Jain A,Cheifetz AS. Patients With Low Drug Levels or Antibodies to a Prior Anti-Tumor Necrosis Factor Are More Likely to Develop Antibodies to a Subsequent Anti-Tumor Necrosis Factor.
2022 ; 20 : 465 -467 .e2[PMID: 33421628 DOI: 10 .1016 /j.cgh.2021 .01 .006 ]
20 Sasson AN, Ananthakrishnan AN. High Anti-Infliximab Antibody Titers Do Not Impact Response to Subsequent Adalimumab Treatment in Inflammatory Bowel Diseases.
2021 [PMID: 34117949 DOI:10 .1007 /s10620 -021 -07088 -x]
21 Matsumoto T, Motoya S, Watanabe K, Hisamatsu T, Nakase H, Yoshimura N, Ishida T, Kato S, Nakagawa T, Esaki M,Nagahori M, Matsui T, Naito Y, Kanai T, Suzuki Y, Nojima M, Watanabe M, Hibi T; DIAMOND study group.Adalimumab Monotherapy and a Combination with Azathioprine for Crohn's Disease: A Prospective, Randomized Trial.
2016 ; 10 : 1259 -1266 [PMID: 27566367 DOI: 10 .1093 /ecco-jcc/jjw152 ]
22 Inokuchi T, Takahashi S, Hiraoka S, Toyokawa T, Takagi S, Takemoto K, Miyaike J, Fujimoto T, Higashi R, Morito Y,Nawa T, Suzuki S, Nishimura M, Inoue M, Kato J, Okada H. Long-term outcomes of patients with Crohn's disease who received infliximab or adalimumab as the first-line biologics.
2019 ; 34 : 1329 -1336 [PMID:30724387 DOI: 10 .1111 /jgh.14624 ]
23 Roda G, Jharap B, Neeraj N, Colombel JF. Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management.
2016 ; 7 : e135 [PMID: 26741065 DOI: 10 .1038 /ctg.2015 .63 ]
 World Journal of Clinical Cases2022年18期
World Journal of Clinical Cases2022年18期
- World Journal of Clinical Cases的其它文章
- Diabetes mellitus susceptibility with varied diseased phenotypes and its comparison with phenome interactome networks
- Sequential chemotherapy and icotinib as first-line treatment for advanced epidermal growth factor receptor-mutated non-small cell lung cancer
- Impact of preoperative carbohydrate loading on gastric volume in patients with type 2 diabetes
- Disseminated strongyloidiasis in a patient with rheumatoid arthritis: A case report
- CYP27A1 mutation in a case of cerebrotendinous xanthomatosis: A case report
- Postoperative multiple metastasis of clear cell sarcoma-like tumor of the gastrointestinal tract in adolescent: A case report
